SCE questions on Lung Transplantation Richard Davidson Causes


















- Slides: 40

SCE questions on Lung Transplantation Richard Davidson

Causes of death in lung transplant What is the most common cause of death in lung Tx recipients beyond the first year? 1. Malignancy 2. Cardiovascular disease 3. Obliterative Bronchiolitis 4. Graft failure 5. Infection

Causes of death in lung transplant What is the most common cause of death in lung Tx recipients beyond the first year? 1. Malignancy 2. Cardiovascular disease 3. Obliterative Bronchiolitis 4. Graft failure 5. Infection

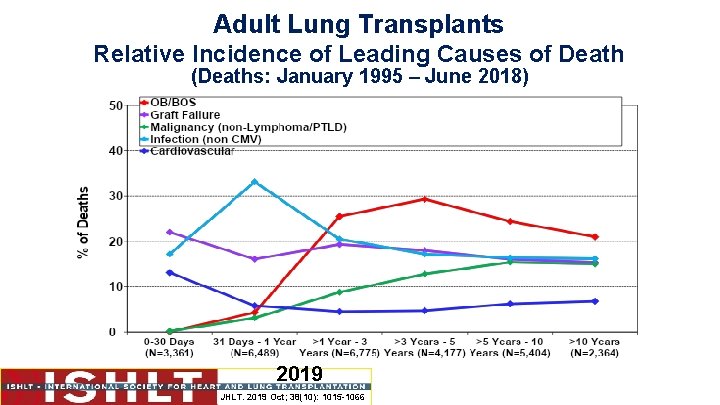
Adult Lung Transplants Relative Incidence of Leading Causes of Death (Deaths: January 1995 – June 2018) 2019 JHLT. 2019 Oct; 38(10): 1015 -1066

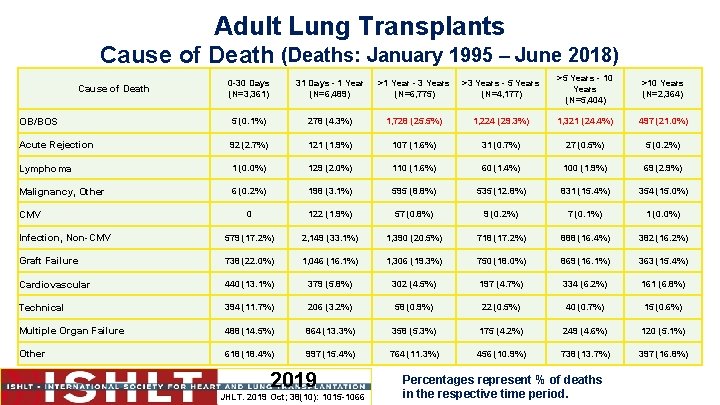
Adult Lung Transplants Cause of Death (Deaths: January 1995 – June 2018) 0 -30 Days (N=3, 361) 31 Days - 1 Year (N=6, 489) >1 Year - 3 Years (N=6, 775) >3 Years - 5 Years (N=4, 177) >5 Years - 10 Years (N=5, 404) >10 Years (N=2, 364) OB/BOS 5 (0. 1%) 278 (4. 3%) 1, 728 (25. 5%) 1, 224 (29. 3%) 1, 321 (24. 4%) 497 (21. 0%) Acute Rejection 92 (2. 7%) 121 (1. 9%) 107 (1. 6%) 31 (0. 7%) 27 (0. 5%) 5 (0. 2%) Lymphoma 1 (0. 0%) 129 (2. 0%) 110 (1. 6%) 60 (1. 4%) 100 (1. 9%) 69 (2. 9%) Malignancy, Other 6 (0. 2%) 198 (3. 1%) 595 (8. 8%) 535 (12. 8%) 831 (15. 4%) 354 (15. 0%) 0 122 (1. 9%) 57 (0. 8%) 9 (0. 2%) 7 (0. 1%) 1 (0. 0%) Infection, Non-CMV 579 (17. 2%) 2, 149 (33. 1%) 1, 390 (20. 5%) 718 (17. 2%) 888 (16. 4%) 382 (16. 2%) Graft Failure 738 (22. 0%) 1, 046 (16. 1%) 1, 306 (19. 3%) 750 (18. 0%) 869 (16. 1%) 363 (15. 4%) Cardiovascular 440 (13. 1%) 379 (5. 8%) 302 (4. 5%) 197 (4. 7%) 334 (6. 2%) 161 (6. 8%) Technical 394 (11. 7%) 206 (3. 2%) 58 (0. 9%) 22 (0. 5%) 40 (0. 7%) 15 (0. 6%) Multiple Organ Failure 488 (14. 5%) 864 (13. 3%) 358 (5. 3%) 175 (4. 2%) 249 (4. 6%) 120 (5. 1%) Other 618 (18. 4%) 997 (15. 4%) 764 (11. 3%) 456 (10. 9%) 738 (13. 7%) 397 (16. 8%) Cause of Death CMV 2019 JHLT. 2019 Oct; 38(10): 1015 -1066 Percentages represent % of deaths in the respective time period.


