Soft Matter Review 10 January 2012 Lecture 1


- Slides: 22

Soft Matter Review 10 January 2012

Lecture 1 Characteristics of Soft Matter (1) Length scales between atomic and macroscopic (sometimes called mesoscopic) (2) The importance of thermal fluctuations and Brownian motion (3) Tendency to self-assemble into hierarchical structures (i. e. ordered on multiple size scales beyond the molecular) (4) Short-range forces and interfaces are important.

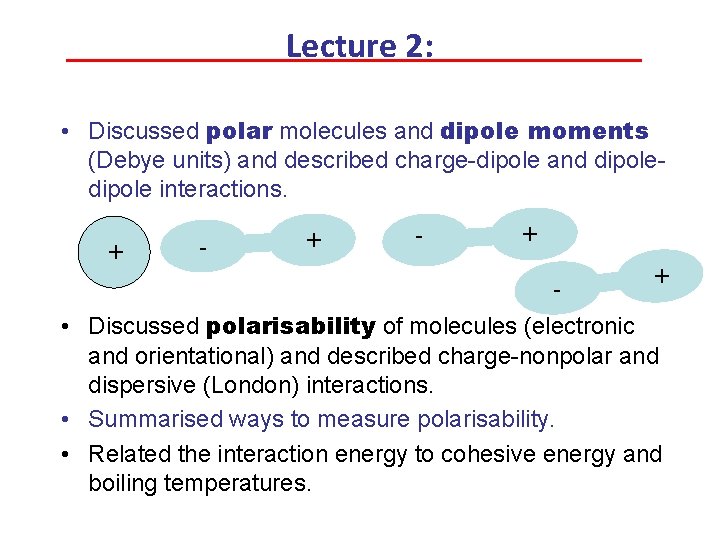
Lecture 2: • Discussed polar molecules and dipole moments (Debye units) and described charge-dipole and dipole interactions. + - + - + • Discussed polarisability of molecules (electronic and orientational) and described charge-nonpolar and dispersive (London) interactions. • Summarised ways to measure polarisability. • Related the interaction energy to cohesive energy and boiling temperatures.

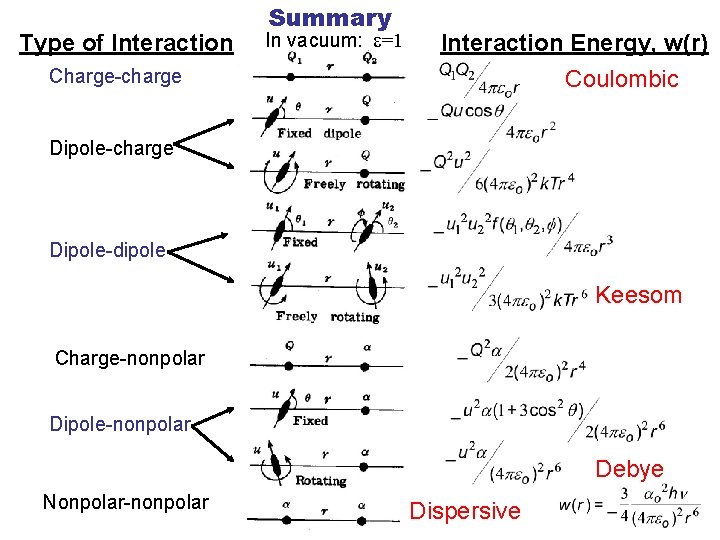
Type of Interaction Charge-charge Summary In vacuum: e=1 Interaction Energy, w(r) Coulombic Dipole-charge Dipole-dipole Keesom Charge-nonpolar Dipole-nonpolar Debye Nonpolar-nonpolar Dispersive

