Scientific Method Measurement Significant Figures Scientific Notation Density




















- Slides: 76

Scientific Method Measurement Significant Figures Scientific Notation Density Edward Wen, Ph. D

We use Chemistry Everywhere • Human body involves chemical reactions (mental/physical) • Electric vehicle utilizes battery (chemistry <-> electricity) • Cooking process is chemical change

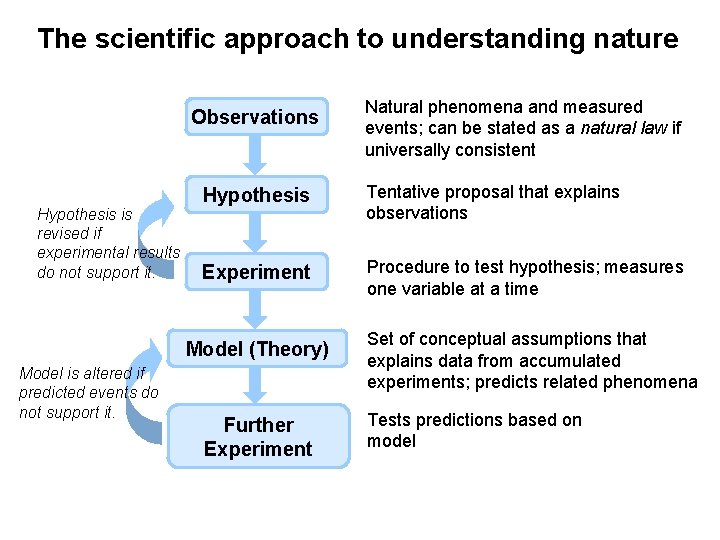
The scientific approach to understanding nature Observations Hypothesis is revised if experimental results do not support it. Hypothesis Tentative proposal that explains observations Experiment Procedure to test hypothesis; measures one variable at a time Model (Theory) Model is altered if predicted events do not support it. Natural phenomena and measured events; can be stated as a natural law if universally consistent Further Experiment Set of conceptual assumptions that explains data from accumulated experiments; predicts related phenomena Tests predictions based on model

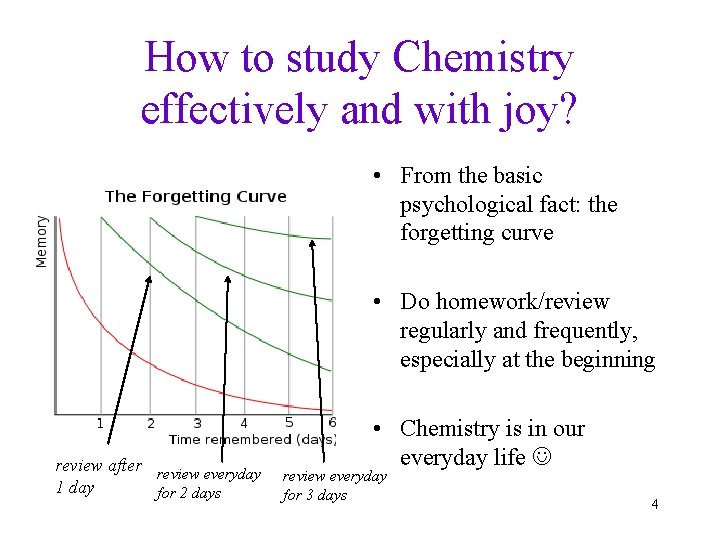
How to study Chemistry effectively and with joy? • From the basic psychological fact: the forgetting curve • Do homework/review regularly and frequently, especially at the beginning review after review everyday 1 day for 2 days • Chemistry is in our everyday life review everyday for 3 days 4

Chapter Outline • • Measurement, Metric system (SI) Conversion factor Scientific Notation Significant figures in Measurement/Calculation • Conversion involving units raised to powers • Density

What is a Measurement? • Quantitative observation • comparison to an agreed upon standard Every measurement has a number and a unit: • 77 Fahrenheit: Room temperature • 7. 5 pounds: Average newborn body weight in the US: • 55 grams: amount of sugar in one can of Coca Cola UNIT: what standard you are comparing your object to the number tells you 1. what multiple of the standard the object measures 2. the uncertainty in the measurement (±) 6

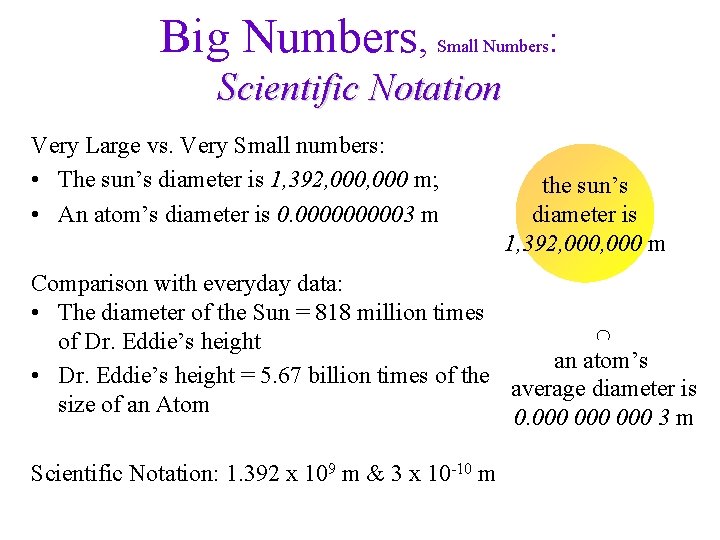
Big Numbers, Small Numbers : Scientific Notation Very Large vs. Very Small numbers: • The sun’s diameter is 1, 392, 000 m; • An atom’s diameter is 0. 000003 m the sun’s diameter is 1, 392, 000 m Comparison with everyday data: • The diameter of the Sun = 818 million times of Dr. Eddie’s height an atom’s • Dr. Eddie’s height = 5. 67 billion times of the average diameter is size of an Atom 0. 000 000 3 m Scientific Notation: 1. 392 x 109 m & 3 x 10 -10 m

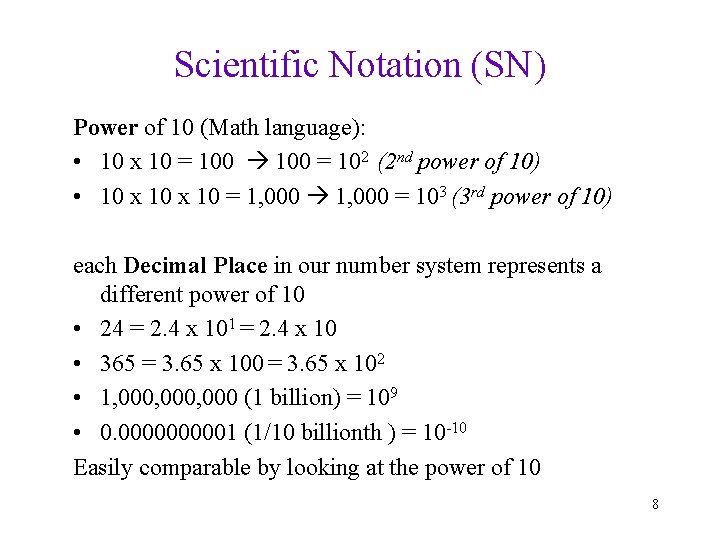
Scientific Notation (SN) Power of 10 (Math language): • 10 x 10 = 100 = 102 (2 nd power of 10) • 10 x 10 = 1, 000 = 103 (3 rd power of 10) each Decimal Place in our number system represents a different power of 10 • 24 = 2. 4 x 101 = 2. 4 x 10 • 365 = 3. 65 x 100 = 3. 65 x 102 • 1, 000, 000 (1 billion) = 109 • 0. 000001 (1/10 billionth ) = 10 -10 Easily comparable by looking at the power of 10 8

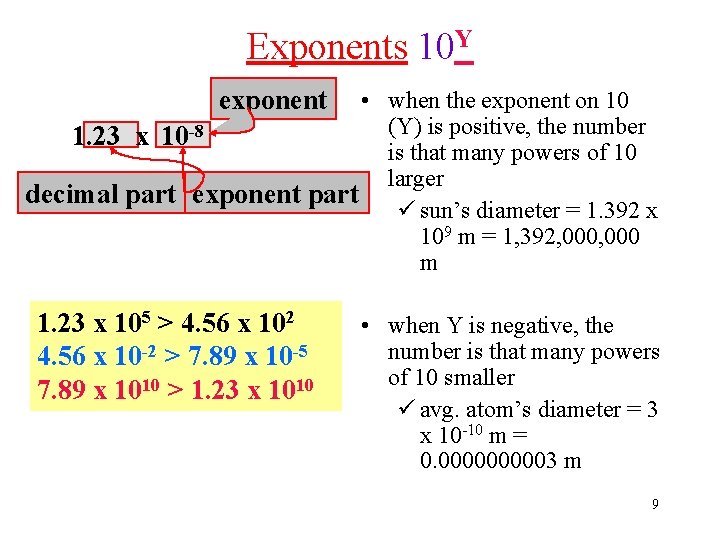
Exponents 10 Y exponent • when the exponent on 10 (Y) is positive, the number 1. 23 x 10 -8 is that many powers of 10 larger decimal part exponent part ü sun’s diameter = 1. 392 x 109 m = 1, 392, 000 m 1. 23 x 105 > 4. 56 x 102 4. 56 x 10 -2 > 7. 89 x 10 -5 7. 89 x 1010 > 1. 23 x 1010 • when Y is negative, the number is that many powers of 10 smaller ü avg. atom’s diameter = 3 x 10 -10 m = 0. 000003 m 9

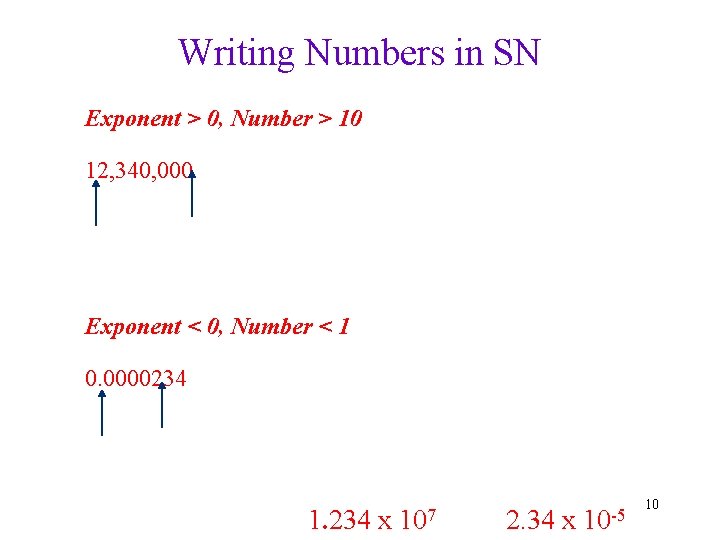
Writing Numbers in SN Exponent > 0, Number > 10 12, 340, 000 Exponent < 0, Number < 1 0. 0000234 1. 234 x 107 2. 34 x 10 -5 10