Survival following lung transplant According to information from the ISHLT registry, which of the following statements is true? 1. Median survival is longer following a single lung transplant 2. Female transplant recipients have a longer median survival 3. Median survival is now over 10 years for a double lung transplant 4. The median survival for CF transplant recipients is not statistically superior to COPD or Alpha-1 -Antitrypsin Deficiency 5. 1 year survival rates for COPD transplant recipients are inferior to CF

Survival following lung transplant According to information from the ISHLT registry, which of the following statements is true? 1. Median survival is longer following a single lung transplant 2. Female transplant recipients have a longer median survival 3. Median survival is now over 10 years for a double lung transplant 4. The median survival for CF transplant recipients is not statistically superior to COPD or Alpha-1 -Antitrypsin Deficiency 5. 1 year survival rates for COPD transplant recipients are inferior to CF

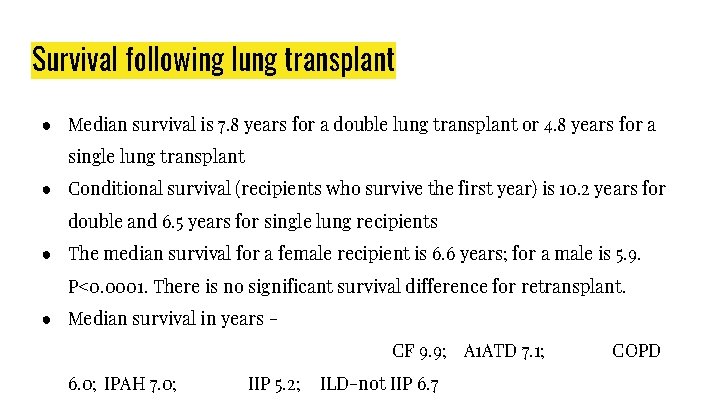
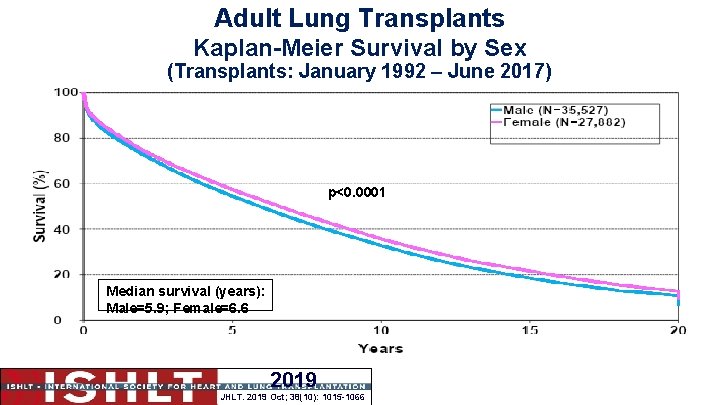
Survival following lung transplant ● Median survival is 7. 8 years for a double lung transplant or 4. 8 years for a single lung transplant ● Conditional survival (recipients who survive the first year) is 10. 2 years for double and 6. 5 years for single lung recipients ● The median survival for a female recipient is 6. 6 years; for a male is 5. 9. P<0. 0001. There is no significant survival difference for retransplant. ● Median survival in years CF 9. 9; A 1 ATD 7. 1; 6. 0; IPAH 7. 0; IIP 5. 2; ILD-not IIP 6. 7 COPD

Adult Lung Transplants Kaplan-Meier Survival by Sex (Transplants: January 1992 – June 2017) p<0. 0001 Median survival (years): Male=5. 9; Female=6. 6 2019 JHLT. 2019 Oct; 38(10): 1015 -1066

