Concentration Important definitions Solution A homogeneous mixture It

















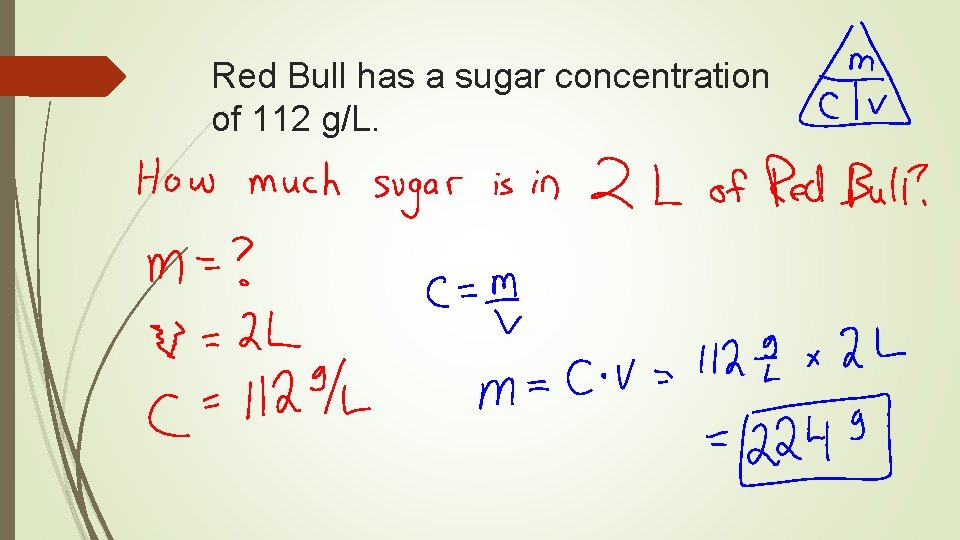












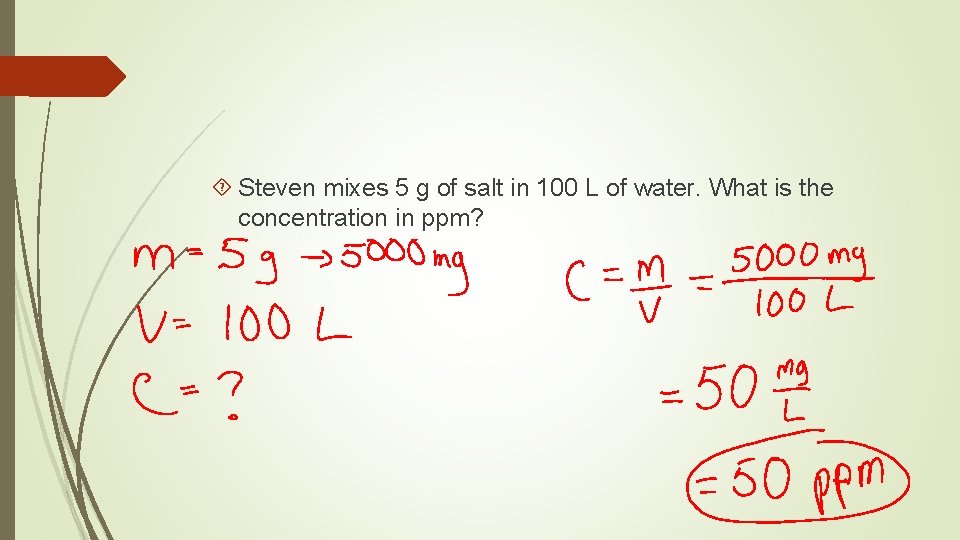


- Slides: 44

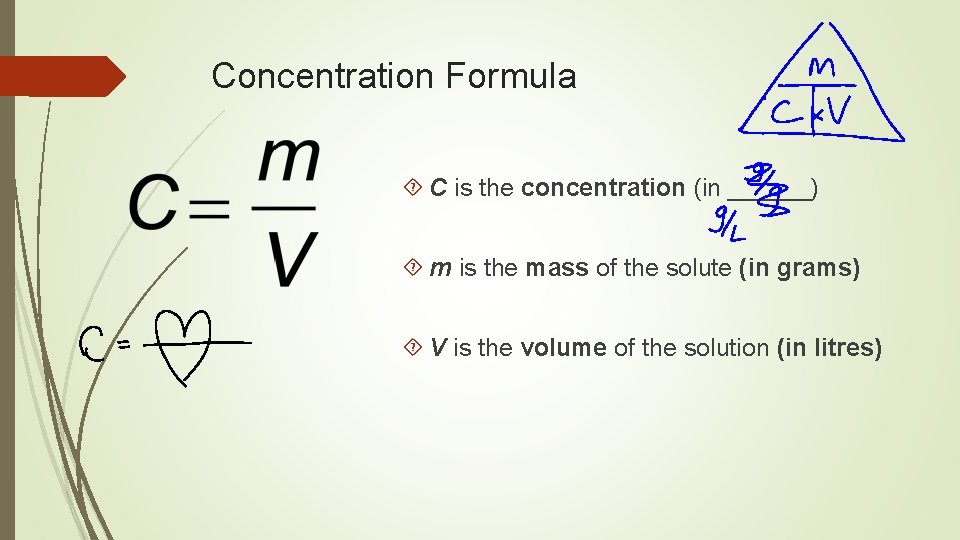
Concentration

Important definitions Solution: A homogeneous mixture. It appears to only be one substance.

Important definitions Solution: A homogeneous mixture. It appears to only be one substance.

Important definitions Solution: A homogeneous mixture. It appears to only be one substance. Solute: The substance that is being dissolved. Solvent: The substance that does the dissolving (usually water).

Concentration is a measurement of how much solute there is in a given amount of solution.