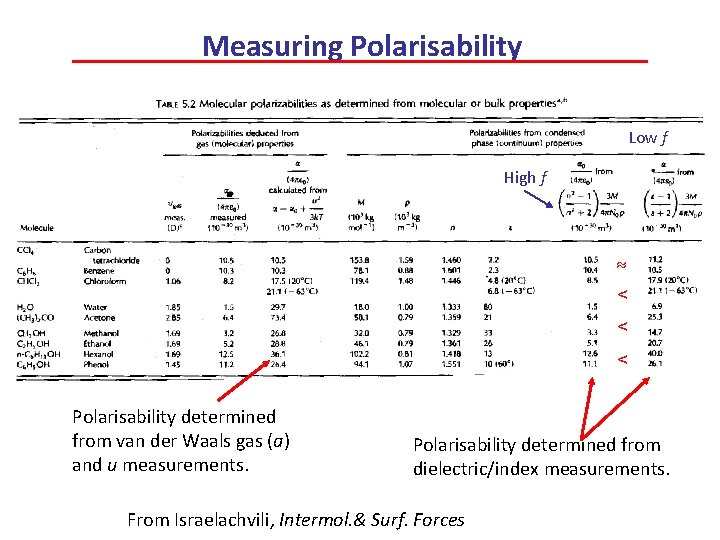
Measuring Polarisability Low f High f < < < Polarisability determined from van der Waals gas (a) and u measurements. Polarisability determined from dielectric/index measurements. From Israelachvili, Intermol. & Surf. Forces

Lecture 3 • Lennard-Jones potential energy for pairs of atoms and for pairs within molecular crystals • Evaluation of the Young’s (elastic) modulus for molecular crystals starting from the L-J potentials • Response of soft matter to shear stress: Hookean (elastic) solids versus Newtonian (viscous) liquids • Description of viscoelasticity with a transition from elastic to viscous response at a characteristic relaxation time, t • An important relationship between elastic and viscous components: h = Got

In the previous lecture: Interaction Potentials: w = -Cr -n • If n <3, molecules interact with all others in the system of size, L. If n >3, molecules interact only with the nearer neighbours. • Gravity: negligible at the molecular level. W(r) = -Cr -1 • Coulombic: relevant for salts, ionic liquids and charged molecules. W(r) = -Cr -1 • van der Waals’ Interaction: three types; usually quite weak; causes attraction between ANY two molecules. W(r) = -Cr -6 • Covalent bonds: usually the strongest type of bond; directional forces - not described by a simple potential. • Hydrogen bonding: stronger than van der Waals bonds; charge attracting resulting from unshielded proton in H.

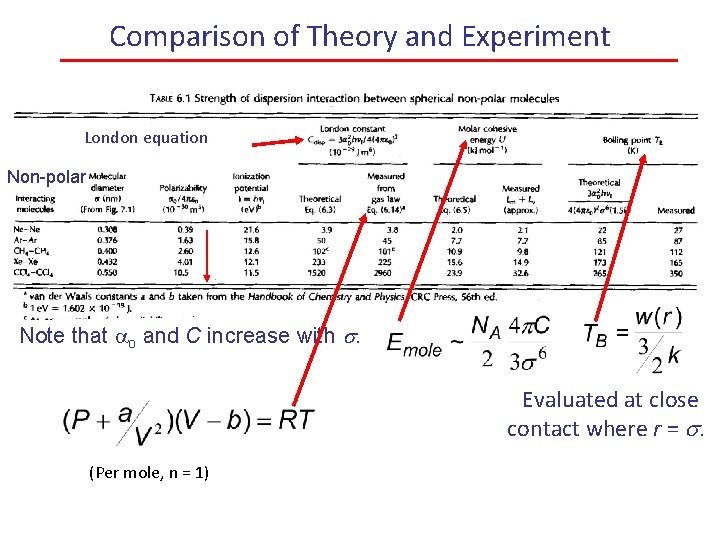
Comparison of Theory and Experiment London equation Non-polar Note that ao and C increase with s. Evaluated at close contact where r = s. (Per mole, n = 1)

Lecture 4 • Viscosity and relaxation times increase strongly with decreasing temperature: Arrhenius and Vogel-Fulcher equations • First and second-order phase transitions are defined by derivatives of Gibbs’ free energy. • The glass transition occurs at a temperature where tconfig texp and is dependent on thermal history. In a glass, tconfig > texp. • Glass structure is described by a radial distribution function. • The Kauzmann temperature could represent the temperature at which there is a first-order phase transition underlying the glass transition – possibly at a temperature of T 0.