Writing a Number in Standard Form Negative exponent: move the decimal point to the left. if you run out of digits, add zeros 1. 234 x 10 -6 = Positive exponent: move decimal point to the right and add trailing zeros: 1. 234 x 1010 = 11 • 0. 000 001 234; 1. 2340000000

Practice • Convert the following numbers to SN: 149, 000 0. 0000844 • Convert the following SN to standard form: 4. 45 x 1012 1. 45 x 10 -7

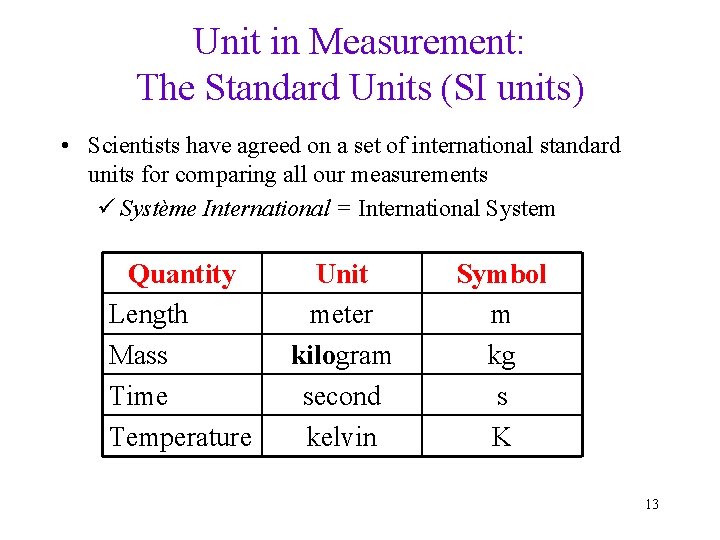
Unit in Measurement: The Standard Units (SI units) • Scientists have agreed on a set of international standard units for comparing all our measurements ü Système International = International System Quantity Length Mass Time Temperature Unit meter kilogram second kelvin Symbol m kg s K 13

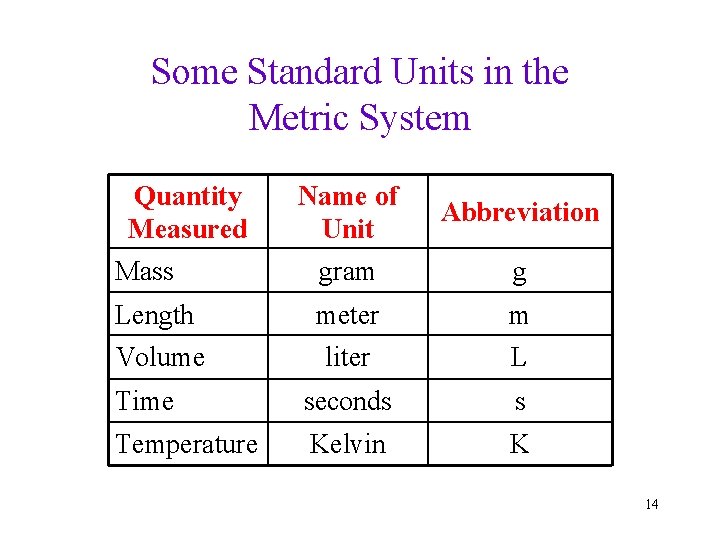
Some Standard Units in the Metric System Quantity Measured Name of Unit Abbreviation Mass gram g Length meter m Volume liter L Time seconds s Temperature Kelvin K 14

Related Units in the SI System All units in the SI system are related to the standard unit by a power of 10 • 1 kg = 103 g • 1 km = 103 m • 1 m = 102 cm • The power of 10 is indicated by a prefix • The prefixes are always the same, regardless of the standard unit 15

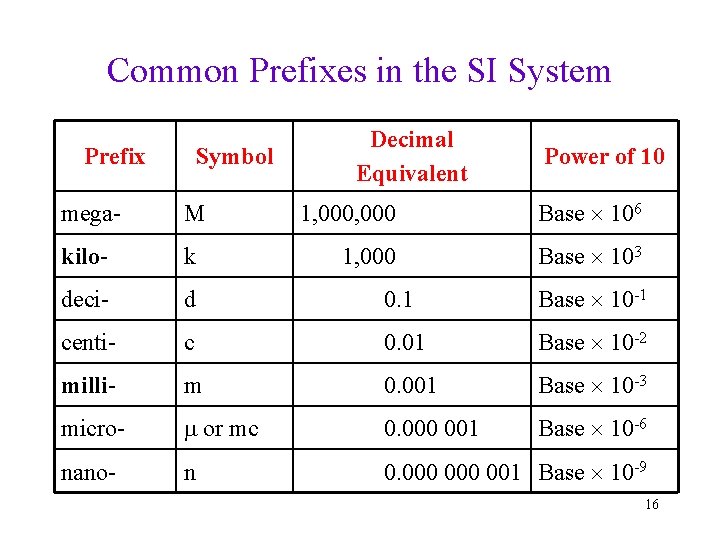
Common Prefixes in the SI System Prefix Symbol Decimal Equivalent Power of 10 1, 000 Base 106 1, 000 Base 103 mega- M kilo- k deci- d 0. 1 Base 10 -1 centi- c 0. 01 Base 10 -2 milli- m 0. 001 Base 10 -3 micro- or mc 0. 000 001 Base 10 -6 nano- n 0. 000 001 Base 10 -9 16

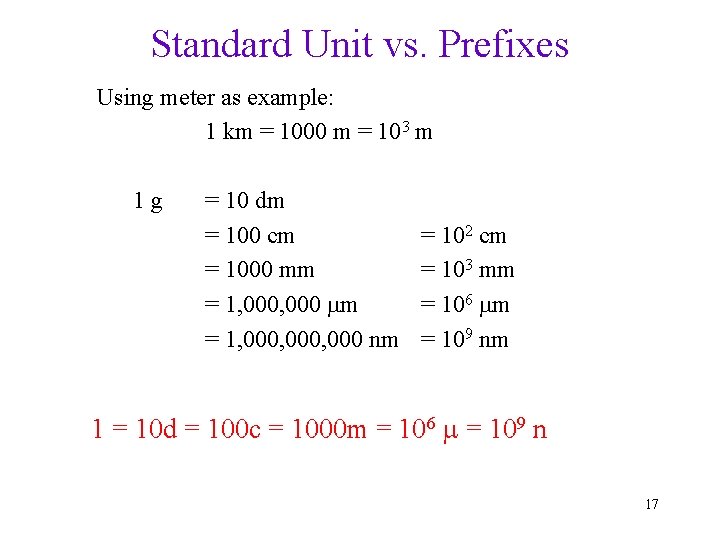
Standard Unit vs. Prefixes Using meter as example: 1 km = 1000 m = 103 m 1 g = 10 dm = 100 cm = 1000 mm = 1, 000, 000 nm = 102 cm = 103 mm = 106 m = 109 nm 1 = 10 d = 100 c = 1000 m = 106 = 109 n 17

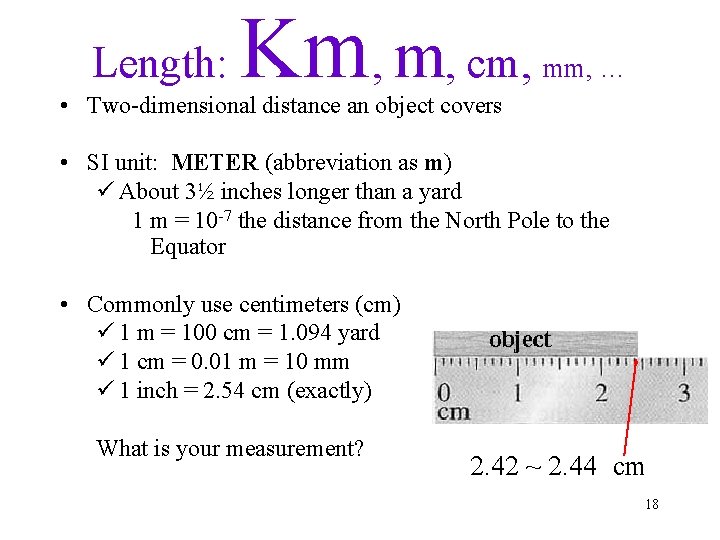
Length: Km, m, cm, mm, … • Two-dimensional distance an object covers • SI unit: METER (abbreviation as m) ü About 3½ inches longer than a yard 1 m = 10 -7 the distance from the North Pole to the Equator • Commonly use centimeters (cm) ü 1 m = 100 cm = 1. 094 yard ü 1 cm = 0. 01 m = 10 mm ü 1 inch = 2. 54 cm (exactly) What is your measurement? 2. 42 ~ 2. 44 cm 18

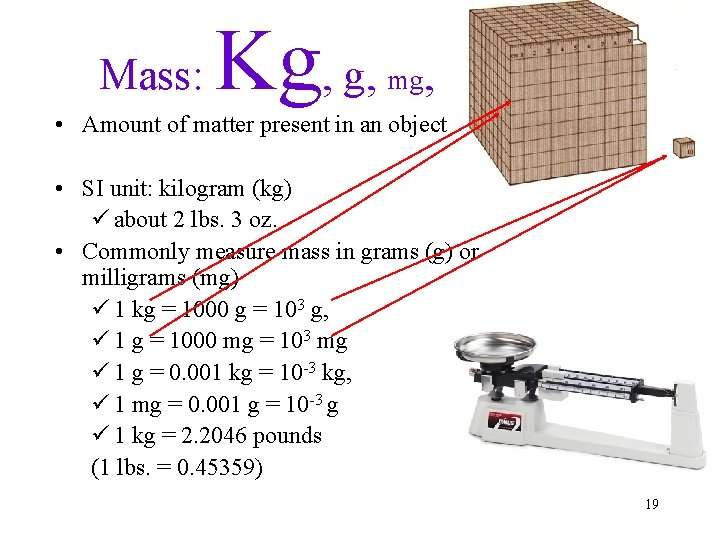
Mass: Kg, g, mg, • Amount of matter present in an object • SI unit: kilogram (kg) ü about 2 lbs. 3 oz. • Commonly measure mass in grams (g) or milligrams (mg) ü 1 kg = 1000 g = 103 g, ü 1 g = 1000 mg = 103 mg ü 1 g = 0. 001 kg = 10 -3 kg, ü 1 mg = 0. 001 g = 10 -3 g ü 1 kg = 2. 2046 pounds (1 lbs. = 0. 45359) 19