2 questions to think about 1. Can you add too much solute to a mixture, such that the solvent can no longer dissolve it?

2 questions to think about 1. Can you add too much solute to a mixture, such that the solvent can no longer dissolve it? 7. 5 L

2 questions to think about 2. When you add a solute to a solvent, does it increase the volume of the solution?

2 questions to think about 2. When you add a solute to a solvent, does it increase the volume of the solution?

2 more definitions Saturation: This is when there is too much solute for a solvent to dissolve. Dilution: This is the act of adding more solvent.

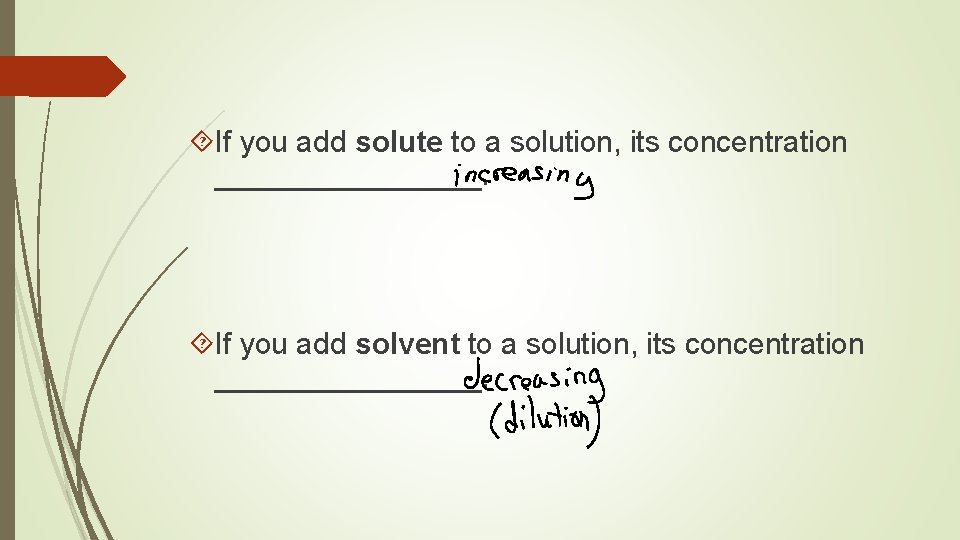
If you add solute to a solution, its concentration ________. If you add solvent to a solution, its concentration ________.

