Selective Atomic Layer Deposition of Ti O 2





- Slides: 15

Selective Atomic Layer Deposition of Ti. O 2 on Silicon/Copperpatterned Substrates UIC REU 2011 AMRe. L, University of Illinois at Chicago Abigail Jablansky Department of Chemical and Biomolecular Engineering, University of Pennsylvania

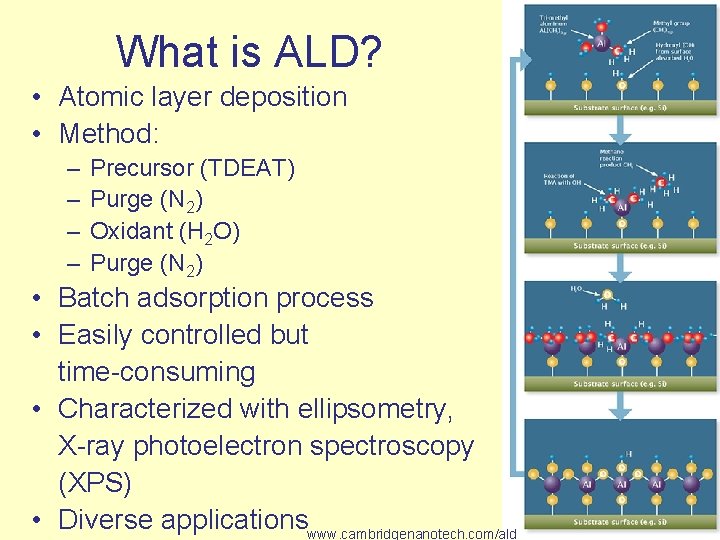
What is ALD? • Atomic layer deposition • Method: – – Precursor (TDEAT) Purge (N 2) Oxidant (H 2 O) Purge (N 2) • Batch adsorption process • Easily controlled but time-consuming • Characterized with ellipsometry, X-ray photoelectron spectroscopy (XPS) • Diverse applicationswww. cambridgenanotech. com/ald

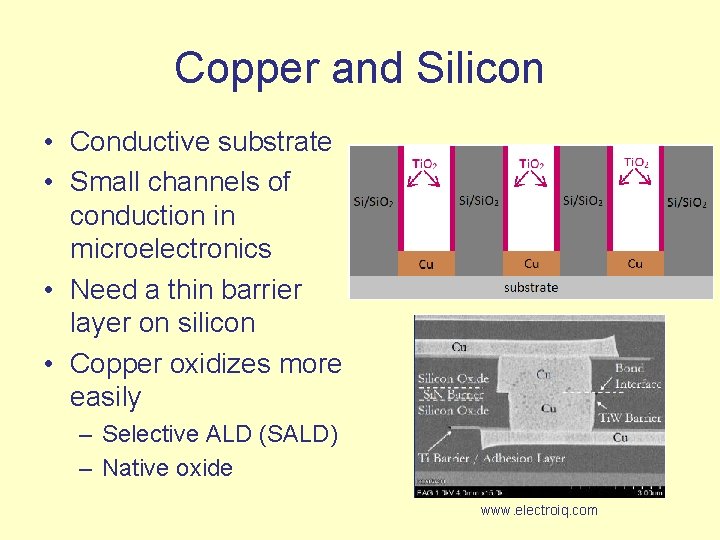
Copper and Silicon • Conductive substrate • Small channels of conduction in microelectronics • Need a thin barrier layer on silicon • Copper oxidizes more easily – Selective ALD (SALD) – Native oxide www. electroiq. com

