Slide 1 of 21 Indications for Use 510k


















- Slides: 21

Slide 1 of 21 Indications for Use 510(k) Submission, Section 4 Rob Packard, President www. Medical. Device. Academy. com rob@13485 cert. com

Slide 2 of 21 Organization of Your 510(k) http: //medicaldeviceacademy. com/510 k-project-management -an-inside-look-at-our-process/ Rob Packard, President www. Medical. Device. Academy. com rob@13485 cert. com

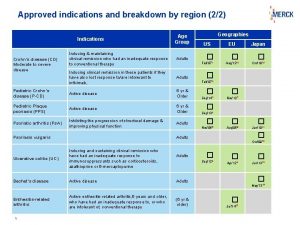
Slide 3 of 21 Where the Indications for Use Appear • • 510(k) Cover letter Vol 4 – Indications for Use Vol 5 – 510(k) Summary Vol 9 – Declaration of Conformity & Summary Reports Vol 10 – Executive Summary Vol 11 – Device Description Vol 12 – Substantial Equivalence Vol 13 – Labeling (must appear in the IFU) Rob Packard, President www. Medical. Device. Academy. com rob@13485 cert. com

Slide 4 of 21 Why I Prepare Section 4 First • This section is short and mandatory. • This section can be completed even if the design is in progress. • The header identifies the title of the submission which will be used in all other sections. • The content identifies the proposed primary predicate. • The content identifies the indications for use stated in the applicable regulation. Rob Packard, President www. Medical. Device. Academy. com rob@13485 cert. com

Slide 5 of 21 Product Classification http: //medicaldeviceacademy. com/fda-device-classification/ 1. Identify a device similar to yours 2. Use the registration and listing database http: //bit. ly/CDRH-Registration-Listing-Database 3. Identify the 3 -letter product code 4. Click on the code to go to the product classification page 5. Click on the TPLC link http: //bit. ly/FDATPLC Rob Packard, President www. Medical. Device. Academy. com rob@13485 cert. com

Slide 6 of 21 Case Study Example • Topical skin adhesive • Special controls guidance • Multiple predicates to choose within the MPN product code http: //medicaldeviceacademy. com/510 k-submission-fda-case-study/ Rob Packard, President www. Medical. Device. Academy. com rob@13485 cert. com

Slide 7 of 21 21 CFR 878. 4010 Rob Packard, President www. Medical. Device. Academy. com rob@13485 cert. com

Slide 8 of 21 Indications from Regulation “A device intended for topical closure of surgical incisions, including laparoscopic incisions, and simple traumatic lacerations that have easily approximated skin edges. Tissue adhesives for the topical approximation of skin may be used in conjunction with, but not in place of, deep dermal stitches. ” Rob Packard, President www. Medical. Device. Academy. com rob@13485 cert. com

Slide 9 of 21 Primary Predicate 510(k) Summary If the company submitted a 510(k) Summary after February 1996, then the Indications for Use page will be included in the 510(k) Summary as a single page. If the company submitted a 510(k) Statement, then you will need to find the Indications from another source: Option 1 – request a copy of submission Option 2 – search their website Rob Packard, President www. Medical. Device. Academy. com rob@13485 cert. com

Slide 10 of 21 Indications from Primary Predicate “Surgiseal™ Topical Skin Adhesive is intended for topical applications only to hold closed easily approximated skin edges of wounds from surgical incisions, including punctures from minimally invasive surgery, simple, thoroughly cleansed, trauma induced lacerations. Surgiseal™ Topical Skin Adhesive may be used in conjunction with, but not in place of, dermal sutures. ” Rob Packard, President www. Medical. Device. Academy. com rob@13485 cert. com

Slide 11 of 21 Indications for Subject Device “Krazyseal™ Topical Skin Adhesive is intended for topical applications only to hold closed easily approximated skin edges of wounds from surgical incisions, including punctures from minimally invasive surgery, simple, thoroughly cleansed, trauma induced lacerations. Krazyseal™ Topical Skin Adhesive may be used in conjunction with, but not in place of, dermal sutures. ” Rob Packard, President www. Medical. Device. Academy. com rob@13485 cert. com

Slide 12 of 21 Section 2 - Table of Contents http: //bit. ly/510 k-Format • 20 Sections • Create a Template (http: //bit. ly/510 k-To. C) • e. Copy Guidance (http: //bit. ly/FDA-e. Copy) • RTA Checklist (http: //bit. ly/Acceptance-Checklist) Rob Packard, President www. Medical. Device. Academy. com rob@13485 cert. com

Slide 13 of 21 Content of Volume 4, Doc 1 • • Copy of Indications from Applicable Regulation Copy of Indications from Primary Predicate Copy of Indications for Subject Device Reference to Volume 4, Doc 2 Rob Packard, President www. Medical. Device. Academy. com rob@13485 cert. com