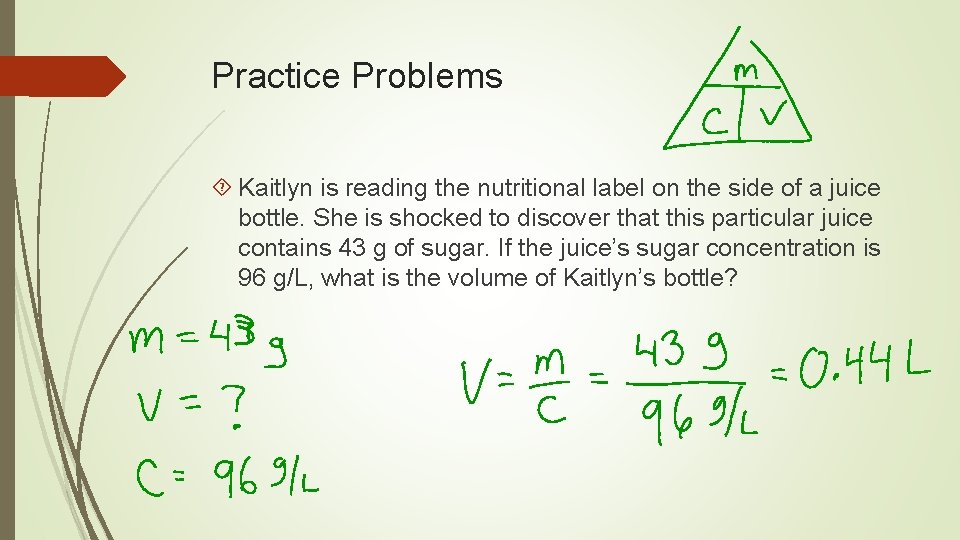
Concentration Formula C is the concentration (in ______) m is the mass of the solute (in grams) V is the volume of the solution (in litres)

Yard Foot Gallon Quart Pound Ounce Inch Mile

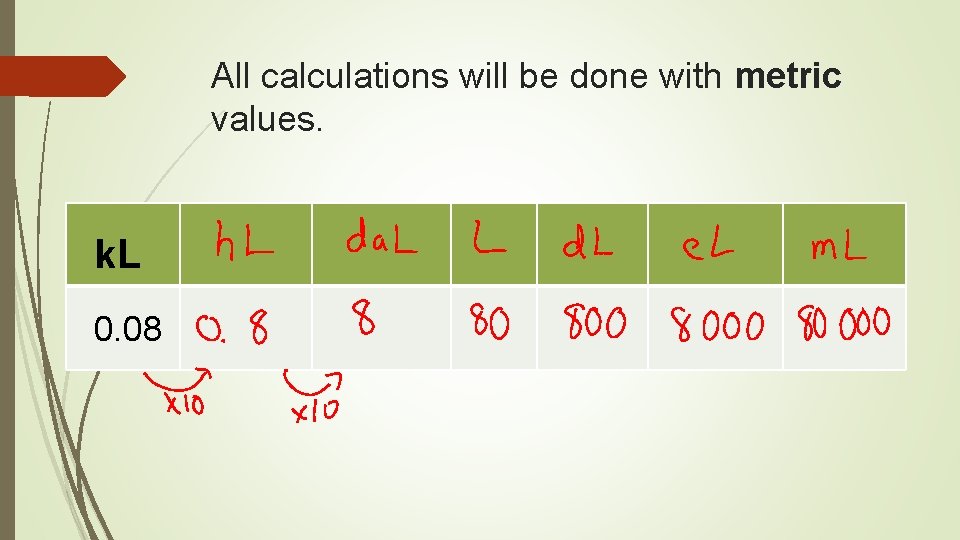
All calculations will be done with metric values.

