SCAI 2019 May 22 2019 Are Drug Eluting




- Slides: 29

SCAI 2019 May 22, 2019 Are Drug Eluting Stents the Future of SFA Treatment? Sahil A. Parikh, MD, FACC, FSCAI Associate Professor of Medicine Director, Endovascular Services Center for Interventional Vascular Therapy New York-Presbyterian Hospital Columbia University Irving Medical Center Columbia University Vagelos College of Physicians and Surgeons

Disclosures Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below. Affiliation/Financial Relationship • Grant/Research Support • Consulting Fees/Honoraria • Advisory Board Company • Tri. Reme Medical, Shockwave Medical, NIH, Surmodics, Silk Road Medical (CEC); Boston Scientific (DSMB) • Terumo, Abiomed • Abbott, Boston Scientific, Medtronic, CSI, Philips

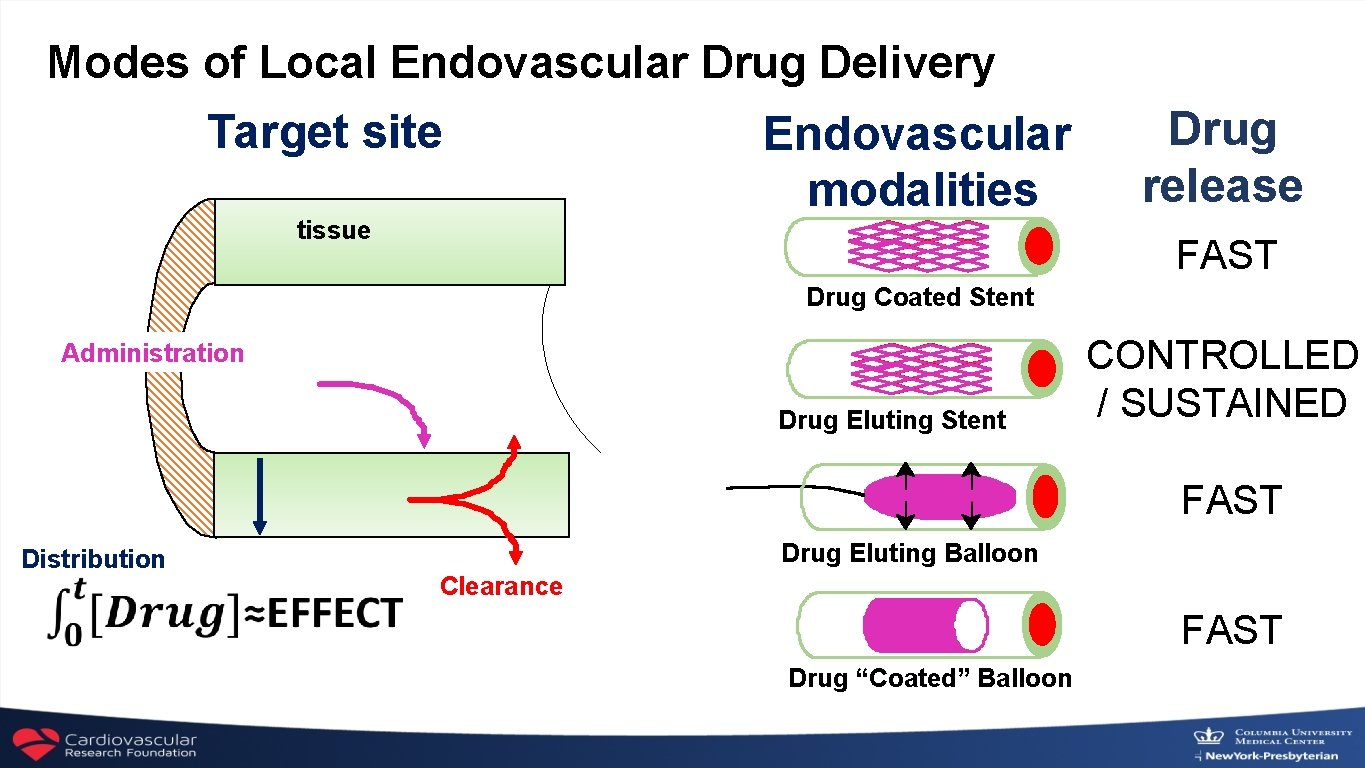
Modes of Local Endovascular Drug Delivery Target site tissue Endovascular modalities Drug release FAST Drug Coated Stent Administration Drug Eluting Stent CONTROLLED / SUSTAINED FAST Distribution Drug Eluting Balloon Clearance FAST Drug “Coated” Balloon