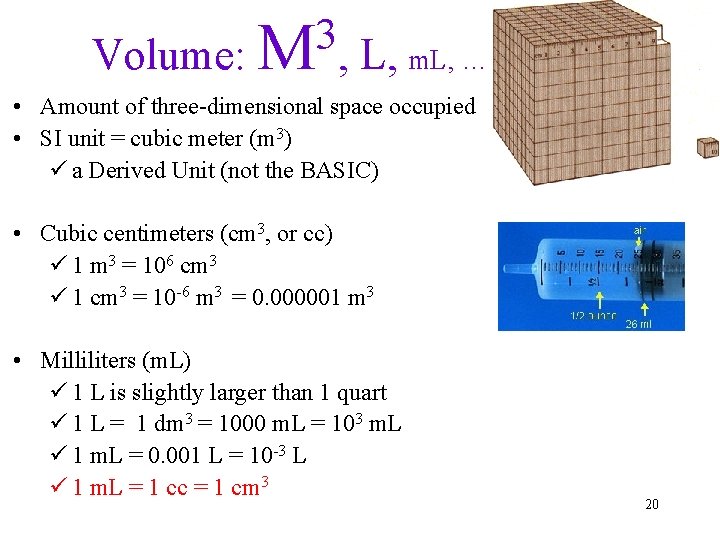
3 Volume: M , L, m. L, … • Amount of three-dimensional space occupied • SI unit = cubic meter (m 3) ü a Derived Unit (not the BASIC) • Cubic centimeters (cm 3, or cc) ü 1 m 3 = 106 cm 3 ü 1 cm 3 = 10 -6 m 3 = 0. 000001 m 3 • Milliliters (m. L) ü 1 L is slightly larger than 1 quart ü 1 L = 1 dm 3 = 1000 m. L = 103 m. L ü 1 m. L = 0. 001 L = 10 -3 L ü 1 m. L = 1 cc = 1 cm 3 20

Prefixes Used to Modify Standard Unit • kilo = 1000 times base unit = 103 ü 1 kg = 1000 g = 103 g • deci = 0. 1 times the base unit = 10 -1 ü 1 d. L = 0. 1 L = 10 -1 L; 1 L = 10 d. L • centi = 0. 01 times the base unit = 10 -2 ü 1 cm = 0. 01 m = 10 -2 m; 1 m = 100 cm • milli = 0. 001 times the base unit = 10 -3 ü 1 mg = 0. 001 g = 10 -3 g; 1 g = 1000 mg • micro = 10 -6 times the base unit ü 1 m = 10 -6 m; 106 m = 1 m • nano = 10 -9 times the base unit ü 1 n. L = 10 -9 L; 109 n. L = 1 L 21

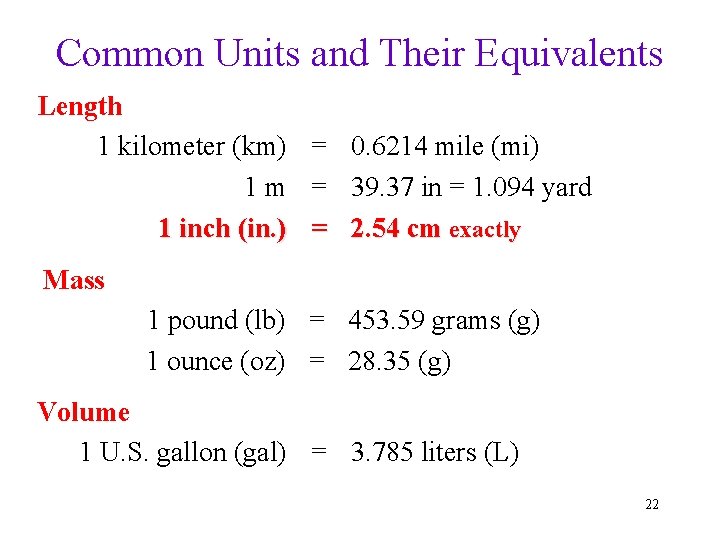
Common Units and Their Equivalents Length 1 kilometer (km) = 0. 6214 mile (mi) 1 m = 39. 37 in = 1. 094 yard 1 inch (in. ) = 2. 54 cm exactly Mass 1 pound (lb) = 453. 59 grams (g) 1 ounce (oz) = 28. 35 (g) Volume 1 U. S. gallon (gal) = 3. 785 liters (L) 22

Use of Units • Always write every number with its associated unit • Always include units in your calculations üyou can do the same kind of operations on units as you can with numbers • cm × cm = cm 2 • cm + cm = cm • cm ÷ cm = 1 üusing units as a guide to problem solving 23

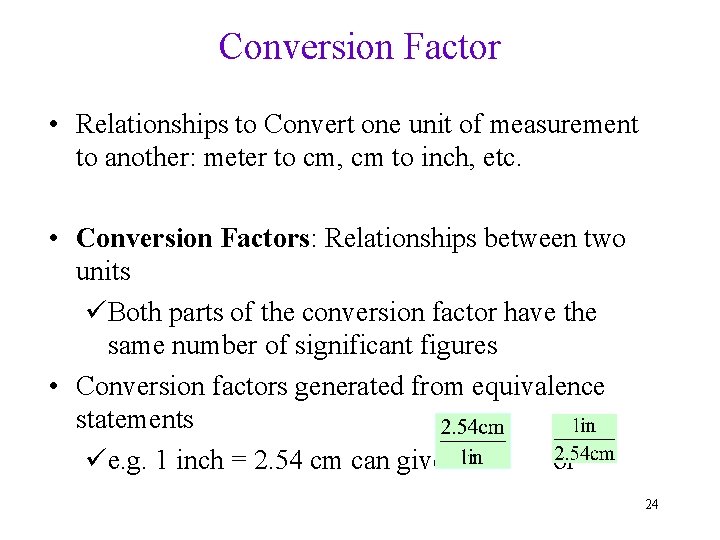
Conversion Factor • Relationships to Convert one unit of measurement to another: meter to cm, cm to inch, etc. • Conversion Factors: Relationships between two units üBoth parts of the conversion factor have the same number of significant figures • Conversion factors generated from equivalence statements üe. g. 1 inch = 2. 54 cm can give or 24

We have been using the Conversion Factor ALL THE TIME! • How are we converting #cents into #dollars? Why? ü From 1 dollar = 100 cents 45, 000 cents x 1 dollar = 450 dollars 100 cents Conversion Factor 25

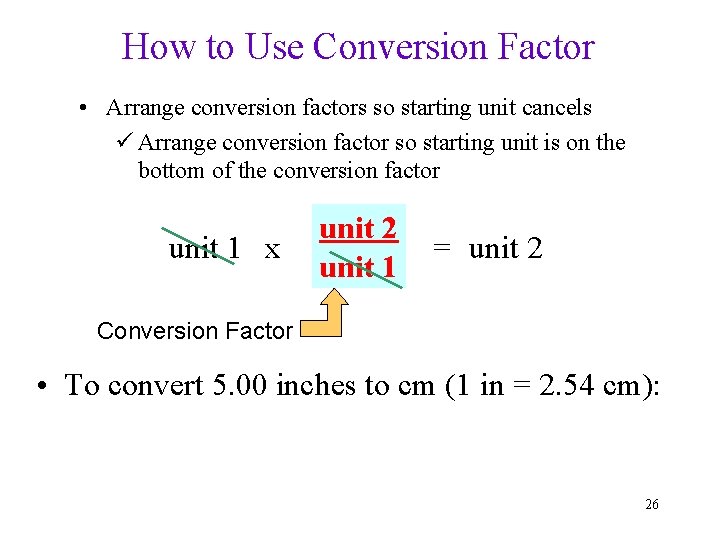
How to Use Conversion Factor • Arrange conversion factors so starting unit cancels ü Arrange conversion factor so starting unit is on the bottom of the conversion factor unit 1 x unit 2 unit 1 = unit 2 Conversion Factor • To convert 5. 00 inches to cm (1 in = 2. 54 cm): 26

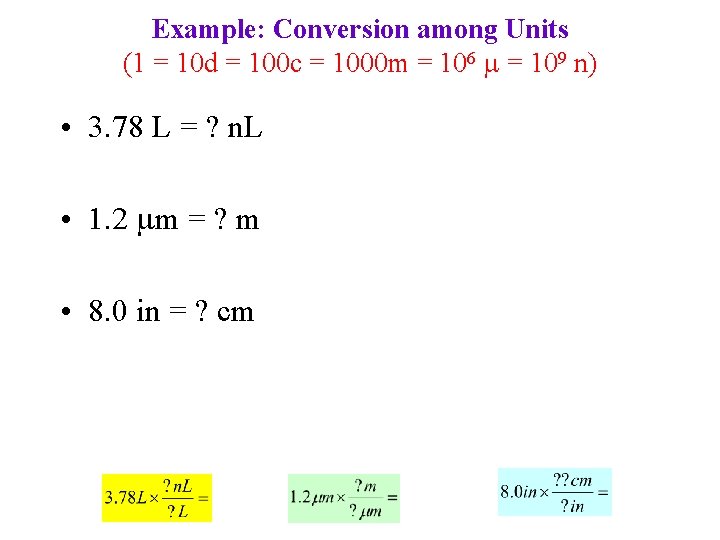
Example: Conversion among Units (1 = 10 d = 100 c = 1000 m = 106 = 109 n) • 3. 78 L = ? n. L • 1. 2 m = ? m • 8. 0 in = ? cm

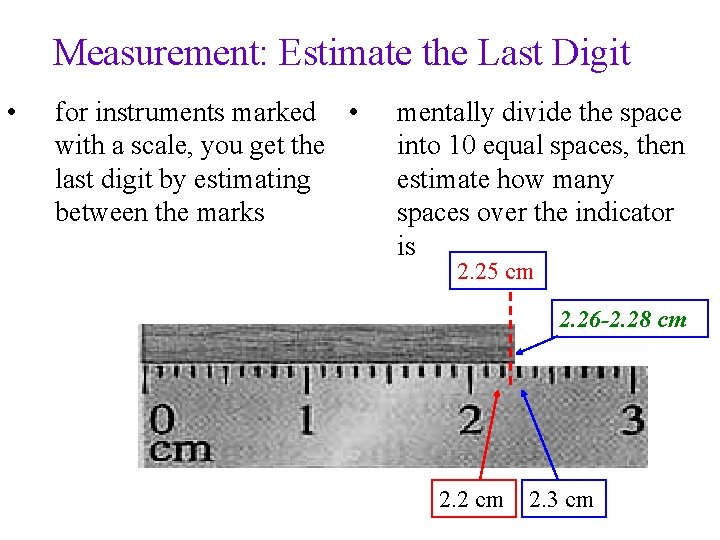
Measurement: Estimate the Last Digit • for instruments marked • with a scale, you get the last digit by estimating between the marks mentally divide the space into 10 equal spaces, then estimate how many spaces over the indicator is 2. 25 cm 2. 26 -2. 28 cm 2. 2 cm 2. 3 cm