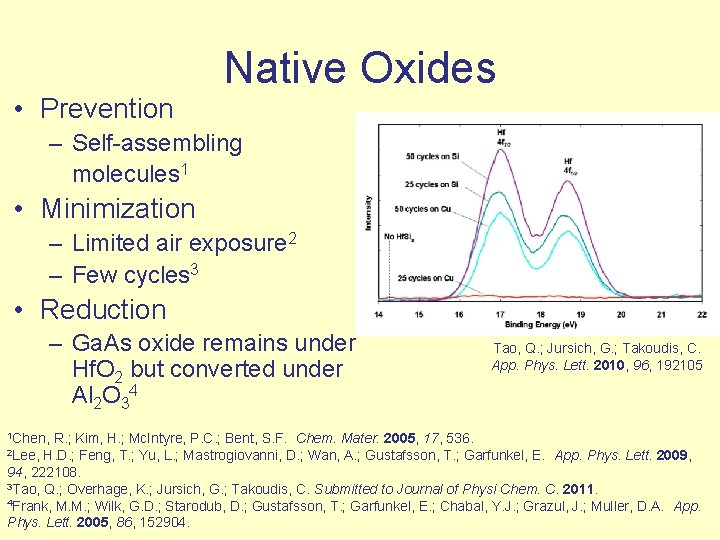
Native Oxides • Prevention – Self-assembling molecules 1 • Minimization – Limited air exposure 2 – Few cycles 3 • Reduction – Ga. As oxide remains under Hf. O 2 but converted under Al 2 O 34 1 Chen, Tao, Q. ; Jursich, G. ; Takoudis, C. App. Phys. Lett. 2010, 96, 192105 R. ; Kim, H. ; Mc. Intyre, P. C. ; Bent, S. F. Chem. Mater. 2005, 17, 536. 2 Lee, H. D. ; Feng, T. ; Yu, L. ; Mastrogiovanni, D. ; Wan, A. ; Gustafsson, T. ; Garfunkel, E. App. Phys. Lett. 2009, 94, 222108. 3 Tao, Q. ; Overhage, K. ; Jursich, G. ; Takoudis, C. Submitted to Journal of Physi Chem. C. 2011. 4 Frank, M. M. ; Wilk, G. D. ; Starodub, D. ; Gustafsson, T. ; Garfunkel, E. ; Chabal, Y. J. ; Grazul, J. ; Muller, D. A. App. Phys. Lett. 2005, 86, 152904.

Copper Oxides • Cu 2 O (cuprous oxide) – Linear – Most stable copper compounds at high T – Forms ammine under NH 35 • Cu. O (cupric oxide) – Square planar – Decomposes at high T to Cu 2 O + O 2 – H 2 or CO reduction at 250 o. C 5 • Cu 2 O forms first, then Cu. O if stable 6 • Reduction methods 5 Cotton, F. A. ; Wilkinson, G. Advanced Inorganic Chemistry, 2 nd ed. New York: Interscience Publishers, 1966, pp. 894 -902. 6 Zhu, Y. ; Mimura, K. ; Lim, J. ; Isshiki, M. ; Jiang, Q. Metal. and Mineral Trans. A. 2006, 37 A, 1231.

Project Description • ALD of Ti. O 2 onto Si/Cu wafers – Precursor: tetrakis(diethylamino)titanium (TDEAT) – Oxidizer: water • Compare 24 -hr Cu (1 nm native oxide) exposure to 1 -hr 7 • Minimize exposure from reactor to ellipsometer, x-ray photoelectron spectroscopy (XPS) 7 Tao, Q. Ph. D Dissertation, University of Illinois at Chicago, 2011.

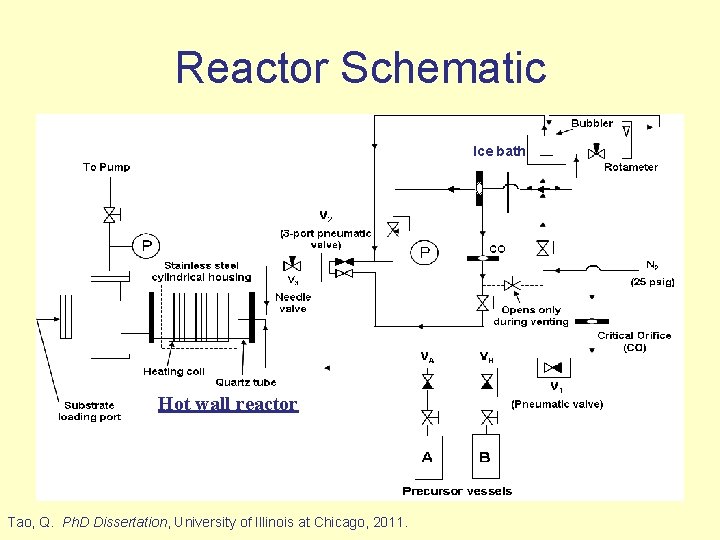
Reactor Schematic Ice bath Hot wall reactor Tao, Q. Ph. D Dissertation, University of Illinois at Chicago, 2011.

