Solution Concentration Review A solution is a homogeneous











- Slides: 21

Solution Concentration

Review ¬A solution is a homogeneous mixture. ¬The solvent is the major component of the solution. ¬The solute is the minor component and active ingredient. ¬A saturated solution holds the maximum amount of solute that is theoretically possible for a given temperature.

How would you describe this picture?

Solution Concentration ¬Is one glass of tea stronger than the other? – What’s true about the “stronger” glass of tea? – How much tea does it have in it compared to the other glass?

Solution Concentration ¬Concentration – a ratio comparing the amount of solute to the amount of solution. ¬Many ways of expressing concentration: – Molarity (M) is the one we will be dealing with

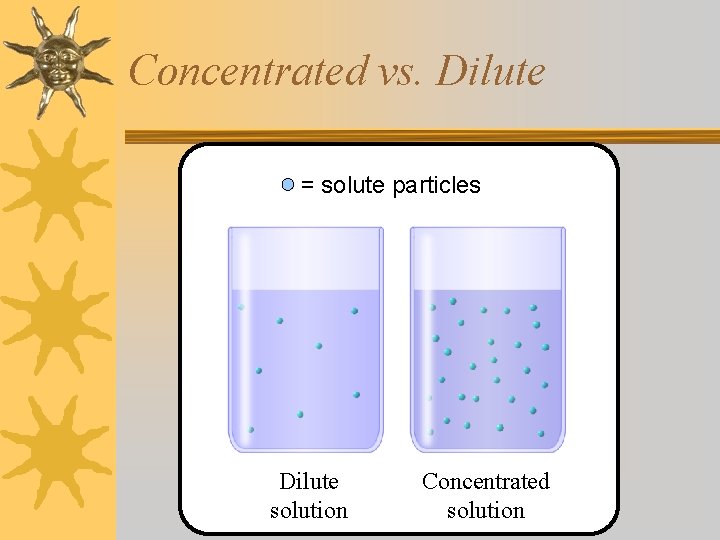
Concentrated vs. Dilute ¬The words “concentrated” and “dilute” are opposites. ¬EX: The dark tea is more concentrated than the light tea. ¬EX: The light tea is more dilute than the dark tea.

Concentrated vs. Dilute = solute particles Dilute solution Concentrated solution

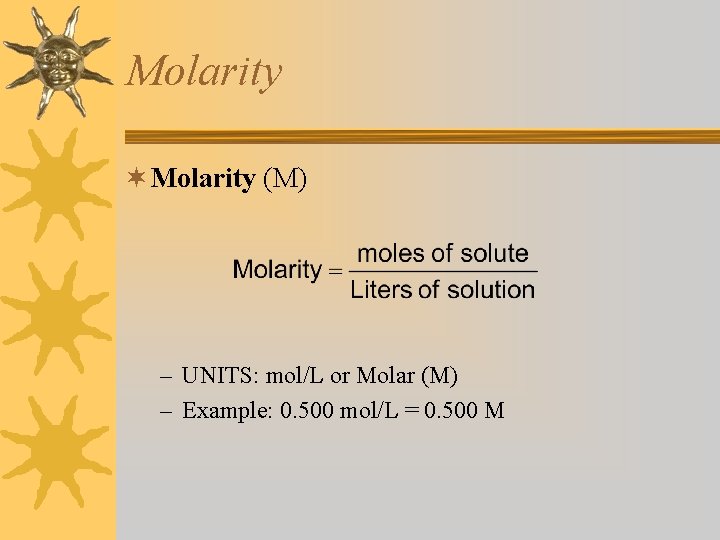
Molarity ¬ Molarity (M) – UNITS: mol/L or Molar (M) – Example: 0. 500 mol/L = 0. 500 M

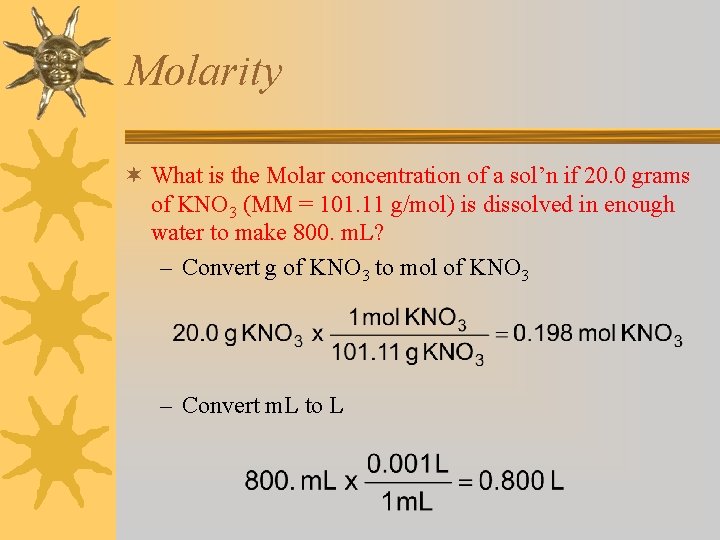
Molarity ¬ What is the Molar concentration of a sol’n if 20. 0 grams of KNO 3 (MM = 101. 11 g/mol) is dissolved in enough water to make 800. m. L? – Convert g of KNO 3 to mol of KNO 3 – Convert m. L to L