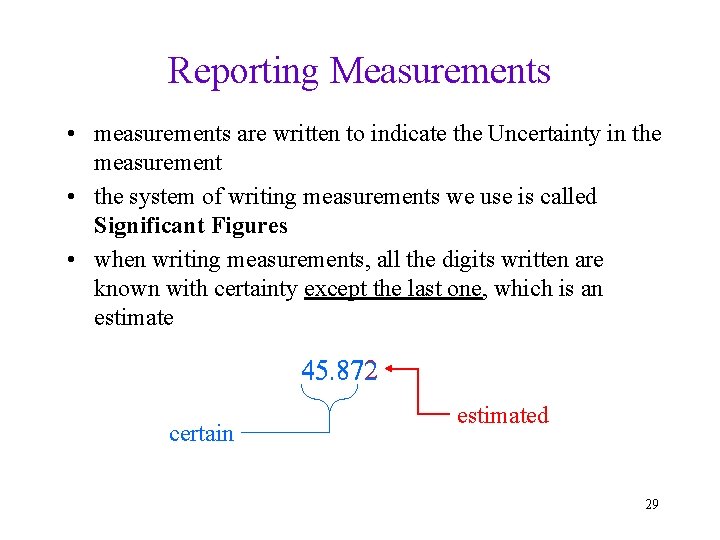
Reporting Measurements • measurements are written to indicate the Uncertainty in the measurement • the system of writing measurements we use is called Significant Figures • when writing measurements, all the digits written are known with certainty except the last one, which is an estimate 45. 872 certain estimated 29

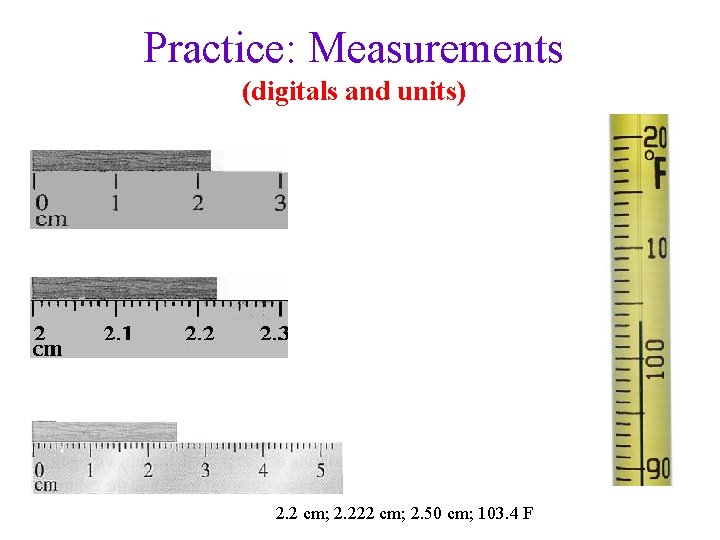
Practice: Measurements (digitals and units) 2. 2 cm; 2. 222 cm; 2. 50 cm; 103. 4 F

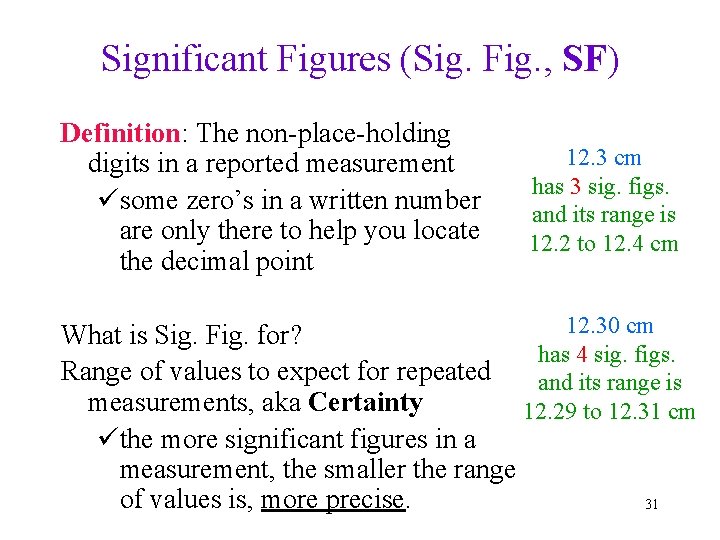
Significant Figures (Sig. Fig. , SF) Definition: The non-place-holding digits in a reported measurement üsome zero’s in a written number are only there to help you locate the decimal point 12. 3 cm has 3 sig. figs. and its range is 12. 2 to 12. 4 cm 12. 30 cm What is Sig. Fig. for? has 4 sig. figs. Range of values to expect for repeated and its range is measurements, aka Certainty 12. 29 to 12. 31 cm üthe more significant figures in a measurement, the smaller the range of values is, more precise. 31

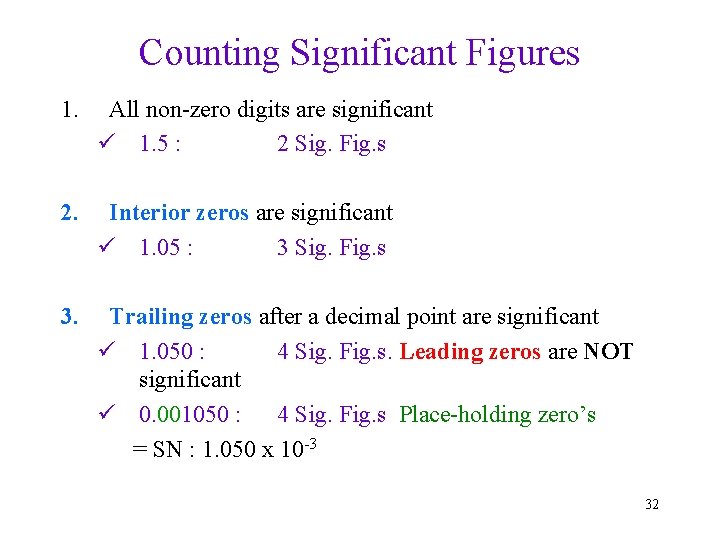
Counting Significant Figures 1. All non-zero digits are significant ü 1. 5 : 2 Sig. Fig. s 2. Interior zeros are significant ü 1. 05 : 3 Sig. Fig. s 3. Trailing zeros after a decimal point are significant ü 1. 050 : 4 Sig. Fig. s. Leading zeros are NOT significant ü 0. 001050 : 4 Sig. Fig. s Place-holding zero’s = SN : 1. 050 x 10 -3 32

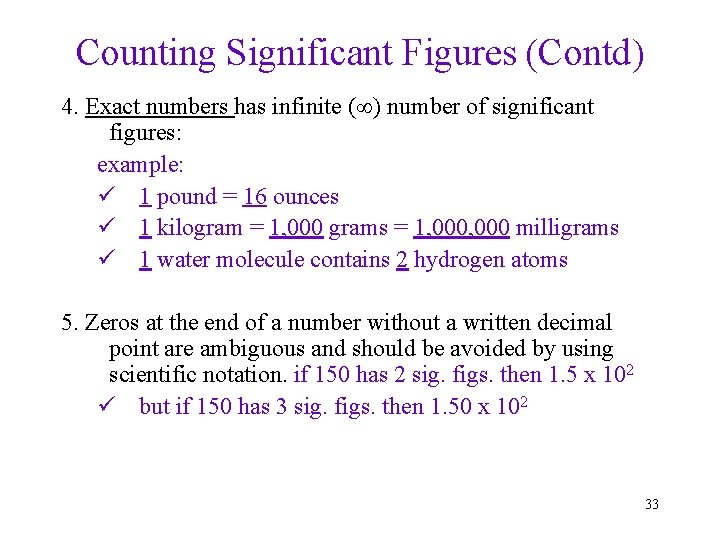
Counting Significant Figures (Contd) 4. Exact numbers has infinite ( ) number of significant figures: example: ü 1 pound = 16 ounces ü 1 kilogram = 1, 000 grams = 1, 000 milligrams ü 1 water molecule contains 2 hydrogen atoms 5. Zeros at the end of a number without a written decimal point are ambiguous and should be avoided by using scientific notation. if 150 has 2 sig. figs. then 1. 5 x 102 ü but if 150 has 3 sig. figs. then 1. 50 x 102 33

Example– How many Sig. Figs? 1. 080 L 23, 000 students 0. 200 cm. 2. 970 × 105 kg 1 dozen = 12 34 • 4; ambiguous or 2; 3; 4; both exact

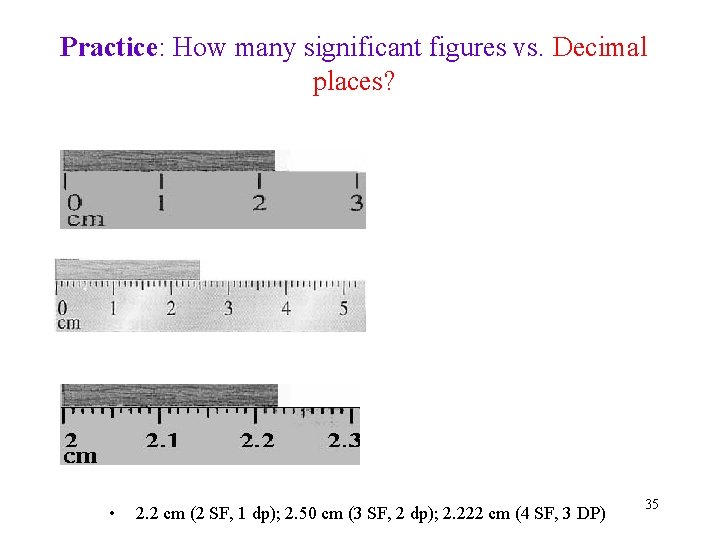
Practice: How many significant figures vs. Decimal places? • 2. 2 cm (2 SF, 1 dp); 2. 50 cm (3 SF, 2 dp); 2. 222 cm (4 SF, 3 DP) 35

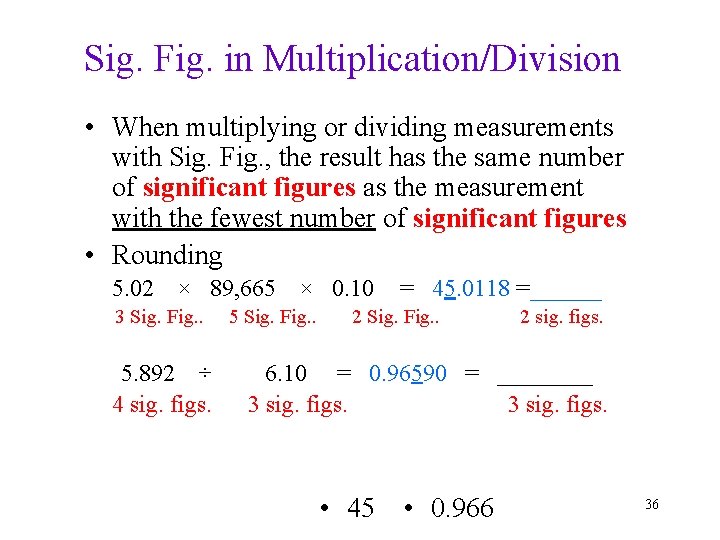
Sig. Fig. in Multiplication/Division • When multiplying or dividing measurements with Sig. Fig. , the result has the same number of significant figures as the measurement with the fewest number of significant figures • Rounding 5. 02 × 89, 665 × 0. 10 3 Sig. Fig. . 5. 892 ÷ 4 sig. figs. 5 Sig. Fig. . = 45. 0118 =______ 2 Sig. Fig. . 2 sig. figs. 6. 10 = 0. 96590 = ____ 3 sig. figs. • 45 • 0. 966 36