Slide 14 of 21 Content of Volume 4, Doc 2 • 510(k) Number – if known (typically not known) – Leave it blank or type “Unknown” if a 510(k) number has not been assigned • Device Name – the brand can change anytime; therefore, I always recommend using a simple descriptive title for the submission • Indications for Use – should match the predicate and be very similar to the regulation • Type of Use – you can pick one or both Rob Packard, President www. Medical. Device. Academy. com rob@13485 cert. com

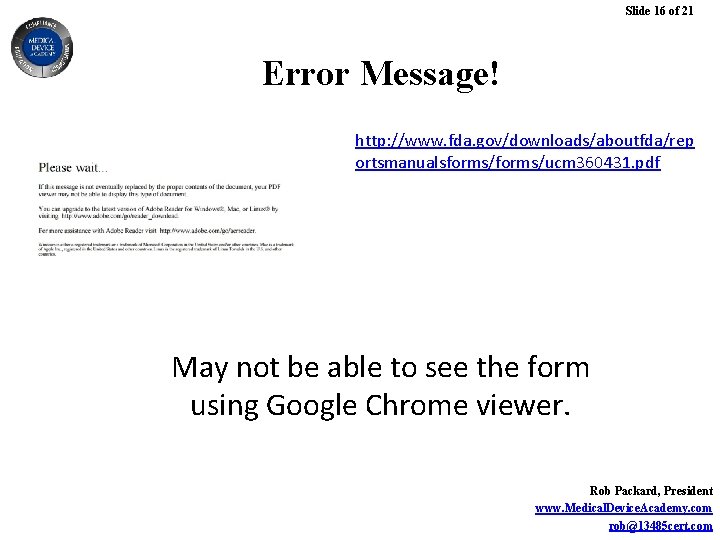
Slide 15 of 21 Where to Download FDA Form 3881 http: //www. fda. gov/downloads/aboutfda/rep ortsmanualsforms/ucm 360431. pdf You need Adobe Acrobat to complete this in a way that you can make changes and save as a PDF. Otherwise you need to print and scan from Adobe Reader. Rob Packard, President www. Medical. Device. Academy. com rob@13485 cert. com

Slide 16 of 21 Error Message! http: //www. fda. gov/downloads/aboutfda/rep ortsmanualsforms/ucm 360431. pdf May not be able to see the form using Google Chrome viewer. Rob Packard, President www. Medical. Device. Academy. com rob@13485 cert. com

Slide 17 of 21 Broader Indications • Expanding the indications: – Different Patient Population (e. g. , pediatrics) – Different part of the body (e. g. , extremities) • Making Performance Claims – Healing time – Reduction in adverse events • Claims and Indications like the above often require clinical evidence Rob Packard, President www. Medical. Device. Academy. com rob@13485 cert. com

Slide 18 of 21 More Narrow Indications • You can always select an more narrow indication, however, you may have difficulty in later submissions if you try to use your own device as a predicate. Rob Packard, President www. Medical. Device. Academy. com rob@13485 cert. com

Slide 19 of 21 510(k) Project Management Webinar http: //medicaldeviceacademy. com/510 k-projectmanagement-webinar/ Rob Packard, President www. Medical. Device. Academy. com rob@13485 cert. com

Slide 20 of 21 Q&A rob@13485 cert. com Rob Packard, President www. Medical. Device. Academy. com rob@13485 cert. com

Slide 21 of 21 Do You Need Help with Your 510(k)? rob 13485 rob@13485 cert. com Rob Packard +1. 802. 281. 4381 Rob Packard, President www. Medical. Device. Academy. com rob@13485 cert. com
 510k cover letter template
510k cover letter template How to heel toe dance
How to heel toe dance Binomials factoring
Binomials factoring Kontinuitetshantering i praktiken
Kontinuitetshantering i praktiken Typiska novell drag
Typiska novell drag Nationell inriktning för artificiell intelligens
Nationell inriktning för artificiell intelligens Ekologiskt fotavtryck
Ekologiskt fotavtryck Shingelfrisyren
Shingelfrisyren En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Personalliggare bygg undantag
Personalliggare bygg undantag Tidbok för yrkesförare
Tidbok för yrkesförare Anatomi organ reproduksi
Anatomi organ reproduksi Förklara densitet för barn
Förklara densitet för barn Datorkunskap för nybörjare
Datorkunskap för nybörjare Stig kerman
Stig kerman Hur skriver man en debattartikel
Hur skriver man en debattartikel Magnetsjukhus
Magnetsjukhus Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Påbyggnader för flakfordon
Påbyggnader för flakfordon Tryck formel
Tryck formel Svenskt ramverk för digital samverkan
Svenskt ramverk för digital samverkan Kyssande vind analys
Kyssande vind analys