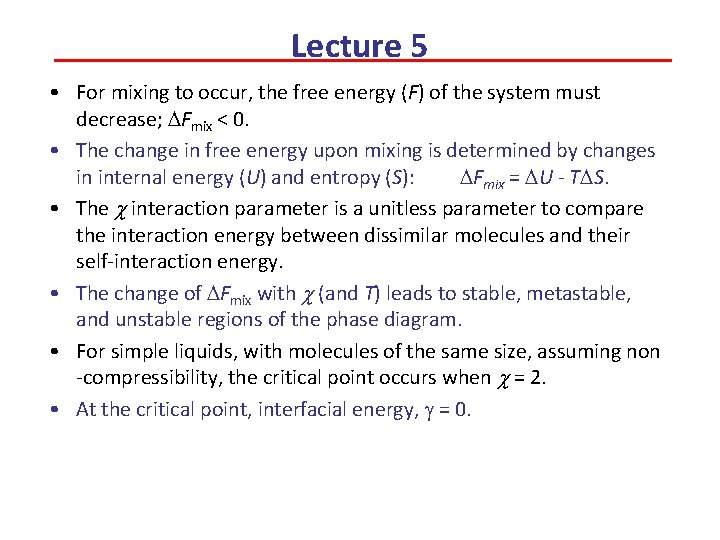
Lecture 5 • For mixing to occur, the free energy (F) of the system must decrease; DFmix < 0. • The change in free energy upon mixing is determined by changes in internal energy (U) and entropy (S): DFmix = DU - TDS. • The c interaction parameter is a unitless parameter to compare the interaction energy between dissimilar molecules and their self-interaction energy. • The change of DFmix with c (and T) leads to stable, metastable, and unstable regions of the phase diagram. • For simple liquids, with molecules of the same size, assuming non -compressibility, the critical point occurs when c = 2. • At the critical point, interfacial energy, g = 0.

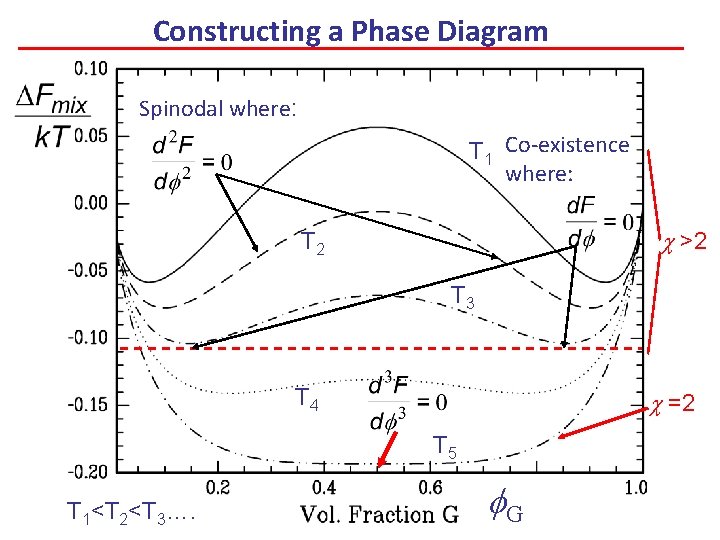
Constructing a Phase Diagram Spinodal where: T 1 Co-existence where: c >2 T 3 T 4 c =2 T 5 T 1<T 2<T 3…. f. G

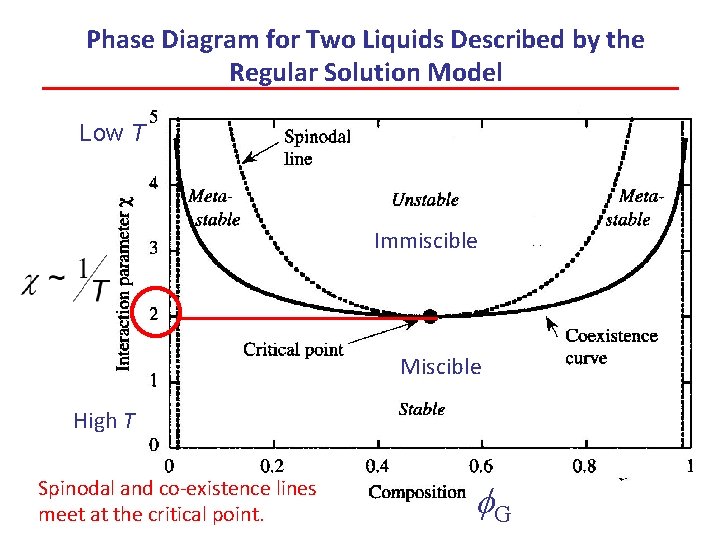
Phase Diagram for Two Liquids Described by the Regular Solution Model Low T Immiscible Miscible High T Spinodal and co-existence lines meet at the critical point. f. G