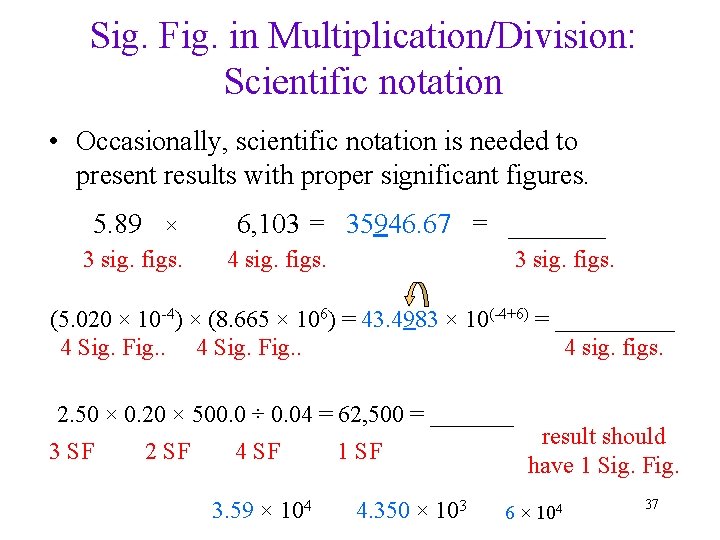
Sig. Fig. in Multiplication/Division: Scientific notation • Occasionally, scientific notation is needed to present results with proper significant figures. 5. 89 × 3 sig. figs. 6, 103 = 35946. 67 = _______ 3 sig. figs. 4 sig. figs. (5. 020 × 10 -4) × (8. 665 × 106) = 43. 4983 × 10(-4+6) = _____ 4 Sig. Fig. . 4 sig. figs. 2. 50 × 0. 20 × 500. 0 ÷ 0. 04 = 62, 500 = _______ 3 SF 2 SF 4 SF 3. 59 × 104 1 SF 4. 350 × 103 result should have 1 Sig. Fig. 6 × 104 37

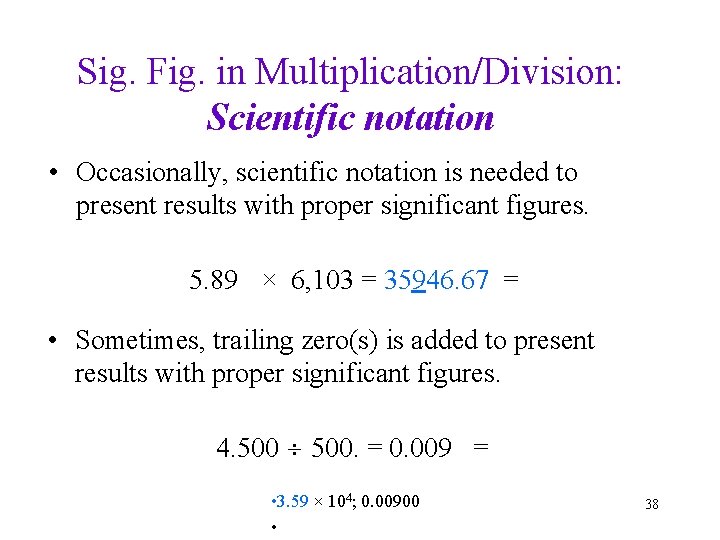
Sig. Fig. in Multiplication/Division: Scientific notation • Occasionally, scientific notation is needed to present results with proper significant figures. 5. 89 × 6, 103 = 35946. 67 = • Sometimes, trailing zero(s) is added to present results with proper significant figures. 4. 500. = 0. 009 = • 3. 59 × 104; 0. 00900 • 38

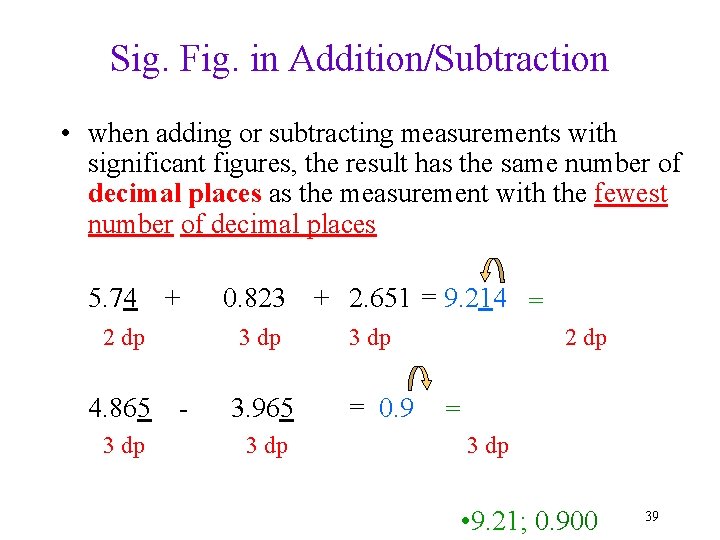
Sig. Fig. in Addition/Subtraction • when adding or subtracting measurements with significant figures, the result has the same number of decimal places as the measurement with the fewest number of decimal places 5. 74 + 2 dp 4. 865 3 dp 0. 823 3 dp - 3. 965 3 dp + 2. 651 = 9. 214 = 3 dp = 0. 9 2 dp = 3 dp • 9. 21; 0. 900 39

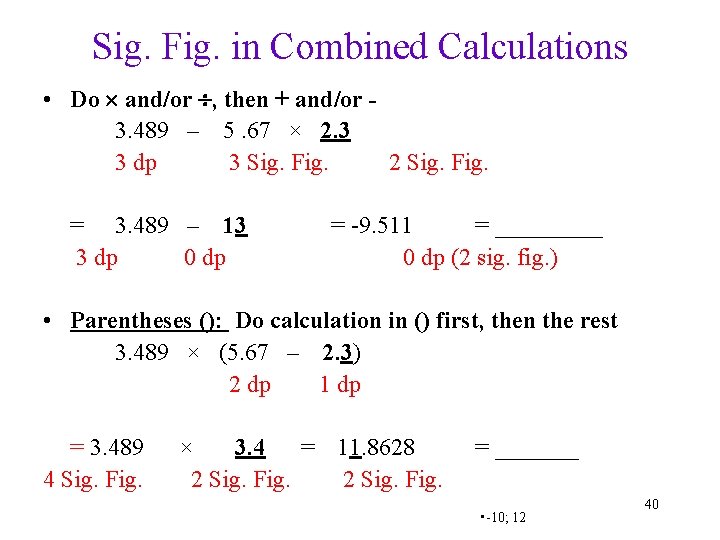
Sig. Fig. in Combined Calculations • Do and/or , then + and/or 3. 489 – 5. 67 × 2. 3 3 dp 3 Sig. Fig. 2 Sig. Fig. = 3. 489 – 13 3 dp 0 dp = -9. 511 = _____ 0 dp (2 sig. fig. ) • Parentheses (): Do calculation in () first, then the rest 3. 489 × (5. 67 – 2. 3) 2 dp 1 dp = 3. 489 4 Sig. Fig. × 3. 4 = 11. 8628 2 Sig. Fig. = _______ • -10; 12 40

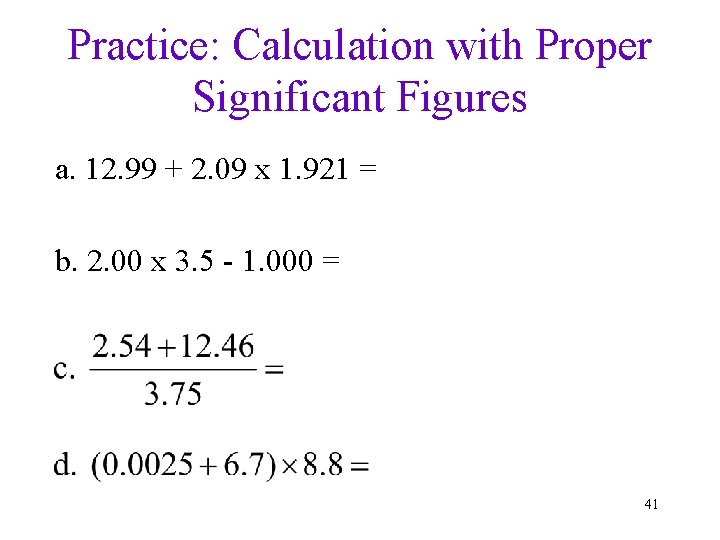
Practice: Calculation with Proper Significant Figures a. 12. 99 + 2. 09 x 1. 921 = b. 2. 00 x 3. 5 - 1. 000 = 41

How to solve Unit Conversion Problems 1) Write down Given Amount and Unit 2) Write down what you want to Find and Unit 3) Write down needed Conversion Factors or Equations 4) Design a Solution Map for the Problem ü order Conversions to cancel previous units or ü arrange Equation so Find amount is isolated. Example: from Equation A = b c to solve for b 42

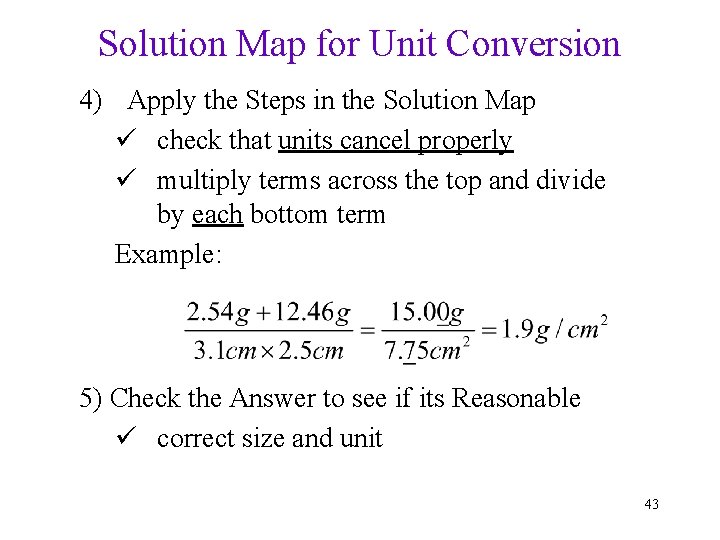
Solution Map for Unit Conversion 4) Apply the Steps in the Solution Map ü check that units cancel properly ü multiply terms across the top and divide by each bottom term Example: 5) Check the Answer to see if its Reasonable ü correct size and unit 43