Molarity ¬ What is the Molar concentration of a sol’n if 0. 198 mol KNO 3 is dissolved in enough water to make 0. 800 L?

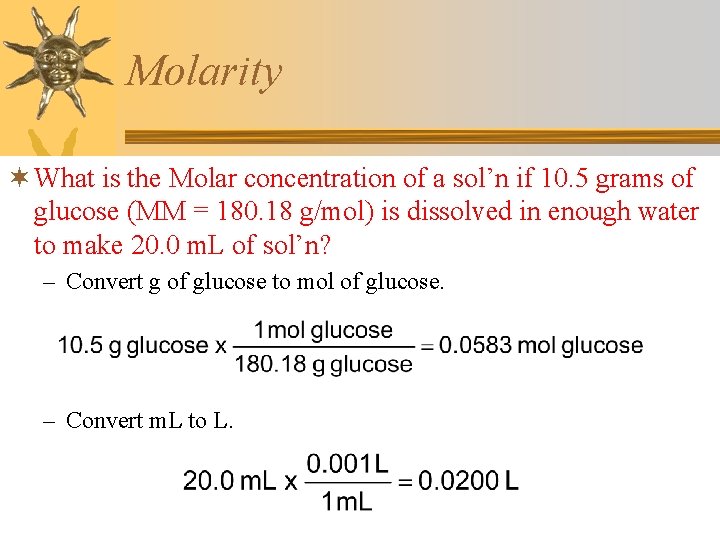
Molarity ¬ What is the Molar concentration of a sol’n if 10. 5 grams of glucose (MM = 180. 18 g/mol) is dissolved in enough water to make 20. 0 m. L of sol’n? – Convert g of glucose to mol of glucose. – Convert m. L to L.

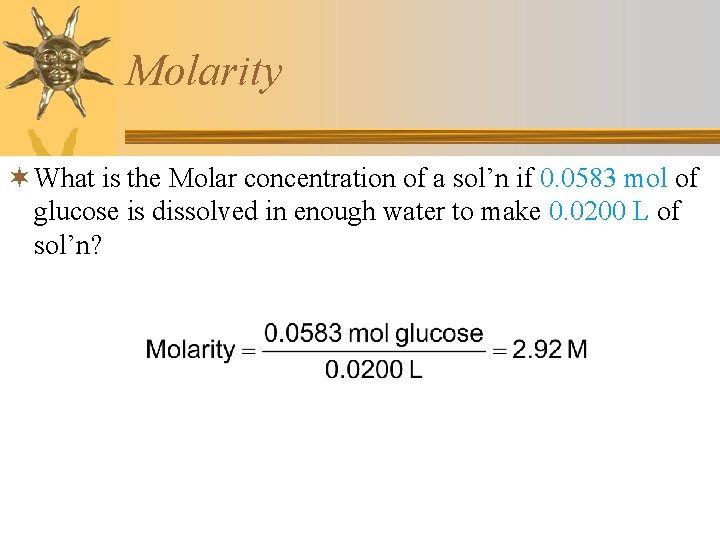
Molarity ¬ What is the Molar concentration of a sol’n if 0. 0583 mol of glucose is dissolved in enough water to make 0. 0200 L of sol’n?