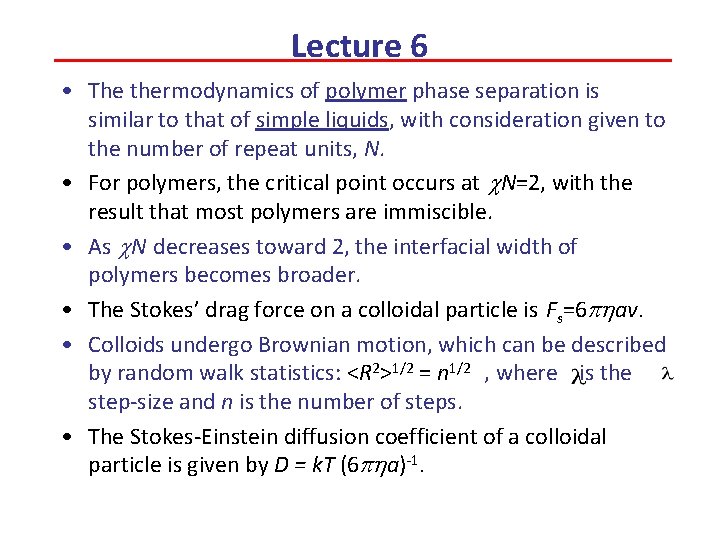
Lecture 6 • The thermodynamics of polymer phase separation is similar to that of simple liquids, with consideration given to the number of repeat units, N. • For polymers, the critical point occurs at c. N=2, with the result that most polymers are immiscible. • As c. N decreases toward 2, the interfacial width of polymers becomes broader. • The Stokes’ drag force on a colloidal particle is Fs=6 phav. • Colloids undergo Brownian motion, which can be described by random walk statistics: <R 2>1/2 = n 1/2 , where is the step-size and n is the number of steps. • The Stokes-Einstein diffusion coefficient of a colloidal particle is given by D = k. T (6 pha)-1.

Lecture 7 • The viscosity of colloidal dispersions depends on the volume fraction of the particles (Einstein equation): • The Peclet number, Pe, describes the competition between particle disordering because of Brownian diffusion and particle ordering under a shear stress. • At high Pe (high shear strain rate), the particles are more ordered; shear thinning behaviour occurs and h decreases. • van der Waals’ energy acting between a colloidal particle and a semi- slab (or another particle) can be calculated by summing up the intermolecular energy between the constituent molecules. • Macroscopic interactions can be related to the molecular level. • The Hamaker constant, A, contains information about molecular density (r) and the strength of intermolecular interactions (via the London constant, C): A = p 2 r 2 C

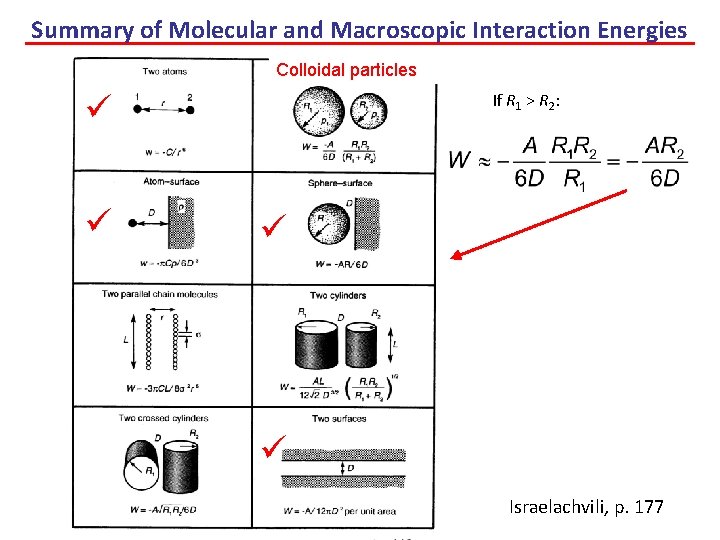
Summary of Molecular and Macroscopic Interaction Energies Colloidal particles If R 1 > R 2: Israelachvili, p. 177