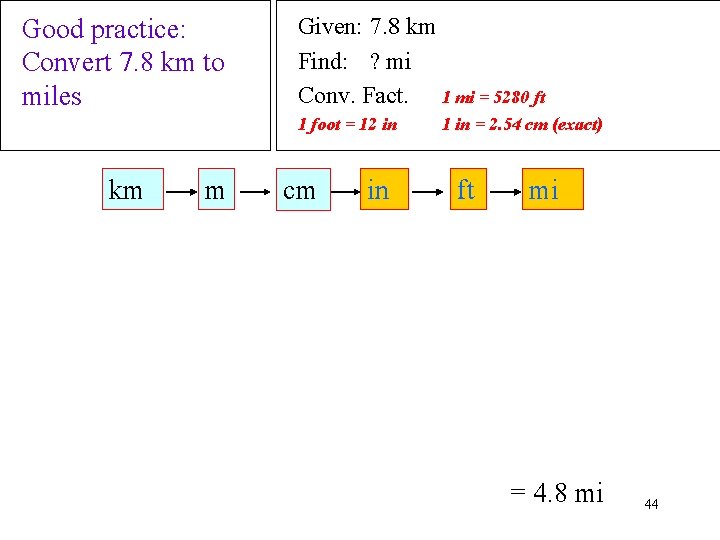
Good practice: Convert 7. 8 km to miles Given: 7. 8 km Find: ? mi Conv. Fact. 1 mi = 5280 ft 1 foot = 12 in km m cm in 1 in = 2. 54 cm (exact) ft mi = 4. 8 mi 44

Know Your Scientific Calculator 45

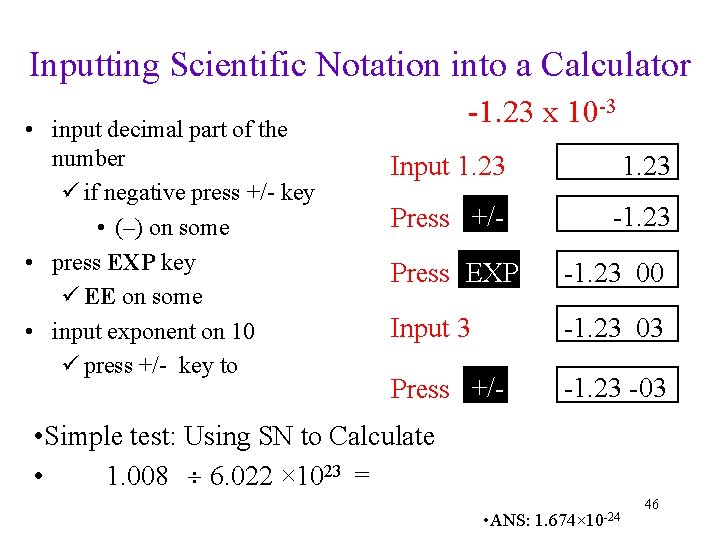
Inputting Scientific Notation into a Calculator • input decimal part of the number ü if negative press +/- key • (–) on some • press EXP key ü EE on some • input exponent on 10 ü press +/- key to -1. 23 x 10 -3 Input 1. 23 Press +/- -1. 23 Press EXP -1. 23 00 Input 3 -1. 23 03 Press +/- -1. 23 -03 • Simple test: Using SN to Calculate • 1. 008 6. 022 × 1023 = • ANS: 1. 674× 10 -24 46

Mass & Volume: • two main characteristics of matter • even though mass and volume are individual properties - for a given type of matter they are related to each other! Density (ratio of mass vs. volume): for a certain matter, its density is one of the characteristic to distinguish from one another 47

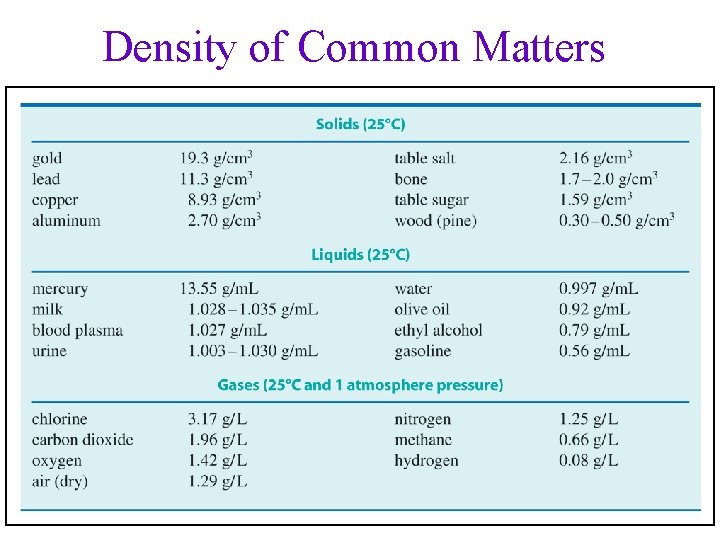
Unit for density • Solids = g/cm 3 ü 1 cm 3 = 1 m. L • Liquids = g/m. L: Density of water = 1. 00 g/m. L • Gases = g/L: Density of Air ~ 1. 3 g/L Volume of a solid can be determined by water displacement • Density : solids > liquids >>> gases üexcept ice and dry wood are less dense than liquid water! 48

Density of Common Matters 49

About Density • Temperature affects the density: Heating objects causes objects to expand, density üThe Lava Lamp: heating/cooling • In a heterogeneous mixture, the denser object sinks üWhy do hot air balloons rise? üThe “Gold Rush”: Extracting gold particle from sand üDensity of gasoline changes over the day! 50

Density and Volume Styrofoam vs. Quarter: Both of these items have a mass of 23 grams, but they have very different volumes; therefore, their densities are different as well. 51

Density and Buoyancy • • Average density of human body = 1. 0 g/cm 3 Average density of sea water = 1. 03 g/m. L Density of mercury, liquid metal, = 13. 6 g/m. L Density of copper penny = 8. 9 g/cm 3 52

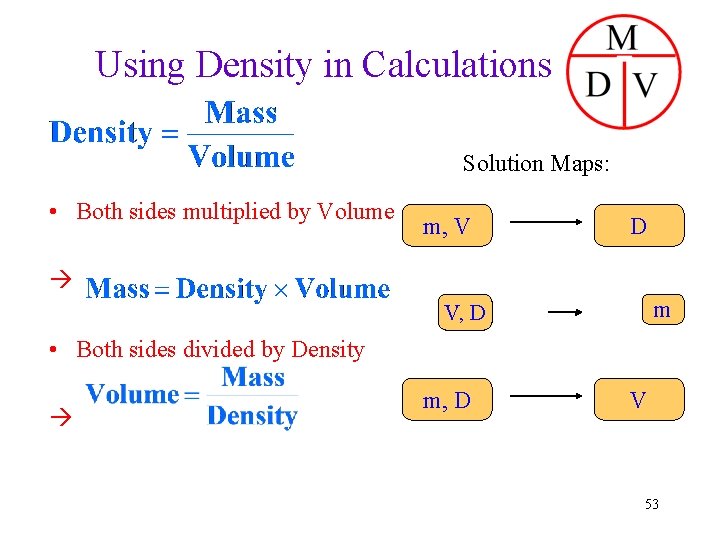
Using Density in Calculations Solution Maps: • Both sides multiplied by Volume m, V D m V, D • Both sides divided by Density m, D V 53

Application of Density A geologist found a piece of golden color mineral. He decided to measure the density to help identify if it is gold. He first measured the mass of mineral as 10. 20 grams. Then he measured its volume as 2. 01 cm 3. What is the density of this mineral? Is it gold? (photo credit: https: //www. analyticalsci. com/store/p 415/Fools_Gold. html) Data: Density pure gold = 19. 3 g/cm 3 54

Test results Given: Mass = 10. 20 grams Volume = 2. 01 cm 3 Density Au = 19. 3 g/cm 3 Find: Density in grams/cm 3 Density 5. 08 g/cm 3

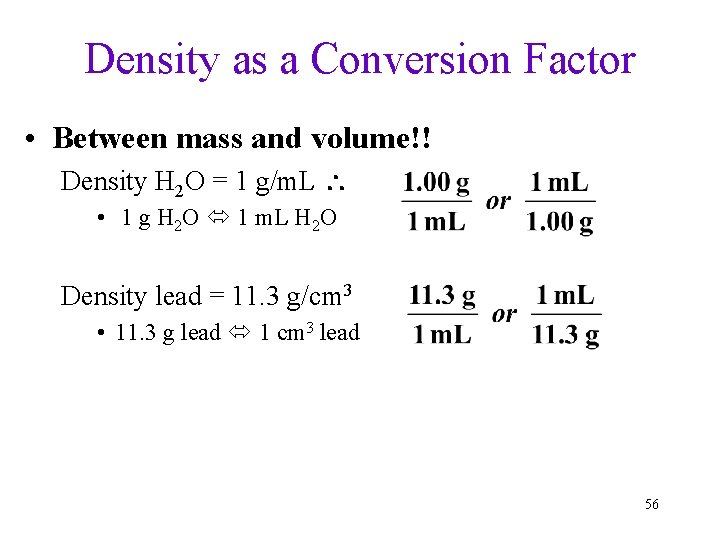
Density as a Conversion Factor • Between mass and volume!! Density H 2 O = 1 g/m. L • 1 g H 2 O 1 m. L H 2 O Density lead = 11. 3 g/cm 3 • 11. 3 g lead 1 cm 3 lead 56

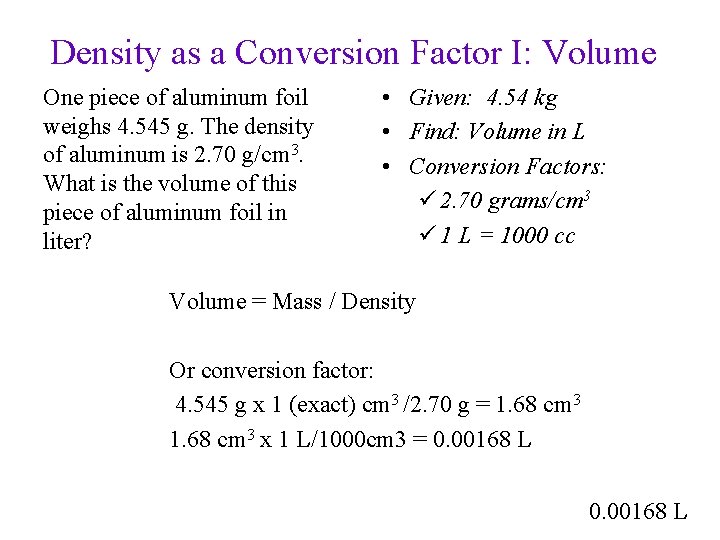
Density as a Conversion Factor I: Volume One piece of aluminum foil weighs 4. 545 g. The density of aluminum is 2. 70 g/cm 3. What is the volume of this piece of aluminum foil in liter? • Given: 4. 54 kg • Find: Volume in L • Conversion Factors: ü 2. 70 grams/cm 3 ü 1 L = 1000 cc 0. 00168 L