Experimental Setup

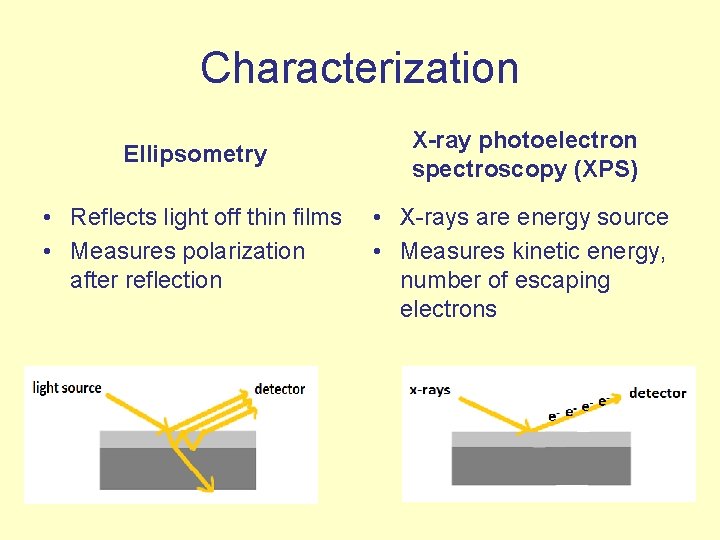
Characterization Ellipsometry • Reflects light off thin films • Measures polarization after reflection X-ray photoelectron spectroscopy (XPS) • X-rays are energy source • Measures kinetic energy, number of escaping electrons

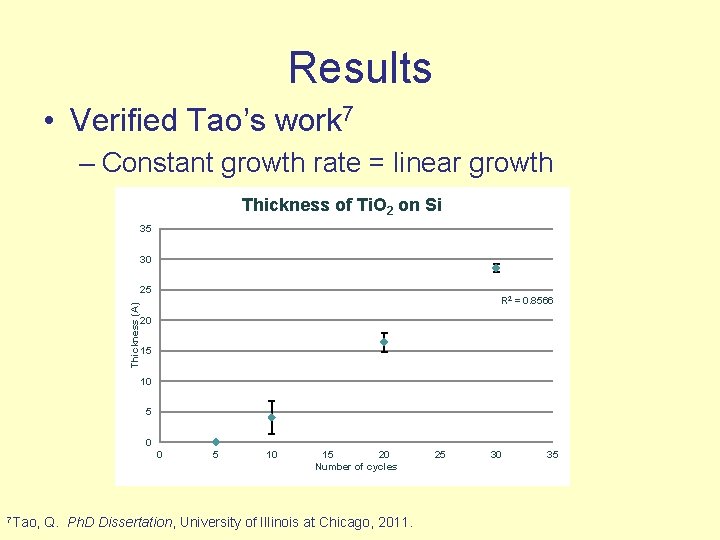
Results • Verified Tao’s work 7 – Constant growth rate = linear growth Thickness of Ti. O 2 on Si 35 30 Thickness (A) 25 R 2 = 0. 8566 20 15 10 5 0 0 7 Tao, 5 10 15 20 Number of cycles Q. Ph. D Dissertation, University of Illinois at Chicago, 2011. 25 30 35