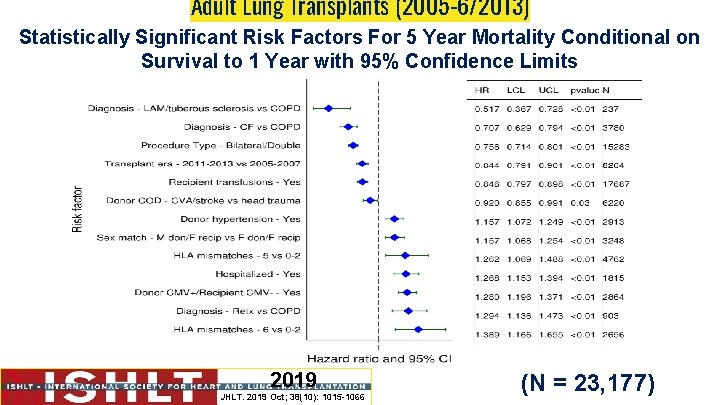
Risk factors for mortality Which of the following confers the greatest risk to 5 year mortality following transplant? 1. Recipient with history of blood transfusions prior to lung transplant 2. Donor +ve / Recipient -ve CMV mismatch 3. Donor hypertension 4. Donor cause of death stroke vs head trauma 5. Recipient transplant indication LAM vs COPD

Risk factors for mortality Which of the following confers the greatest risk to 5 year mortality following transplant? 1. Recipient with history of blood transfusions prior to lung transplant 2. Donor +ve / Recipient -ve CMV mismatch 3. Donor hypertension 4. Donor cause of death stroke vs head trauma 5. Recipient transplant indication LAM vs COPD

Adult Lung Transplants (2005 -6/2013) Statistically Significant Risk Factors For 5 Year Mortality Conditional on Survival to 1 Year with 95% Confidence Limits 2019 JHLT. 2019 Oct; 38(10): 1015 -1066 (N = 23, 177)

Adult Lung Transplants (2005 -6/2013) Statistically Significant Risk Factors For 5 Year Mortality Continuous Factors (see figures) Recipient age (years) Donor age (years) Recipient creatinine (mg/d. L) Recipient oxygen requirement (L/min) Recipient FVC% predicted Transplant center volume within 3 yrs Recipient bilirubin (mg/dl) Recipient PRA (%) Donor-recipient weight difference (kg) Donor-recipient height difference (cm) Interaction between height difference (cm) and diagnosis Note: Interaction between donor-recipient height difference and procedure type was retained in the final model but was not statistically significant (p-value = 0. 3399). 2019 JHLT. 2019 Oct; 38(10): 1015 -1066

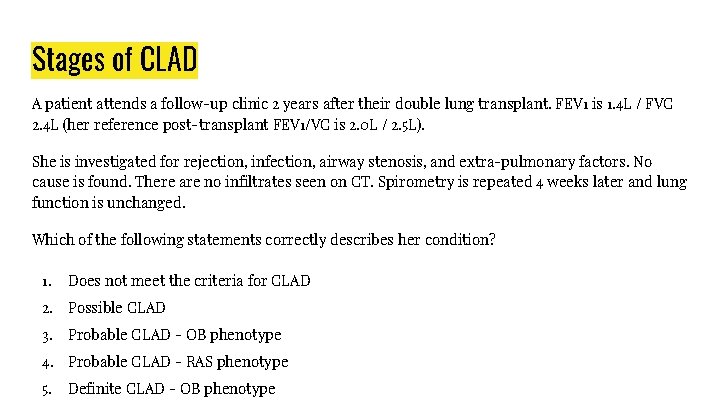
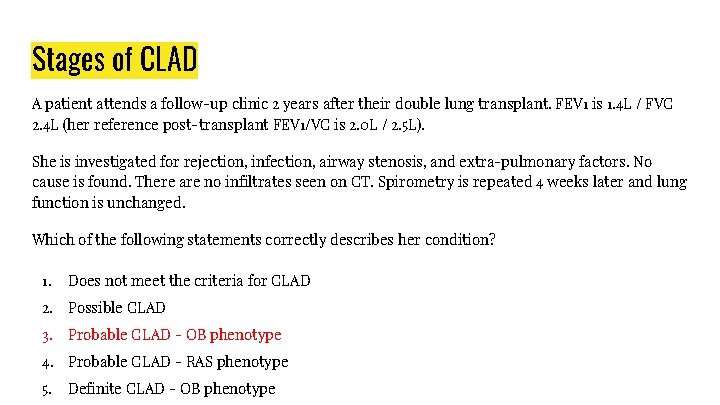
Stages of CLAD A patient attends a follow-up clinic 2 years after their double lung transplant. FEV 1 is 1. 4 L / FVC 2. 4 L (her reference post-transplant FEV 1/VC is 2. 0 L / 2. 5 L). She is investigated for rejection, infection, airway stenosis, and extra-pulmonary factors. No cause is found. There are no infiltrates seen on CT. Spirometry is repeated 4 weeks later and lung function is unchanged. Which of the following statements correctly describes her condition? 1. Does not meet the criteria for CLAD 2. Possible CLAD 3. Probable CLAD - OB phenotype 4. Probable CLAD - RAS phenotype 5. Definite CLAD - OB phenotype