All calculations will be done with metric values. mg 475

All calculations will be done with metric values. k. L 0. 08

Workbook p. 25

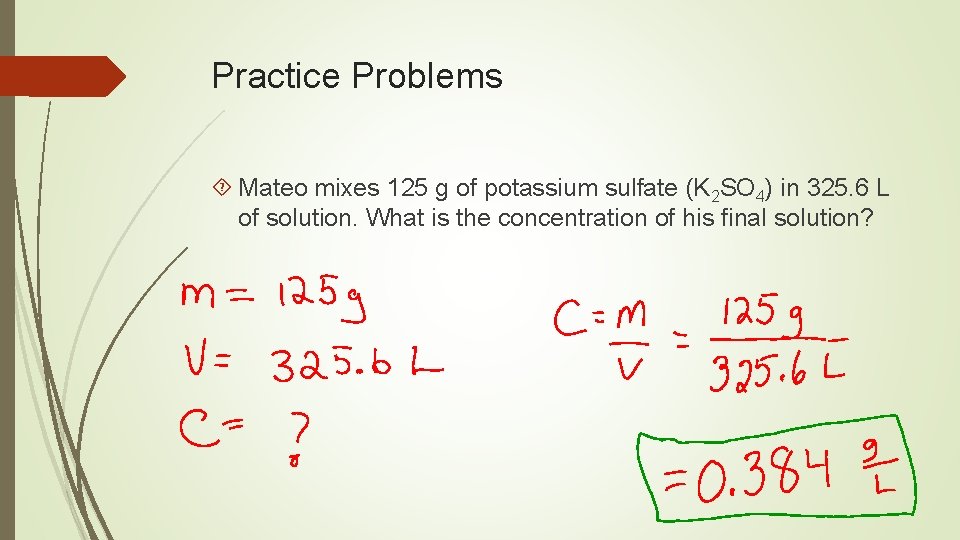
Practice Problems Mateo mixes 125 g of potassium sulfate (K 2 SO 4) in 325. 6 L of solution. What is the concentration of his final solution?

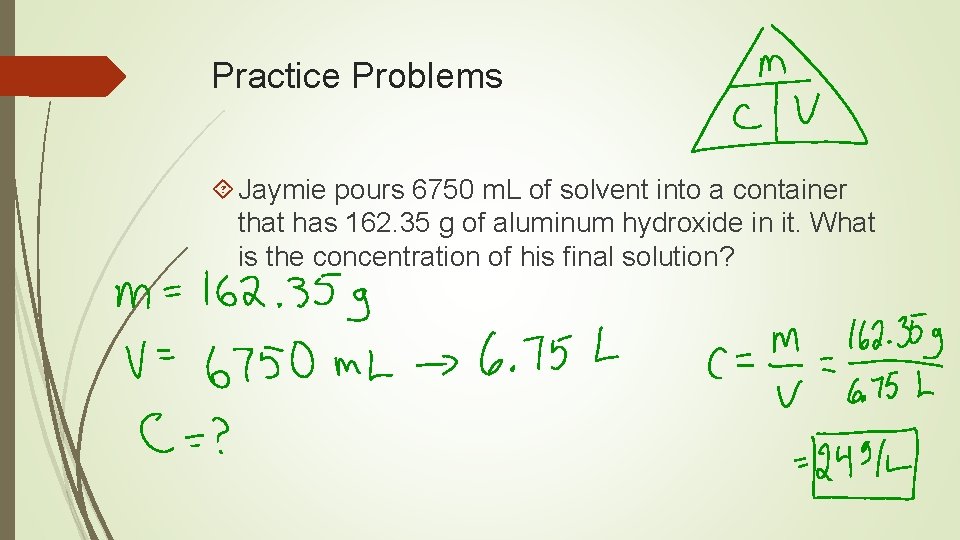
Practice Problems Jaymie pours 6750 m. L of solvent into a container that has 162. 35 g of aluminum hydroxide in it. What is the concentration of his final solution?

Practice Problems Alex adds 20 000 mg of Gatorade powder to 4 L of water. What is its concentration?

Practice Problems Kaitlyn is reading the nutritional label on the side of a juice bottle. She is shocked to discover that this particular juice contains 43 g of sugar. If the juice’s sugar concentration is 96 g/L, what is the volume of Kaitlyn’s bottle?

Practice Problems Kaitlyn is reading the nutritional label on the side of a juice bottle. She is shocked to discover that this particular juice contains 43 g of sugar. If the juice’s sugar concentration is 96 g/L, what is the volume of Kaitlyn’s bottle? True story!

Practice Problems Saad has been asked to prepare a saltwater solution that has a concentration of 35 g/L. He has only 30 m. L of water to work with. How much salt should he pour in?

Practice Problems If the concentration of chlorine in swimming pool water is greater than 0. 003 g/L, swimmers run the risk of developing irritated eyes and skin. Tiffany is the manager of a swimming pool. She has just hired a lifeguard who has added 8000 g of chlorine to 2500 k. L of water. Should Tiffany fire her?