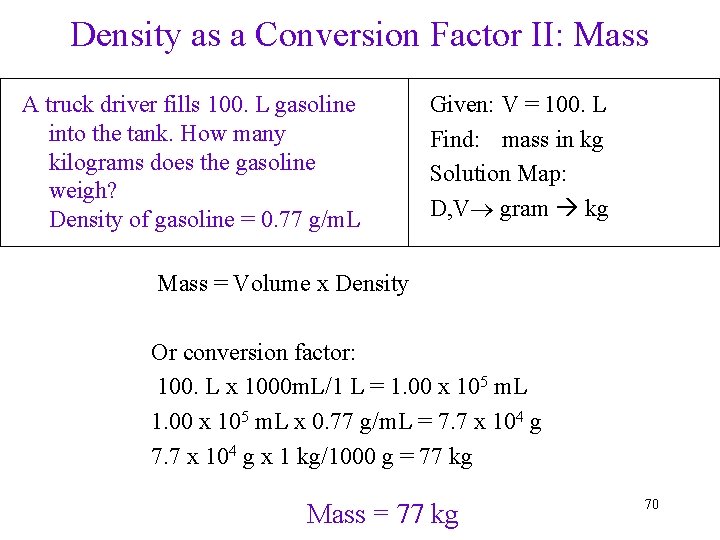
Density as a Conversion Factor II: Mass A truck driver fills 100. L gasoline into the tank. How many kilograms does the gasoline weigh? Density of gasoline = 0. 77 g/m. L Given: V = 100. L Find: mass in kg Solution Map: D, V gram kg Mass = 77 kg 58

Example: Conversion between nonstandard Units (1 = 10 d = 100 c = 1000 m = 106 = 109 n) • 3. 78 n. L = ? L • 1. 2 mm = ? km

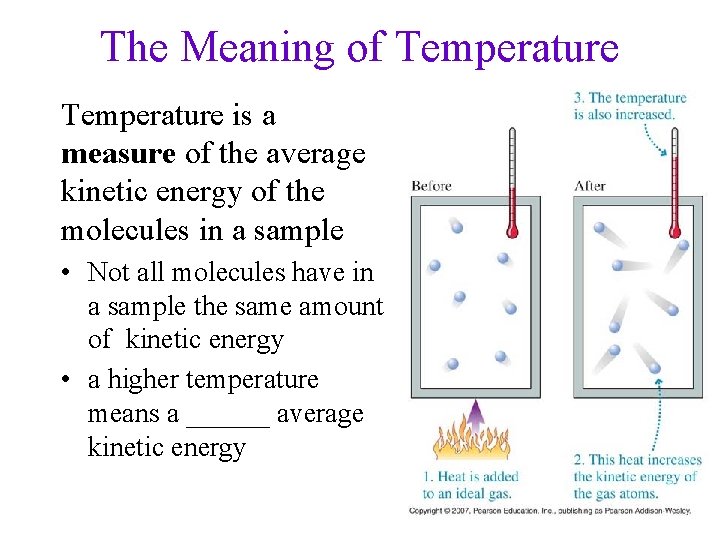
The Meaning of Temperature is a measure of the average kinetic energy of the molecules in a sample • Not all molecules have in a sample the same amount of kinetic energy • a higher temperature means a ______ average kinetic energy 60

Celsius Temperature Scale Two reference points: • Freezing point of distilled water (0°C) • Boiling point of distilled water (100°C) ümore reproducible standards ümost commonly used in science • Room temperature is about 25°C 61

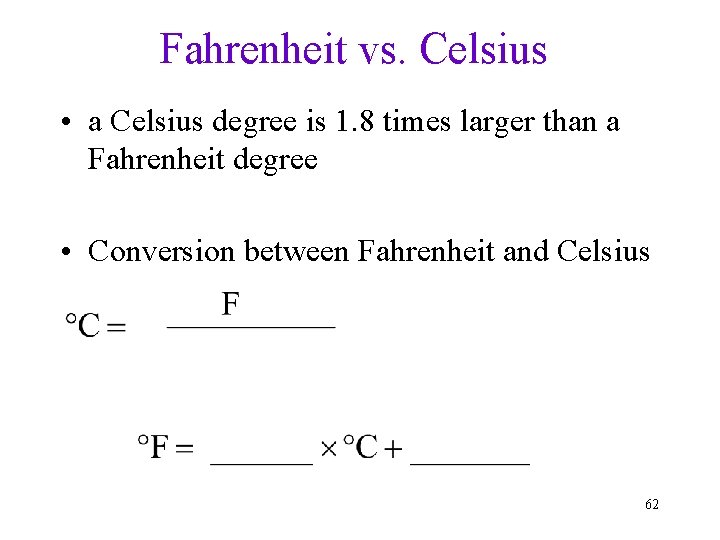
Fahrenheit vs. Celsius • a Celsius degree is 1. 8 times larger than a Fahrenheit degree • Conversion between Fahrenheit and Celsius 62

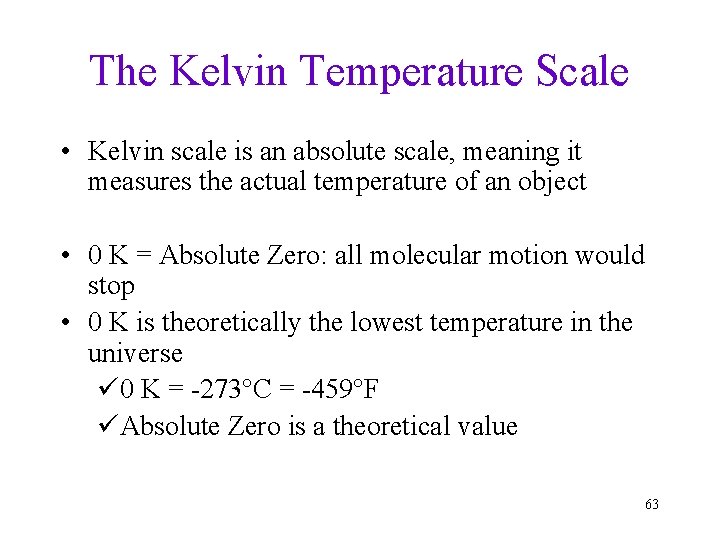
The Kelvin Temperature Scale • Kelvin scale is an absolute scale, meaning it measures the actual temperature of an object • 0 K = Absolute Zero: all molecular motion would stop • 0 K is theoretically the lowest temperature in the universe ü 0 K = -273°C = -459°F üAbsolute Zero is a theoretical value 63

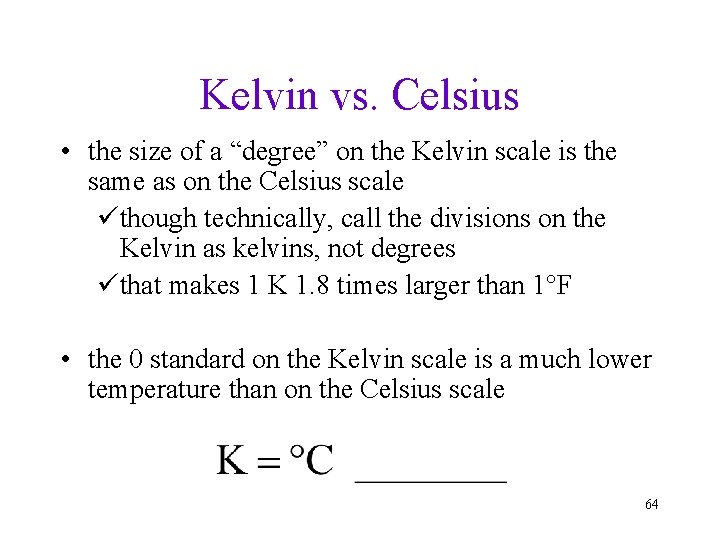
Kelvin vs. Celsius • the size of a “degree” on the Kelvin scale is the same as on the Celsius scale üthough technically, call the divisions on the Kelvin as kelvins, not degrees üthat makes 1 K 1. 8 times larger than 1°F • the 0 standard on the Kelvin scale is a much lower temperature than on the Celsius scale 64

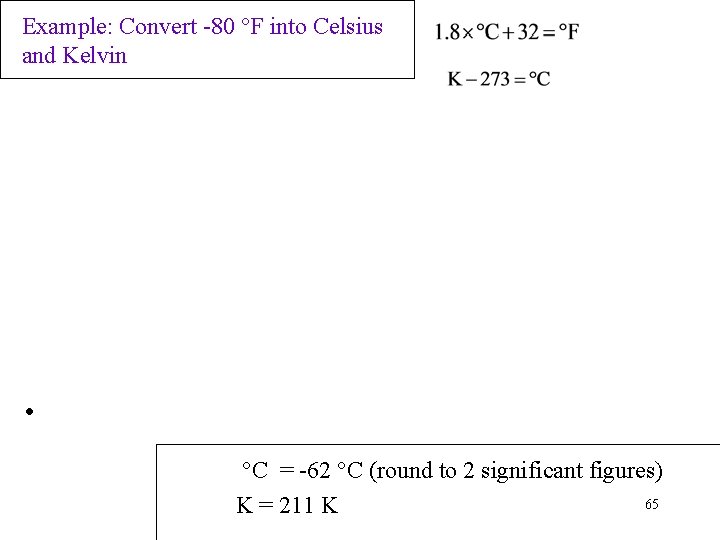
Example: Convert -80 °F into Celsius and Kelvin • °C = -62 °C (round to 2 significant figures) 65 K = 211 K

Practice • Convert the following numbers to SN: 149, 000 0. 0000844 • Convert the following SN to standard form: 4. 45 x 1012 1. 45 x 10 -7

More practice on Conversion 1. Proper dosage of a drug is 3. 5 mg/kg of body weight. Calculate the milligrams of this drug for a 138 -lb individual? (1 lb = 454 g). KEY: 2. 2× 102 mg (2 SF) 2. 100. mg ibuprofen/5 m. L Motrin. Calculate the grams of ibuprofen in 1. 5 teaspoons of Motrin. (1 teaspoon = 5. 0 m. L) KEY: 0. 15 g (2 SF) 67

Density as a Conversion Factor I: Volume One piece of aluminum foil weighs 4. 545 g. The density of aluminum is 2. 70 g/cm 3. What is the volume of this piece of aluminum foil in liter? • Given: 4. 54 kg • Find: Volume in L • Conversion Factors: ü 2. 70 grams/cm 3 ü 1 L = 1000 cc Volume = Mass / Density Or conversion factor: 4. 545 g x 1 (exact) cm 3 /2. 70 g = 1. 68 cm 3 x 1 L/1000 cm 3 = 0. 00168 L