Stages of CLAD A patient attends a follow-up clinic 2 years after their double lung transplant. FEV 1 is 1. 4 L / FVC 2. 4 L (her reference post-transplant FEV 1/VC is 2. 0 L / 2. 5 L). She is investigated for rejection, infection, airway stenosis, and extra-pulmonary factors. No cause is found. There are no infiltrates seen on CT. Spirometry is repeated 4 weeks later and lung function is unchanged. Which of the following statements correctly describes her condition? 1. Does not meet the criteria for CLAD 2. Possible CLAD 3. Probable CLAD - OB phenotype 4. Probable CLAD - RAS phenotype 5. Definite CLAD - OB phenotype


Stages of CLAD The same patient returns 3 months after their initial drop in lung function. Her Spirometry is static - FEV 1/VC 1. 4/2. 4 (baseline 2. 0/2. 5). CLAD is confirmed and the phenotype is OB. What stage of CLAD does she have? 1. CLAD 0 2. CLAD 1 3. CLAD 2 4. CLAD 3 5. CLAD 4

Stages of CLAD The same patient returns 3 months after their initial drop in lung function. Her Spirometry is static - FEV 1/VC 1. 4/2. 4 (baseline 2. 0/2. 5). CLAD is confirmed and the phenotype is OB. What stage of CLAD does she have? 1. CLAD 0 2. CLAD 1 3. CLAD 2 4. CLAD 3 5. CLAD 4


Surgery Which of the following statements regarding surgical considerations ahead of lung transplantation is false: 1. Pleurodesis is neither an absolute nor a relative contraindication to lung transplantation 2. The choice of intervention in pneumothorax is unlikely to have a bearing on future acceptance for transplantation 3. In otherwise well-selected patients, medium and long-term outcomes are unaffected by previous chest procedures 4. In older patients (>65 years old) with other comorbidities, previous intrapleural procedures should be taken into account 5. Lung volume reduction surgery is associated with poorer outcomes

Surgery Which of the following statements regarding surgical considerations ahead of lung transplantation is false: 1. Pleurodesis is neither an absolute nor a relative contraindication to lung transplantation 2. The choice of intervention in pneumothorax is unlikely to have a bearing on future acceptance for transplantation 3. In otherwise well-selected patients, medium and long-term outcomes are unaffected by previous chest procedures 4. In older patients (>65 years old) with other comorbidities, previous intrapleural procedures should be taken into account 5. Lung volume reduction surgery is associated with poorer outcomes

● Pleurodesis is the most troublesome situation but is not a contraindication. ● Pneumothorax in a patient who may become a future transplant recipient should be given the best immediate management. The choice of intervention is unlikely to affect future acceptance for transplantation. ● In otherwise well-selected patients, medium-term and long-term outcome is not affected by previous chest procedures. ● Conversely, older patients (>65 years old) with other comorbidities have poorer outcomes, and the previous intrapleural procedure should be taken into account during selection. ● Several reports have examined the specific issue of previous LVRS. Early experience indicated that LVRS had no effect, but in a more recent account, 25 of 177 patients who received transplants for chronic obstructive pulmonary disease (COPD) had undergone previous LVRS and had poorer outcomes. There were the expected higher rates of bleeding and early morbidity but also significantly worse early graft function and poorer results in older, frailer patients.