Troubleshooting • Temperature – Increases along path to reactor – Keep oxidizer cold • Pressure – “Resting pressure” around 0. 176 torr – Cycles during deposition • N 2 tank, H 2 O level in bubbler • Check ellipsometer • Precursor level, clogged pipes

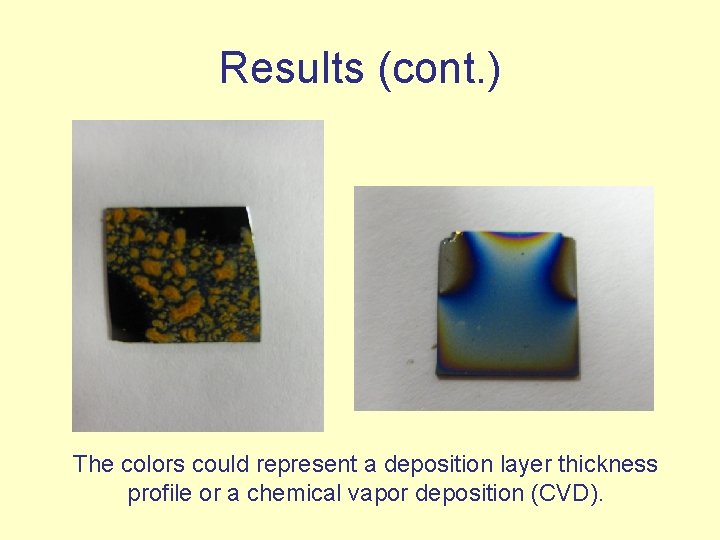
Results (cont. ) The colors could represent a deposition layer thickness profile or a chemical vapor deposition (CVD).

Summary • Objective: SALD of Ti. O 2 on Si for microelectronic applications • Method: reduce native oxide on Cu – Minimize air exposure (in progress) – In situ reduction (future work) • • Characterization: ellipsometry, XPS Results to date verify prior research Not enough data to conclude about Ti. O 2 on copper Troubleshooting, design setbacks are important parts of engineering

Acknowledgements • National Science Foundation, EEC-NSF Grant # 1062943 • CMMI-NSF Grant # 1134753 • Jorge I. Rossero A. • Runshen Xu • Arman Butt • Dr. Jursich • Dr. Takoudis

References • Chen, R. ; Kim, H. ; Mc. Intyre, P. C. ; Bent, S. F. Chem. Mater. 2005, 17, 536. • Lee, H. D. ; Feng, T. ; Yu, L. ; Mastrogiovanni, D. ; Wan, A. ; Gustafsson, T. ; Garfunkel, E. App. Phys. Lett. 2009, 94, 222108. • Tao, Q. ; Jursich, G. ; Takoudis, C. App. Phys. Lett. 2010, 96, 192105 • Tao, Q. ; Overhage, K. ; Jursich, G. ; Takoudis, C. Submitted to Journal of Phys. Chem. C. 2011. • Frank, M. M. ; Wilk, G. D. ; Starodub, D. ; Gustafsson, T. ; Garfunkel, E. ; Chabal, Y. J. ; Grazul, J. ; Muller, D. A. App. Phys. Lett. 2005, 86, 152904. • Cotton, F. A. ; Wilkinson, G. Advanced Inorganic Chemistry, 2 nd ed. New York: Interscience Publishers, 1966, pp. 894 -902. • Zhu, Y. ; Mimura, K. ; Lim, J. ; Isshiki, M. ; Jiang, Q. Metal. and Mineral Trans. A. 2006, 37 A, 1231. • Tao, Q. Ph. D Dissertation, University of Illinois at Chicago, 2011. • Falkenstein, Z. ; Hakovirta, M. ; Nastasi, M. Thin Solid Films. 2001, 381, 84. • Tompkins, H. G. ; Allara, D. L. J. Colloid and Interface Science. 1974, 49, 410. • Sakata, Y. ; Domen, K. ; Maruya, K. -I. ; Onishi, T. Appl. Spec. 1988, 42, 442.