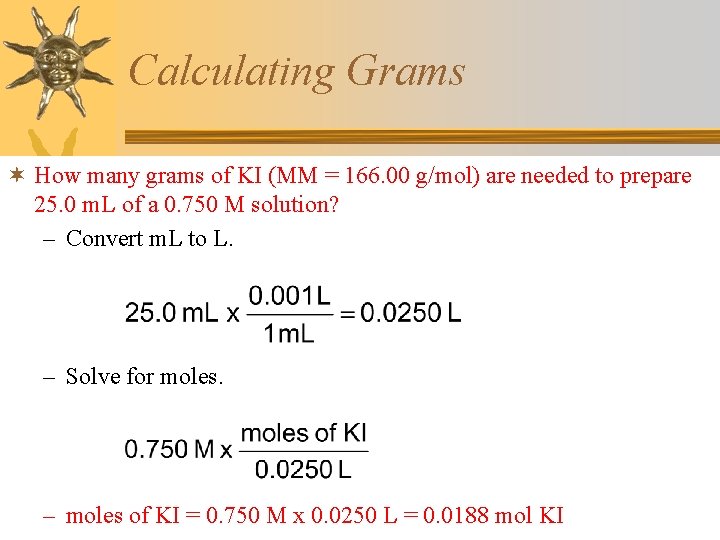
Calculating Grams ¬ How many grams of KI (MM = 166. 00 g/mol) are needed to prepare 25. 0 m. L of a 0. 750 M solution? – Convert m. L to L. – Solve for moles. – moles of KI = 0. 750 M x 0. 0250 L = 0. 0188 mol KI

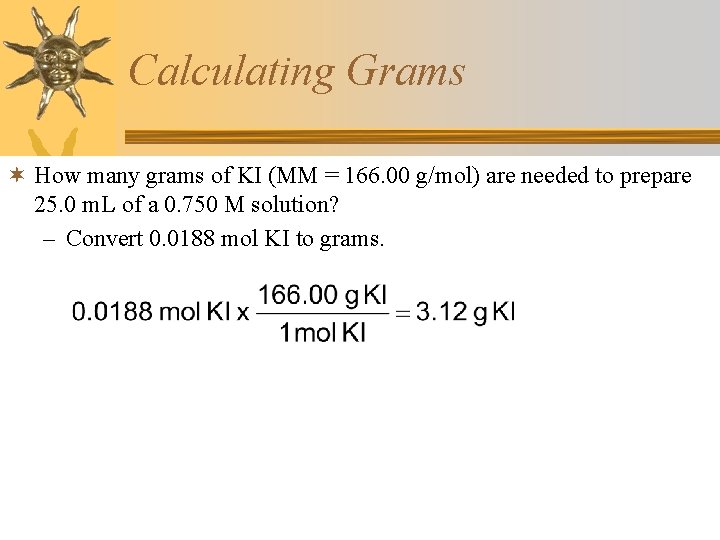
Calculating Grams ¬ How many grams of KI (MM = 166. 00 g/mol) are needed to prepare 25. 0 m. L of a 0. 750 M solution? – Convert 0. 0188 mol KI to grams.

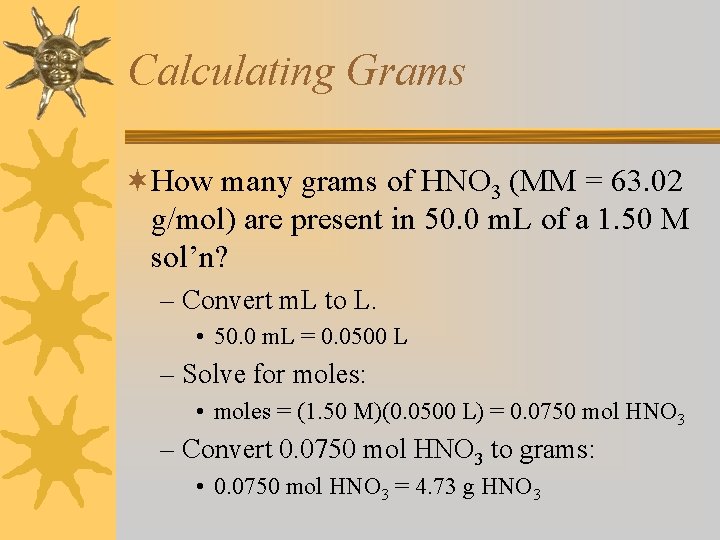
Calculating Grams ¬How many grams of HNO 3 (MM = 63. 02 g/mol) are present in 50. 0 m. L of a 1. 50 M sol’n? – Convert m. L to L. • 50. 0 m. L = 0. 0500 L – Solve for moles: • moles = (1. 50 M)(0. 0500 L) = 0. 0750 mol HNO 3 – Convert 0. 0750 mol HNO 3 to grams: • 0. 0750 mol HNO 3 = 4. 73 g HNO 3

