SIGNIFICANT FIGURES SCIENTIFIC NOTATION Sig Figs Scientific Notation




















- Slides: 27

SIGNIFICANT FIGURES & SCIENTIFIC NOTATION Sig Figs

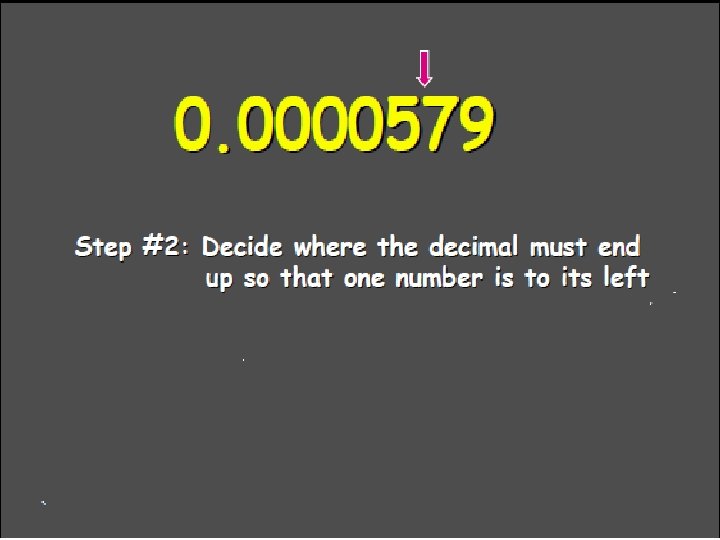
Scientific Notation In science, we often come across either very large or very small numbers, so we use Scientific Notation as a way to simplify them. Some numbers hard to work with: Mass of one atom = 0. 000000000000000091 kg # atoms in 2 grams of hydrogen = 12000000000000 atoms

Scientific Notation Easier: Mass of one electron = 9. 1 x 10 -32 # atoms in 2 g hydrogen = 1. 2 x 1024

In Scientific Notation, a number is written as the product of two numbers: A coefficient, and 10 raised to a power (exponent). The coefficient is always greater than or equal to 1, and less than 10 M x 10 n M = Coefficient between 1 and 10 10 is the base n is the exponent

Scientific Notation Worksheet Numbers > 1 have a positive exponent 5. 2 x 103 Numbers < 1 have a negative exponent 9. 65 x 10 -4

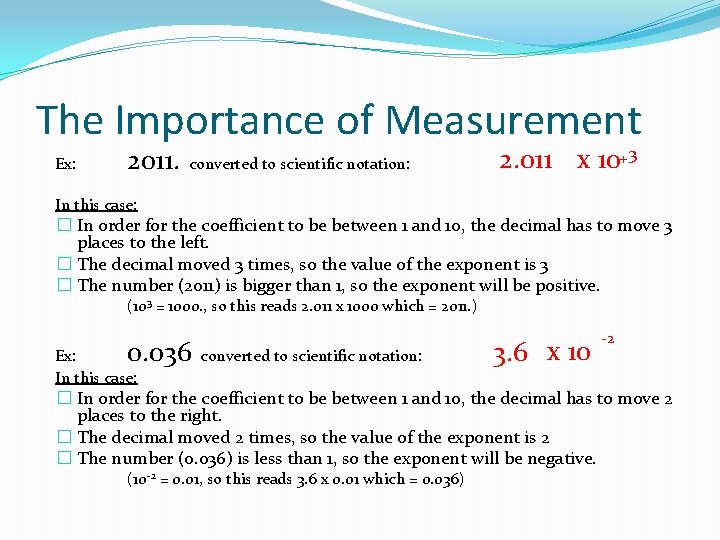
The Importance of Measurement Ex: 2011. converted to scientific notation: 2. 011 x 10+3 In this case: � In order for the coefficient to be between 1 and 10, the decimal has to move 3 places to the left. � The decimal moved 3 times, so the value of the exponent is 3 � The number (2011) is bigger than 1, so the exponent will be positive. (103 = 1000. , so this reads 2. 011 x 1000 which = 2011. ) 0. 036 converted to scientific notation: Ex: In this case: 3. 6 x 10 -2 � In order for the coefficient to be between 1 and 10, the decimal has to move 2 places to the right. � The decimal moved 2 times, so the value of the exponent is 2 � The number (0. 036) is less than 1, so the exponent will be negative. (10 -2 = 0. 01, so this reads 3. 6 x 0. 01 which = 0. 036)