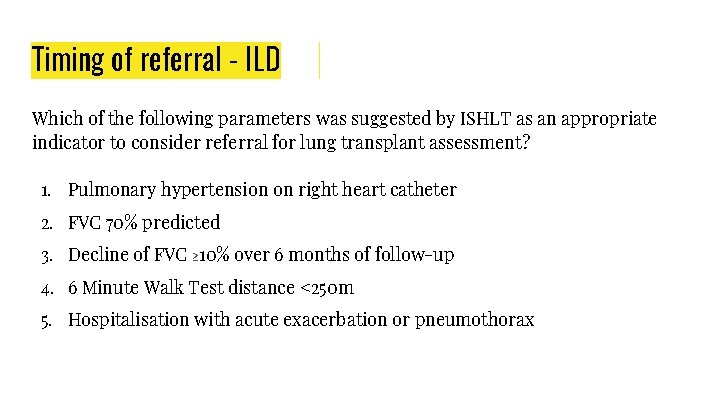
Timing of referral - ILD Which of the following parameters was suggested by ISHLT as an appropriate indicator to consider referral for lung transplant assessment? 1. Pulmonary hypertension on right heart catheter 2. FVC 70% predicted 3. Decline of FVC ≥ 10% over 6 months of follow-up 4. 6 Minute Walk Test distance <250 m 5. Hospitalisation with acute exacerbation or pneumothorax

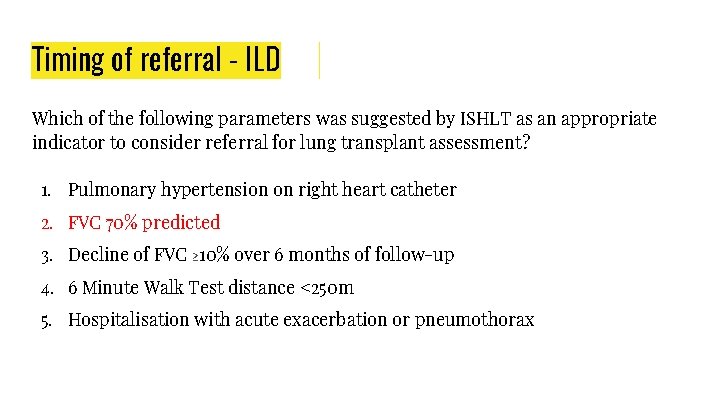
Timing of referral - ILD Which of the following parameters was suggested by ISHLT as an appropriate indicator to consider referral for lung transplant assessment? 1. Pulmonary hypertension on right heart catheter 2. FVC 70% predicted 3. Decline of FVC ≥ 10% over 6 months of follow-up 4. 6 Minute Walk Test distance <250 m 5. Hospitalisation with acute exacerbation or pneumothorax

● Timing of referral: ○ Histopathologic or radiographic evidence of usual interstitial pneumonitis (UIP) or fibrosing non-specific interstitial pneumonitis (NSIP), regardless of lung function. ○ Abnormal lung function: forced vital capacity (FVC) <80% predicted or diffusion capacity of the lung for carbon monoxide (Dlco) <40% predicted. ○ Any dyspnea or functional limitation attributable to lung disease. ○ Any oxygen requirement, even if only during exertion. ○ For inflammatory interstitial lung disease (ILD), failure to improve dyspnea, oxygen requirement, and/or lung function after a clinically indicated trial of medical therapy. ● Timing of listing: ○ Decline in FVC ≥ 10% during 6 months of follow-up (note: a 5% decline is associated with a poorer prognosis and may warrant listing). ○ Decline in Dlco ≥ 15% during 6 months of follow-up. ○ Desaturation to <88% or distance <250 m on 6 -minute-walk test or >50 m decline in 6 -minute-walk distance over a 6 month period. ○ Pulmonary hypertension on right heart catheterization or 2 -dimensional echocardiography. ○ Hospitalization because of respiratory decline, pneumothorax, or acute exacerbation.

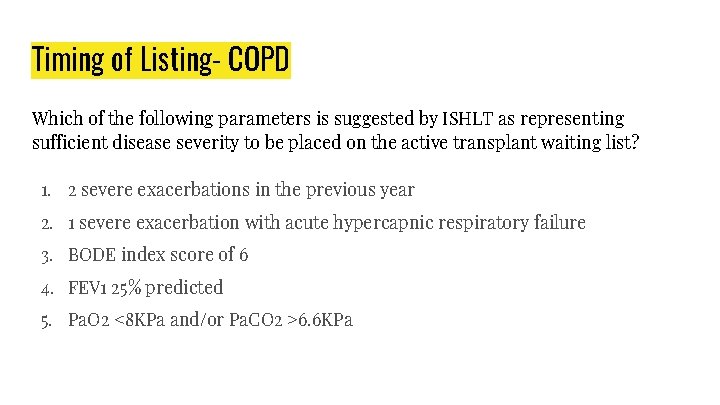
Timing of Listing- COPD Which of the following parameters is suggested by ISHLT as representing sufficient disease severity to be placed on the active transplant waiting list? 1. 2 severe exacerbations in the previous year 2. 1 severe exacerbation with acute hypercapnic respiratory failure 3. BODE index score of 6 4. FEV 1 25% predicted 5. Pa. O 2 <8 KPa and/or Pa. CO 2 >6. 6 KPa