Lecture 8 • Polymer crystals have a hierarchical structure: aligned chains, lamella, spherulites. • Melting point is inversely related to the crystal’s lamellar thickness. • Lamellar thickness is inversely related to the amount of undercooling. • The maximum crystal growth rate usually occurs at temperatures between the melting temperature and the glass transition temperature. • Tacticity and chain branching prevents or interrupts polymer crystal growth.

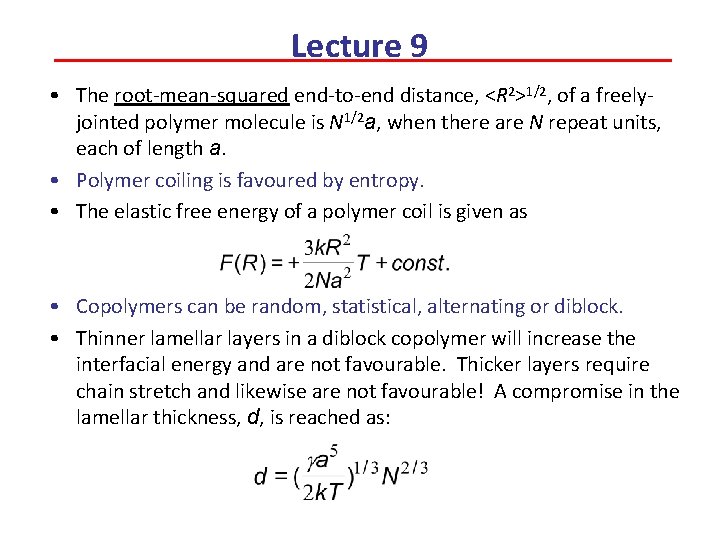
Lecture 9 • The root-mean-squared end-to-end distance, <R 2>1/2, of a freelyjointed polymer molecule is N 1/2 a, when there are N repeat units, each of length a. • Polymer coiling is favoured by entropy. • The elastic free energy of a polymer coil is given as • Copolymers can be random, statistical, alternating or diblock. • Thinner lamellar layers in a diblock copolymer will increase the interfacial energy and are not favourable. Thicker layers require chain stretch and likewise are not favourable! A compromise in the lamellar thickness, d, is reached as:

Lecture 9 • Elastic (entropic) effects cause a polymer molecule to coil up. • Excluded volume effects cause polymer molecules to swell (in a self-avoiding walk). • Polymer-solvent interactions, described by the c-parameter, can favour tight polymer coiling into a globule (large c) or swelling (low c). • Thus there is a competition between three effects! • The radius-of-gyration of a polymer, Rg, is 1/6 of its root-meansquare end-to-end distance <R 2>1/2.

Lecture 9 • When c = 1/2, excluded volume effects are exactly balanced by polymer/solvent interactions. Elastic effects (from an entropic spring) lead to a random coil: <R 2>1/2 ~ a. N 1/2 • When c < 1/2, excluded volume effects dominate over polymer/solvent interactions. In competition with elastic effects, they lead to a swollen coil: <R 2>1/2 ~ a. N 3/5 • When c > 1/2, polymer/solvent interactions are dominant over excluded volume effects. They lead to polymer coiling: a globule results.