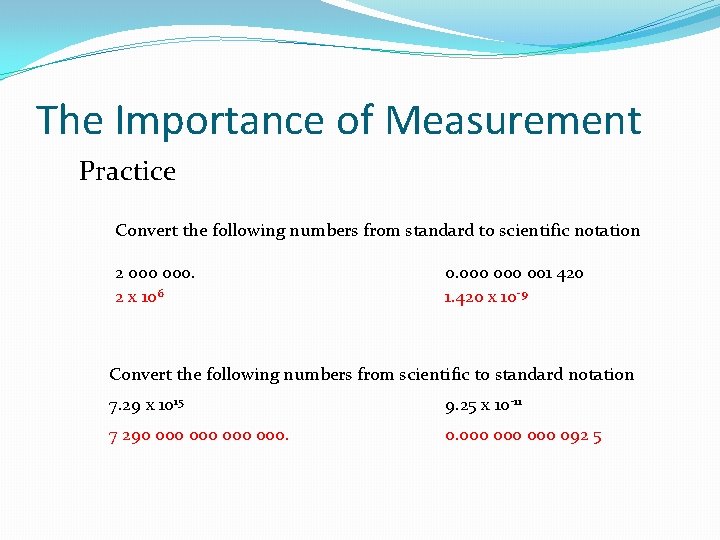
The Importance of Measurement Practice Convert the following numbers from standard to scientific notation 2 000. 2 x 106 0. 000 001 420 1. 420 x 10 -9 Convert the following numbers from scientific to standard notation 7. 29 x 1015 9. 25 x 10 -11 7 290 000 000. 0. 000 000 092 5
















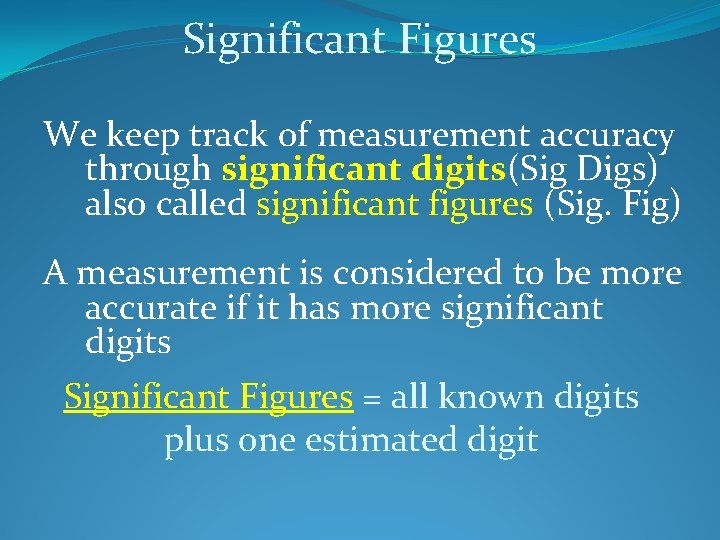
Significant Figures We keep track of measurement accuracy through significant digits(Sig Digs) also called significant figures (Sig. Fig) A measurement is considered to be more accurate if it has more significant digits Significant Figures = all known digits plus one estimated digit

Significant Figures RULES 1) No zeros? All significant. 377 2) All sandwich zeros significant. 307 3) Leading zeros are not significant. 0. 00312 4) If digits are left of a decimal then zeros right of a decimal are significant 3. 00 5) Scientific notation indicates significant figures when numbers end in zero 300 = 3 x 102 or 3. 00 x 102 When you have to guess, zeros don’t count