Concentration Steps

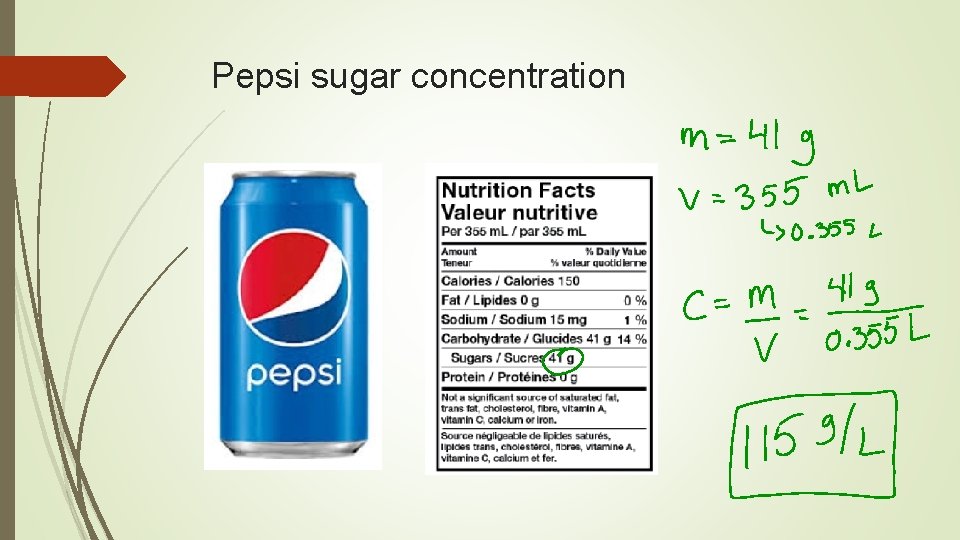
Pepsi sugar concentration

Pepsi sugar concentration
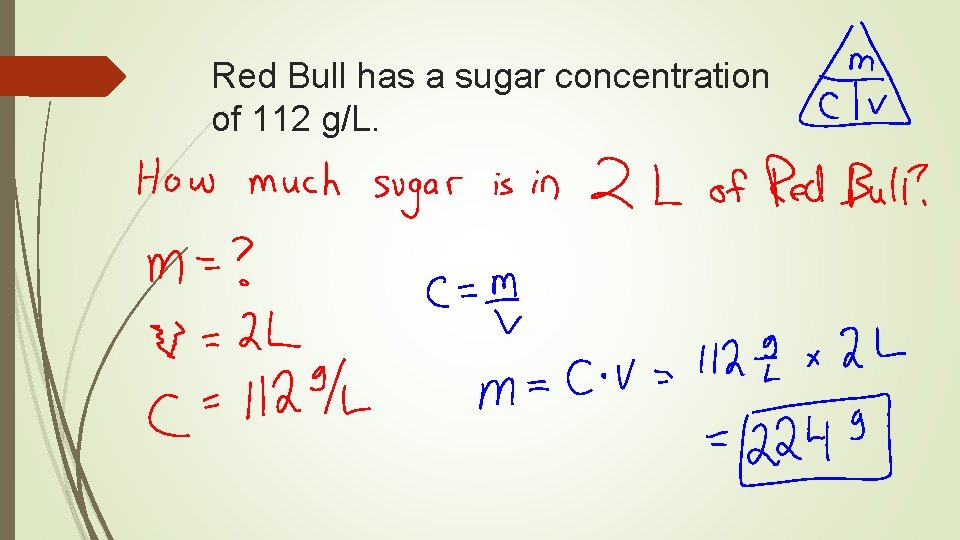
Red Bull has a sugar concentration of 112 g/L.

Steps to answer concentration questions 1. Read the question very carefully. Make sure you… concentrate… on finding all the numbers you need

Steps to answer concentration questions Saad has been asked to prepare a saltwater solution that has a concentration of 35 g/L. He has only 30 m. L of water to work with. How much salt should he pour in?


Steps to answer concentration questions 3. Use your triangle, or whatever other math trick you like, to solve for the unknown variable.

Steps to answer concentration questions Saad has been asked to prepare a saltwater solution that has a concentration of 35 g/L. He has only 30 m. L of water to work with. How much salt should he pour in?

Concentration Warmup Workbook p. 31 #1 -3 Identify the different values and list them (including the units). Is everything in g and L? If so, carry on. If not, convert them to g and L. Decide on which formula you need using the triangle. Again, include your units!! Calculate the missing value… show your units again!