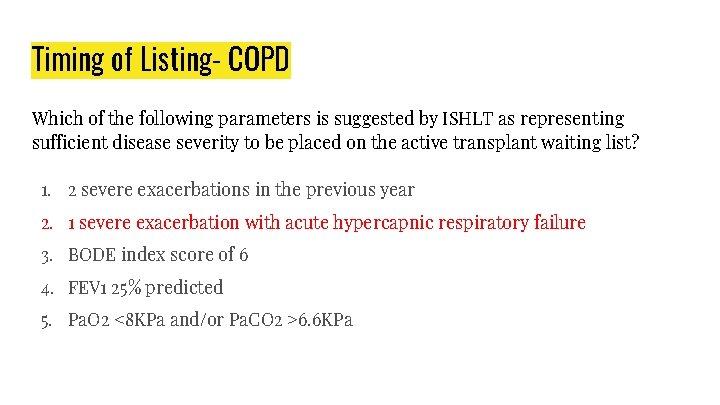
Timing of Listing- COPD Which of the following parameters is suggested by ISHLT as representing sufficient disease severity to be placed on the active transplant waiting list? 1. 2 severe exacerbations in the previous year 2. 1 severe exacerbation with acute hypercapnic respiratory failure 3. BODE index score of 6 4. FEV 1 25% predicted 5. Pa. O 2 <8 KPa and/or Pa. CO 2 >6. 6 KPa

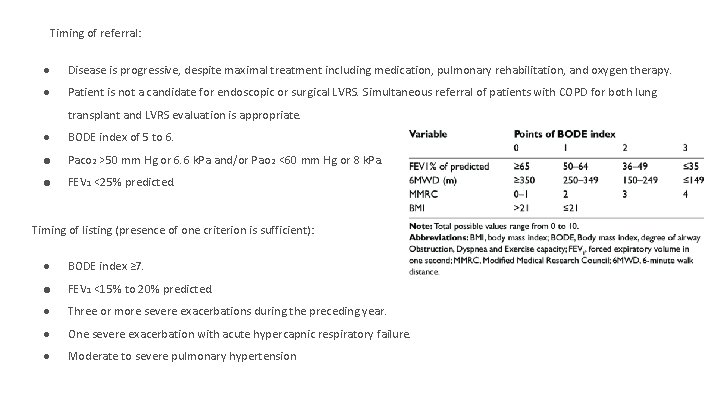
Timing of referral: ● Disease is progressive, despite maximal treatment including medication, pulmonary rehabilitation, and oxygen therapy. ● Patient is not a candidate for endoscopic or surgical LVRS. Simultaneous referral of patients with COPD for both lung transplant and LVRS evaluation is appropriate. ● BODE index of 5 to 6. ● Paco 2 >50 mm Hg or 6. 6 k. Pa and/or Pao 2 <60 mm Hg or 8 k. Pa. ● FEV 1 <25% predicted. Timing of listing (presence of one criterion is sufficient): ● BODE index ≥ 7. ● FEV 1 <15% to 20% predicted. ● Three or more severe exacerbations during the preceding year. ● One severe exacerbation with acute hypercapnic respiratory failure. ● Moderate to severe pulmonary hypertension

Immunosupressive medication mechanisms Which of the following best describes the mechanism of action of Tacrolimus? 1. Binds to cytoplasmic immunophilin and FK-binding protein 12, leading to inhibition of IL-2 and interferon-gamma. Inhibits T cell activation and proliferation. 2. Drug metabolites are incorporated into replicating DNA. Prevents the de novo synthesis of purines and thus interferes with RNA and DNA synthesis. Inhibits replication of T- and B-cells. 3. Inhibit both humoral and cell-mediated immunity. Prevents gene transcription of multiple inflammatory genes decreasing production of cytokines, interleukin (IL)-1, IL-2, IL-6, interferon (IFN)-gamma, and tumor necrosis factor (TNF)-alpha. 4. Activated in the liver. Depletes guanosine nucleotides in T and B lymphocytes, inhibiting proliferation of T and B cells, and the glycosylation and expression of adhesion molecules. 5. Forms a complex to block mechanistic target of rapamycin (m. TOR), a kinase essential for DNA and protein synthesis of T, NK and B cells. Also inhibits fibroblast proliferation.