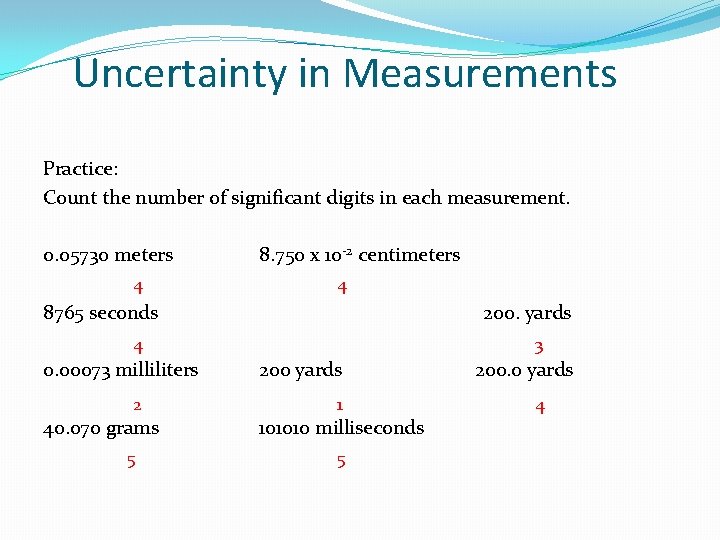
Uncertainty in Measurements Practice: Count the number of significant digits in each measurement. 0. 05730 meters 4 8. 750 x 10 -2 centimeters 4 8765 seconds 200. yards 4 0. 00073 milliliters 2 40. 070 grams 5 3 200 yards 1 101010 milliseconds 5 200. 0 yards 4

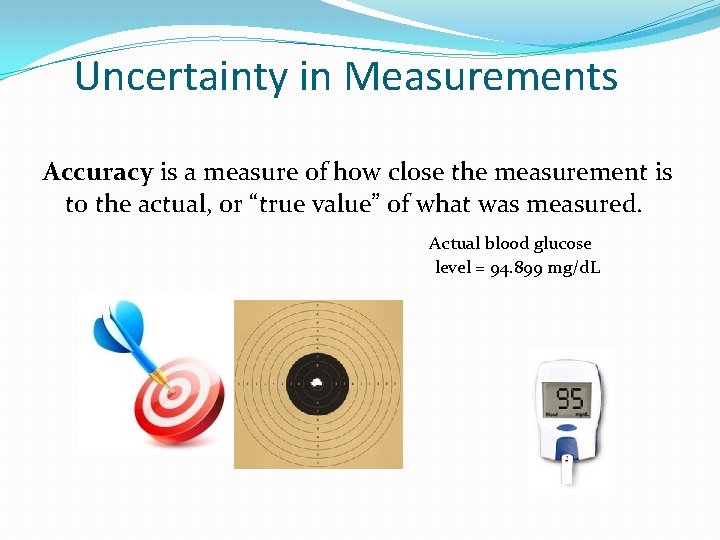
Uncertainty in Measurements Accuracy is a measure of how close the measurement is to the actual, or “true value” of what was measured. Actual blood glucose level = 94. 899 mg/d. L

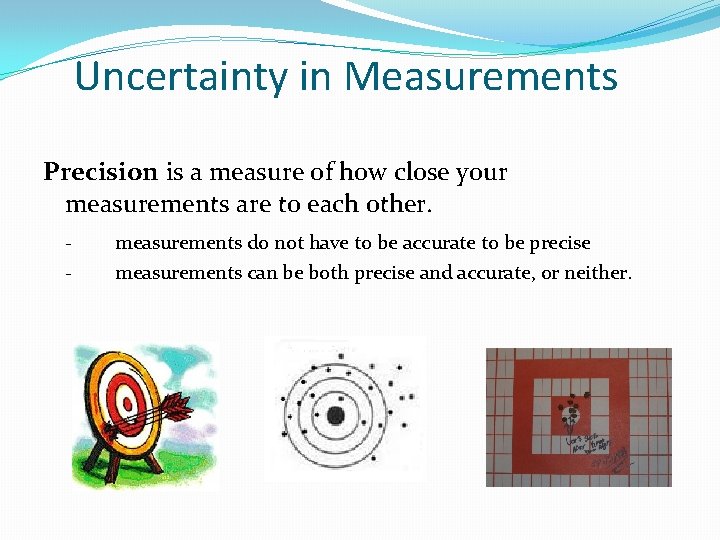
Uncertainty in Measurements Precision is a measure of how close your measurements are to each other. - measurements do not have to be accurate to be precise - measurements can be both precise and accurate, or neither.