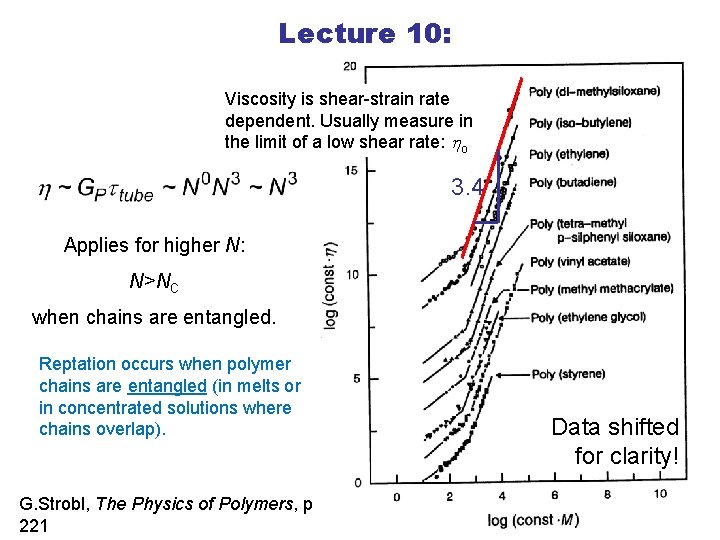
Lecture 10: Viscosity is shear-strain rate dependent. Usually measure in the limit of a low shear rate: ho 3. 4 Applies for higher N: N>NC when chains are entangled. Reptation occurs when polymer chains are entangled (in melts or in concentrated solutions where chains overlap). G. Strobl, The Physics of Polymers, p. 221 Data shifted for clarity!

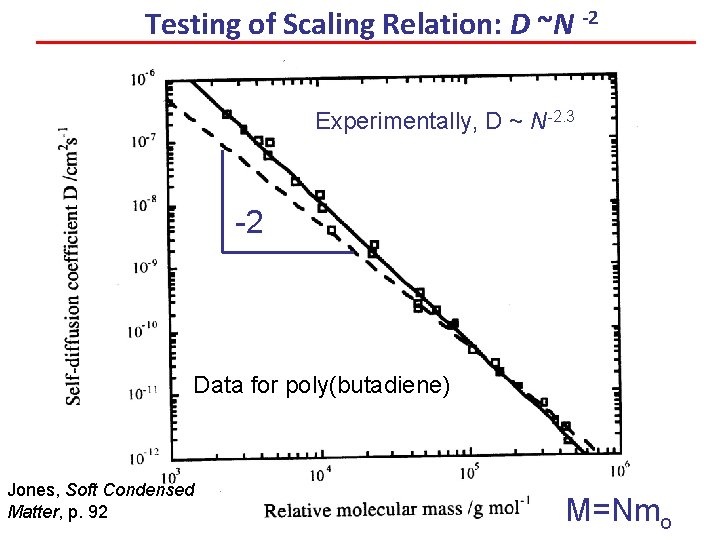
Testing of Scaling Relation: D ~N -2 Experimentally, D ~ N-2. 3 -2 Data for poly(butadiene) Jones, Soft Condensed Matter, p. 92 M=Nmo

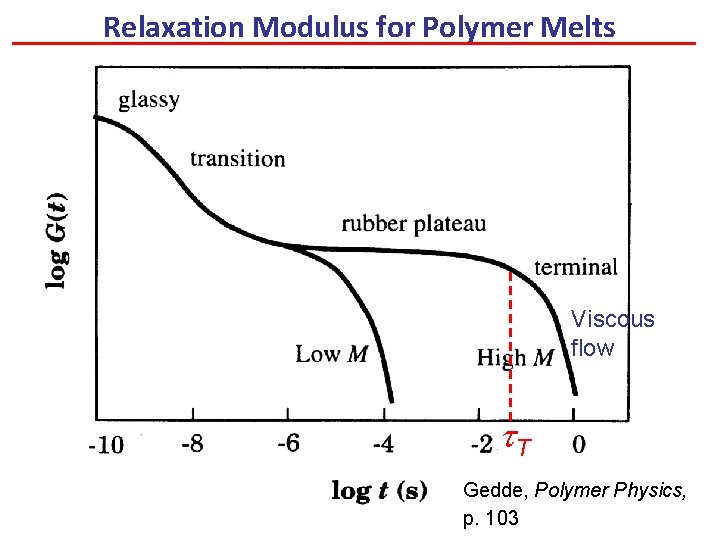
Relaxation Modulus for Polymer Melts Viscous flow t. T Gedde, Polymer Physics, p. 103