Immunosupressive medication mechanisms Which of the following best describes the mechanism of action of Tacrolimus? 1. Binds to cytoplasmic immunophilin and FK-binding protein 12, leading to inhibition of IL-2 and interferon-gamma. Inhibits T cell activation and proliferation. 2. Drug metabolites are incorporated into replicating DNA. Prevents the de novo synthesis of purines and thus interferes with RNA and DNA synthesis. Inhibits replication of T- and B-cells. 3. Inhibit both humoral and cell-mediated immunity. Prevents gene transcription of multiple inflammatory genes decreasing production of cytokines, interleukin (IL)-1, IL-2, IL-6, interferon (IFN)-gamma, and tumor necrosis factor (TNF)-alpha. 4. Activated in the liver. Depletes guanosine nucleotides in T and B lymphocytes, inhibiting proliferation of T and B cells, and the glycosylation and expression of adhesion molecules. 5. Forms a complex to block mechanistic target of rapamycin (m. TOR), a kinase essential for DNA and protein synthesis of T, NK and B cells. Also inhibits fibroblast proliferation.

Immunosupressive medication mechanisms Which of the following best describes the mechanism of action of Tacrolimus? 1. Binds to cytoplasmic immunophilin and FK-binding protein 12, leading to inhibition of IL-2 and interferon-gamma. Inhibits T cell activation and proliferation. Tacrolimus 2. Drug metabolites are incorporated into replicating DNA. Prevents the de novo synthesis of purines and thus interferes with RNA and DNA synthesis. Inhibits replication of T- and B-cells. Azathioprine 3. Inhibit both humoral and cell-mediated immunity. Prevents gene transcription of multiple inflammatory genes decreasing production of cytokines, interleukin (IL)-1, IL-2, IL-6, interferon (IFN)-gamma, and tumor necrosis factor (TNF)-alpha. Prednisolone 4. Activated in the liver. Depletes guanosine nucleotides in T and B lymphocytes, inhibiting proliferation of T and B cells, and the glycosylation and expression of adhesion molecules. MMF 5. Forms a complex to block mechanistic target of rapamycin (m. TOR), a kinase essential for DNA and protein synthesis of T, NK and B cells. Also inhibits fibroblast proliferation. Sirolimus.

Immunosupressive medication interactions Both ciclosporine and tacrolimus are metabolised by CYP 3 A 4 in the liver. Given this, which of these medications is most likely to precipitate acute rejection? 1. Voriconazole 2. Amiodarone 3. Clarithromycin 4. Ritonavir 5. Carbamazepine

Immunosupressive medication interactions Both ciclosporine and tacrolimus are metabolised by CYP 3 A 4 in the liver. Given this, which of these medications is most likely to precipitate acute rejection? 1. Voriconazole 2. Amiodarone 3. Clarithromycin 4. Ritonavir 5. Carbamazepine


Absolute contraindications Which of these is not an absolute contraindication to lung transplantation? 1. BMI 35 2. Active Mycobacteria tuberculosis infection 3. Active cigarette smoker 4. Chronic hepatitis B infection 5. Non-melanomatous skin cancer treated 1 year ago.

Absolute contraindications Which of these is not an absolute contraindication to lung transplantation? 1. BMI 35 2. Active Mycobacteria tuberculosis infection 3. Active cigarette smoker 4. Chronic hepatitis B infection 5. Non-melanomatous skin cancer treated 1 year ago.