Interesting question that would never appear on a test…

Which of the following is/are toxic?

https: //www. youtube. com/watch? v=IPrnd. NZ 4 m 6 w

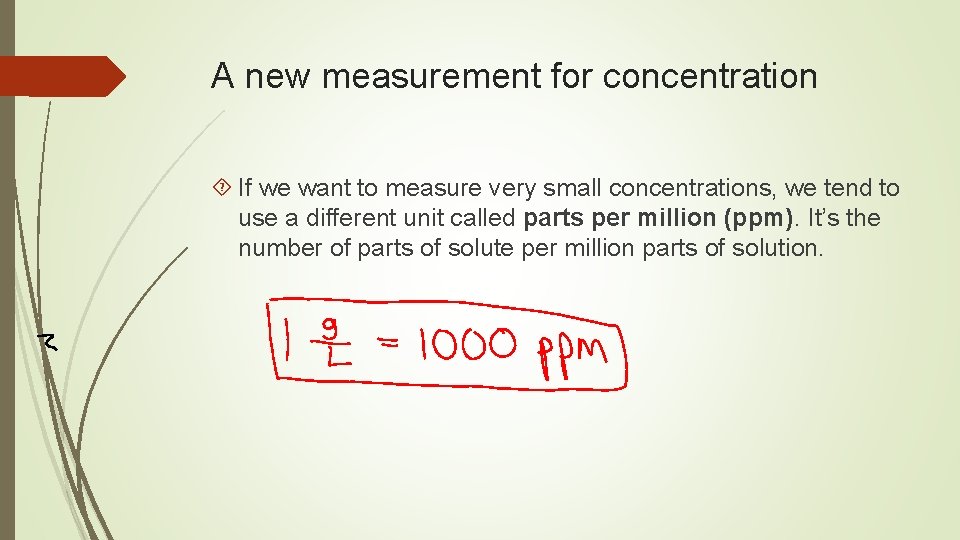
A new measurement for concentration If we want to measure very small concentrations, we tend to use a different unit called parts per million (ppm). It’s the number of parts of solute per million parts of solution.

Calculating ppm The procedure for calculating ppm is the same as g/L, but you need to have a concentration in: mg / L

Practice Problems Alex adds 20 000 mg of Gatorade powder to 4 L of water. What is its concentration?

Ppm Questions 40 g of solute are added to 10 k. L of water. What is the concentration in ppm?
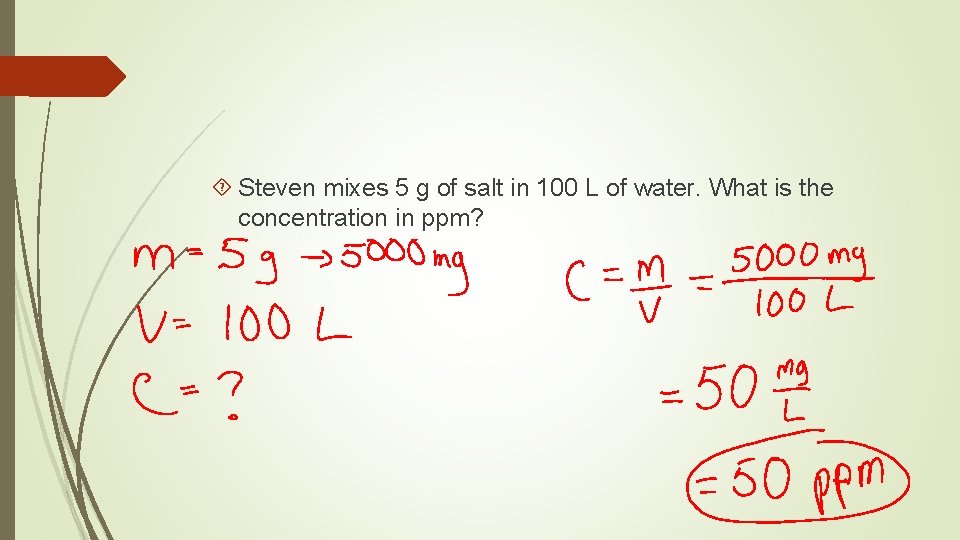
Steven mixes 5 g of salt in 100 L of water. What is the concentration in ppm?

Canadian water quality standards state that the maximum permitted amount of boron in drinking water is 5 ppm. 250 m. L is sent to a lab for analysis. Melanie finds that there is 0. 1 mg of water. Is this water safe to drink?

Workbook page 32 # 6, 8, 9