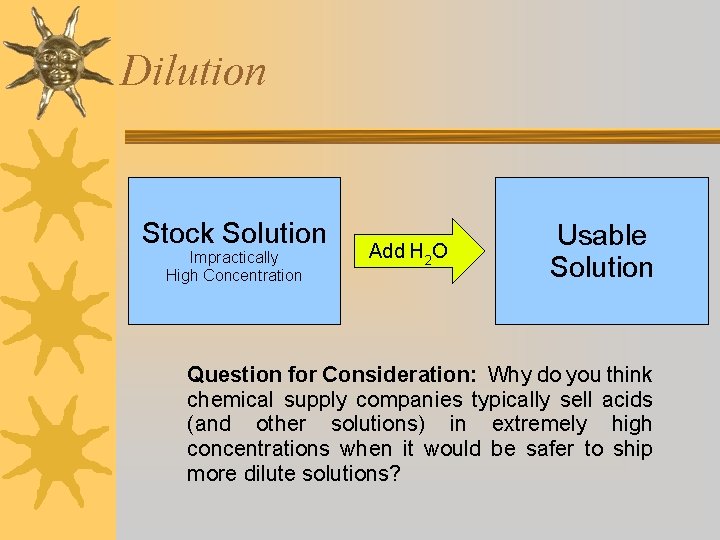
Dilution ¬ Dilute (verb) - to add solvent to a solution. – Decreases sol'n concentration. – M 1 V 1 = M 2 V 2 • • M 1 = initial conc. V 1 = initial volume M 2 = final conc. V 2 = final volume – Assumes no solute is added.

Dilution Stock Solution Impractically High Concentration Add H 2 O Usable Solution Question for Consideration: Why do you think chemical supply companies typically sell acids (and other solutions) in extremely high concentrations when it would be safer to ship more dilute solutions?

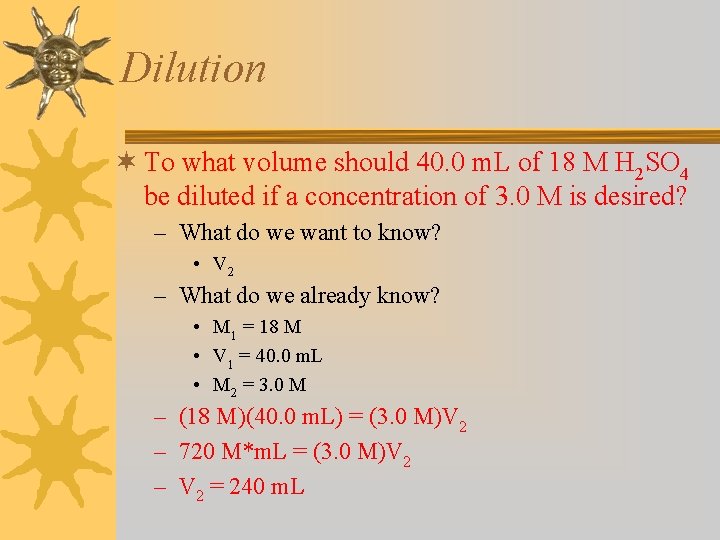
Dilution ¬ To what volume should 40. 0 m. L of 18 M H 2 SO 4 be diluted if a concentration of 3. 0 M is desired? – What do we want to know? • V 2 – What do we already know? • M 1 = 18 M • V 1 = 40. 0 m. L • M 2 = 3. 0 M – (18 M)(40. 0 m. L) = (3. 0 M)V 2 – 720 M*m. L = (3. 0 M)V 2 – V 2 = 240 m. L

Dilution ¬ You are asked to prepare 500. m. L of 0. 250 M HCl, starting with a 12. 0 Molar stock sol'n. How much stock should you use? – What do we want to know? • V 1 – What do we already know? • M 1 = 12. 0 M • M 2 = 0. 250 M • V 2 = 500. m. L – (12. 0 M) V 1 = (0. 250 M)(500. m. L) – (12. 0 M) V 1 = 125 M*m. L – V 1 = 10. 4 m. L

Dilution ¬ To how much water should you add 20. 0 m. L of 5. 00 M HNO 3 to dilute it to 1. 00 M? – What do we want to know? • How much water to add. (V 2 - V 1) – What do we already know? • M 1 = 5. 00 M • V 1 = 20. 0 m. L • M 2 = 1. 00 M – – (5. 00 M)(20. 0 m. L) = (1. 00 M) V 2 100. M*m. L = (1. 00 M) V 2 = 100. m. L Water added = 100. m. L - 20. 0 m. L = 80. m. L

Colligative Properties ¬Colligative properties are properties of solutions that are affected by the number of particles but not the identity of the solute