Absolute contraindications ● Recent malignancy (2 years of disease free survival and low risk of recurrence for instance non-melanomatous skin cancer or in most cases 5 years disease-free, particularly for patients with a history of hematologic malignancy, sarcoma, melanoma, or cancers of the breast, bladder, or kidney. ● Untreatable significant dysfunction of another major organ system (e. g. , heart, liver, kidney, or brain) unless combined organ transplantation can be performed ● Uncorrected atherosclerotic disease with suspected or confirmed end-organ ischemia or dysfunction and/or coronary artery disease not amenable to revascularization. ● Acute medical instability, including, but not limited to, acute sepsis, myocardial infarction, and liver failure. ● Uncorrectable bleeding diathesis. ● Chronic infection with highly virulent and/or resistant microbes that are poorly controlled pre-transplant. ● Evidence of active Mycobacterium tuberculosis infection. ● Significant chest wall or spinal deformity expected to cause severe restriction after transplantation. ● Class II or III obesity (body mass index [BMI] ≥ 35. 0 kg/m 2). ● Current non-adherence to medical therapy or a history of repeated or prolonged episodes of non-adherence to medical therapy that are perceived to increase the risk of non-adherence after transplantation. ● Psychiatric or psychologic conditions associated with the inability to cooperate with the medical/allied health care team and/or adhere with complex medical therapy. ● Absence of an adequate or reliable social support system. ● Severely limited functional status with poor rehabilitation potential ● Substance abuse or dependence (e. g. , alcohol, tobacco, marijuana, or other illicit substances).

Relative contraindications ● Age >65 years in association with low physiologic reserve and/or other relative contraindications. Although there cannot be endorsement of an upper age limit as an absolute contraindication, adults >75 years old are unlikely to be candidates for lung transplantation in most cases. ● Class I obesity (BMI 30. 0– 34. 9 kg/m 2), particularly truncal (central) obesity. ● Progressive or severe malnutrition. ● Severe, symptomatic osteoporosis. ● Extensive prior chest surgery with lung resection. ● Mechanical ventilation and/or extracorporeal life support (ECLS). However, carefully selected candidates without other acute or chronic organ dysfunction may be successfully transplanted. ● Colonization or infection with highly resistant or highly virulent bacteria, fungi, and certain strains of mycobacteria (e. g. , chronic extrapulmonary infection expected to worsen after transplantation). ● For patients infected with hepatitis B and/or C, a lung transplant can be considered in patients without significant clinical, radiologic, or biochemical signs of cirrhosis or portal hypertension and who are stable on appropriate therapy. ● For patients infected with human immunodeficiency virus (HIV), a lung transplant can be considered in patients with controlled disease with undetectable HIV-RNA, and compliant on combined anti-retroviral therapy. ● Infection with Burkholderia cenocepacia, Burkholderia gladioli, and multi-drug–resistant Mycobacterium abscessus if the infection is sufficiently treated preoperatively and there is a reasonable expectation for adequate control postoperatively. ● Atherosclerotic disease burden sufficient to put the candidate at risk for end-organ disease after lung transplantation. ● Other medical conditions that have not resulted in end-stage organ damage, such as diabetes mellitus, systemic hypertension, epilepsy, central venous obstruction, peptic ulcer disease, or gastroesophageal reflux, should be optimally treated before transplantation.

End.
 Clad
Clad Richard davidson andrews university
Richard davidson andrews university Bone marrow transplantation sri lanka
Bone marrow transplantation sri lanka Law of transplantation
Law of transplantation Patrick evrard mont godinne
Patrick evrard mont godinne Cultural transposition
Cultural transposition Transplant
Transplant Stem cell or bone marrow transplantation thailand
Stem cell or bone marrow transplantation thailand Sce respiratory medicine
Sce respiratory medicine Looking for richard stream
Looking for richard stream Siemens sce lehrunterlagen
Siemens sce lehrunterlagen Siemens cooperates with education
Siemens cooperates with education Sce trainer package v17
Sce trainer package v17 Braden scake
Braden scake Sce wdat
Sce wdat Sce care program verification
Sce care program verification Sce e
Sce e Sce tou calculator
Sce tou calculator Attractiveness sce
Attractiveness sce Ho sceso dandoti il braccio parafrasi
Ho sceso dandoti il braccio parafrasi Licter scale
Licter scale Charge ready transport
Charge ready transport Proximate causes of behavior
Proximate causes of behavior Proximate causes vs ultimate causes
Proximate causes vs ultimate causes Transverse wave ex
Transverse wave ex Harley davidson market share
Harley davidson market share Baibae harley davidson
Baibae harley davidson Father sean davidson
Father sean davidson Dnet davidson
Dnet davidson Caitlin d. davidson
Caitlin d. davidson Arthur davidson law
Arthur davidson law Currie v misa
Currie v misa Humongous insurance
Humongous insurance Pi davidson
Pi davidson Davidson police department
Davidson police department Garry berryman
Garry berryman Raquel duarte-davidson
Raquel duarte-davidson Yuda bands designs
Yuda bands designs Value proposition harley davidson
Value proposition harley davidson Eog
Eog Degreeworks emerson college
Degreeworks emerson college