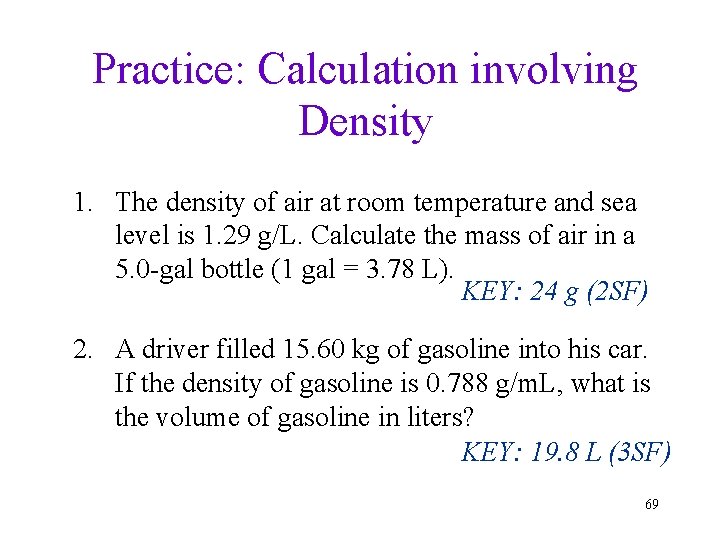
Practice: Calculation involving Density 1. The density of air at room temperature and sea level is 1. 29 g/L. Calculate the mass of air in a 5. 0 -gal bottle (1 gal = 3. 78 L). KEY: 24 g (2 SF) 2. A driver filled 15. 60 kg of gasoline into his car. If the density of gasoline is 0. 788 g/m. L, what is the volume of gasoline in liters? KEY: 19. 8 L (3 SF) 69

Density as a Conversion Factor II: Mass A truck driver fills 100. L gasoline into the tank. How many kilograms does the gasoline weigh? Density of gasoline = 0. 77 g/m. L Given: V = 100. L Find: mass in kg Solution Map: D, V gram kg Mass = Volume x Density Or conversion factor: 100. L x 1000 m. L/1 L = 1. 00 x 105 m. L x 0. 77 g/m. L = 7. 7 x 104 g x 1 kg/1000 g = 77 kg Mass = 77 kg 70

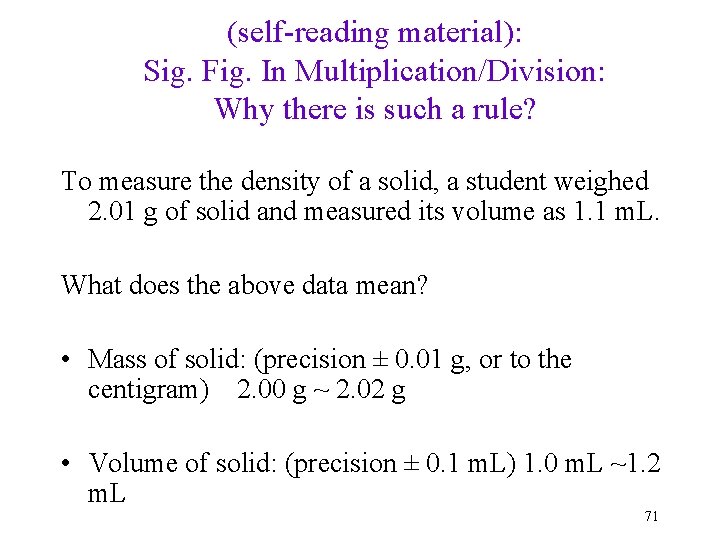
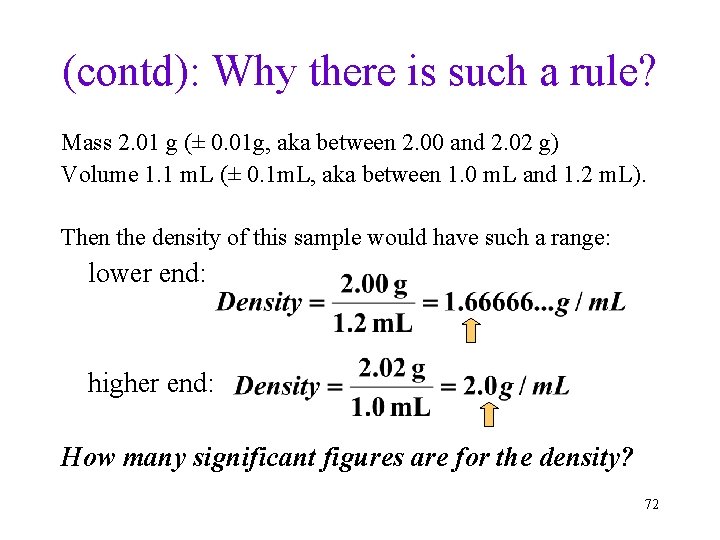
(self-reading material): Sig. Fig. In Multiplication/Division: Why there is such a rule? To measure the density of a solid, a student weighed 2. 01 g of solid and measured its volume as 1. 1 m. L. What does the above data mean? • Mass of solid: (precision ± 0. 01 g, or to the centigram) 2. 00 g ~ 2. 02 g • Volume of solid: (precision ± 0. 1 m. L) 1. 0 m. L ~1. 2 m. L 71

(contd): Why there is such a rule? Mass 2. 01 g (± 0. 01 g, aka between 2. 00 and 2. 02 g) Volume 1. 1 m. L (± 0. 1 m. L, aka between 1. 0 m. L and 1. 2 m. L). Then the density of this sample would have such a range: lower end: higher end: How many significant figures are for the density? 72

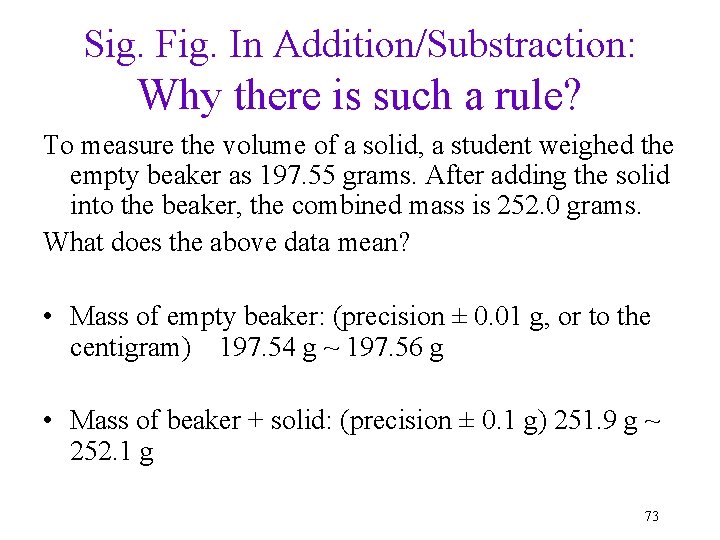
Sig. Fig. In Addition/Substraction: Why there is such a rule? To measure the volume of a solid, a student weighed the empty beaker as 197. 55 grams. After adding the solid into the beaker, the combined mass is 252. 0 grams. What does the above data mean? • Mass of empty beaker: (precision ± 0. 01 g, or to the centigram) 197. 54 g ~ 197. 56 g • Mass of beaker + solid: (precision ± 0. 1 g) 251. 9 g ~ 252. 1 g 73

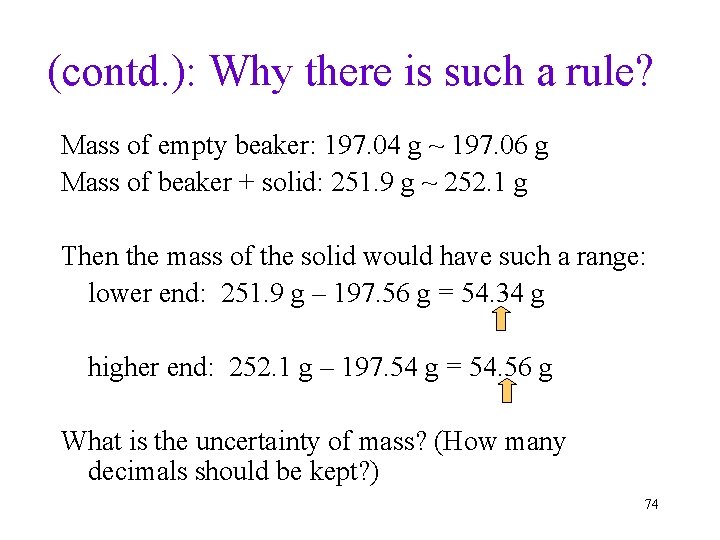
(contd. ): Why there is such a rule? Mass of empty beaker: 197. 04 g ~ 197. 06 g Mass of beaker + solid: 251. 9 g ~ 252. 1 g Then the mass of the solid would have such a range: lower end: 251. 9 g – 197. 56 g = 54. 34 g higher end: 252. 1 g – 197. 54 g = 54. 56 g What is the uncertainty of mass? (How many decimals should be kept? ) 74

Study of Making Best Cookies: Scientific Method • Introduction: https: //www. youtube. com/watch? v=GKGtkzg. Kfkc • PBS documentary on Chemistry: part 1: https: //www. youtube. com/watch? v=l. Yo. Fis_v 4 yk Part 2: https: //www. youtube. com/watch? v=y. G 0 G_Nkw. OMc Part 3: https: //www. youtube. com/watch? v=TKt 3 Vu. MMH_E • Chemistry demystified: https: //www. youtube. com/watch? v=Ydk. Pt 6 DUKu. I

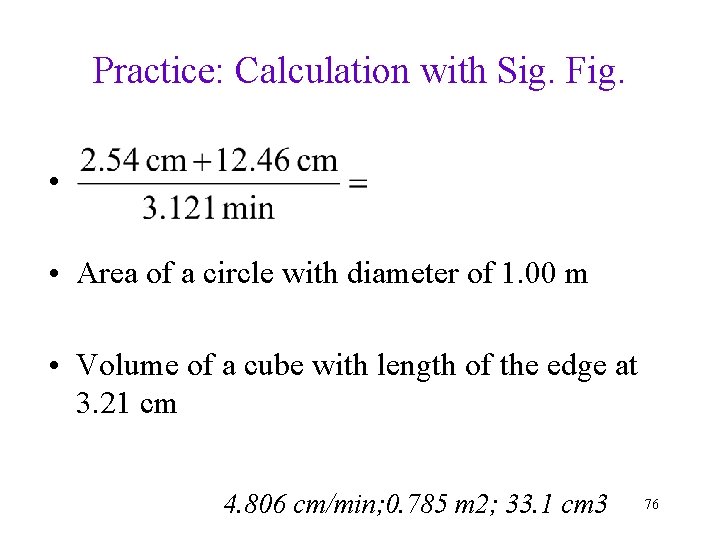
Practice: Calculation with Sig. Fig. • • Area of a circle with diameter of 1. 00 m • Volume of a cube with length of the edge at 3. 21 cm 4. 806 cm/min; 0. 785 m 2; 33. 1 cm 3 76