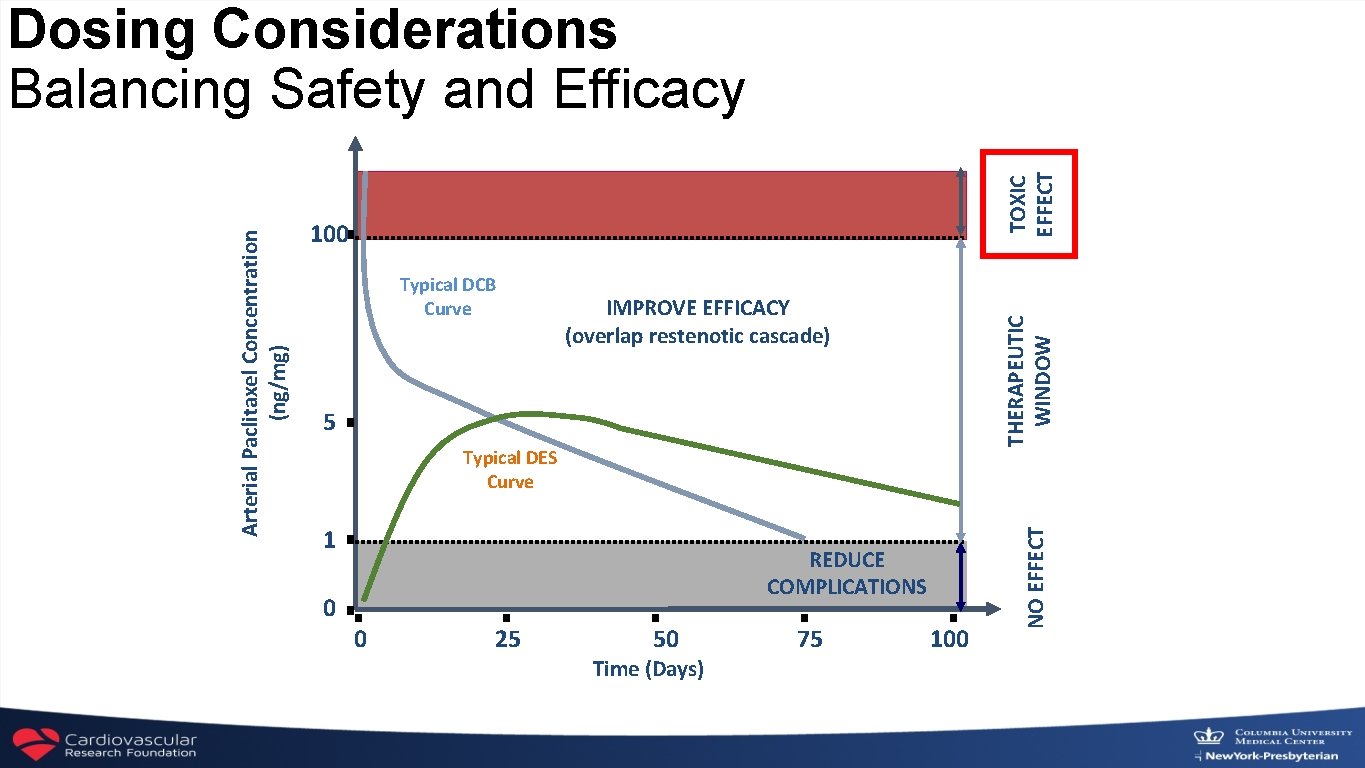
TOXIC EFFECT 100 IMPROVE EFFICACY (overlap restenotic cascade) 5 Typical DES Curve 1 REDUCE COMPLICATIONS 0 0 25 50 Time (Days) 75 100 NO EFFECT Typical DCB Curve THERAPEUTIC WINDOW Arterial Paclitaxel Concentration (ng/mg) Dosing Considerations Balancing Safety and Efficacy

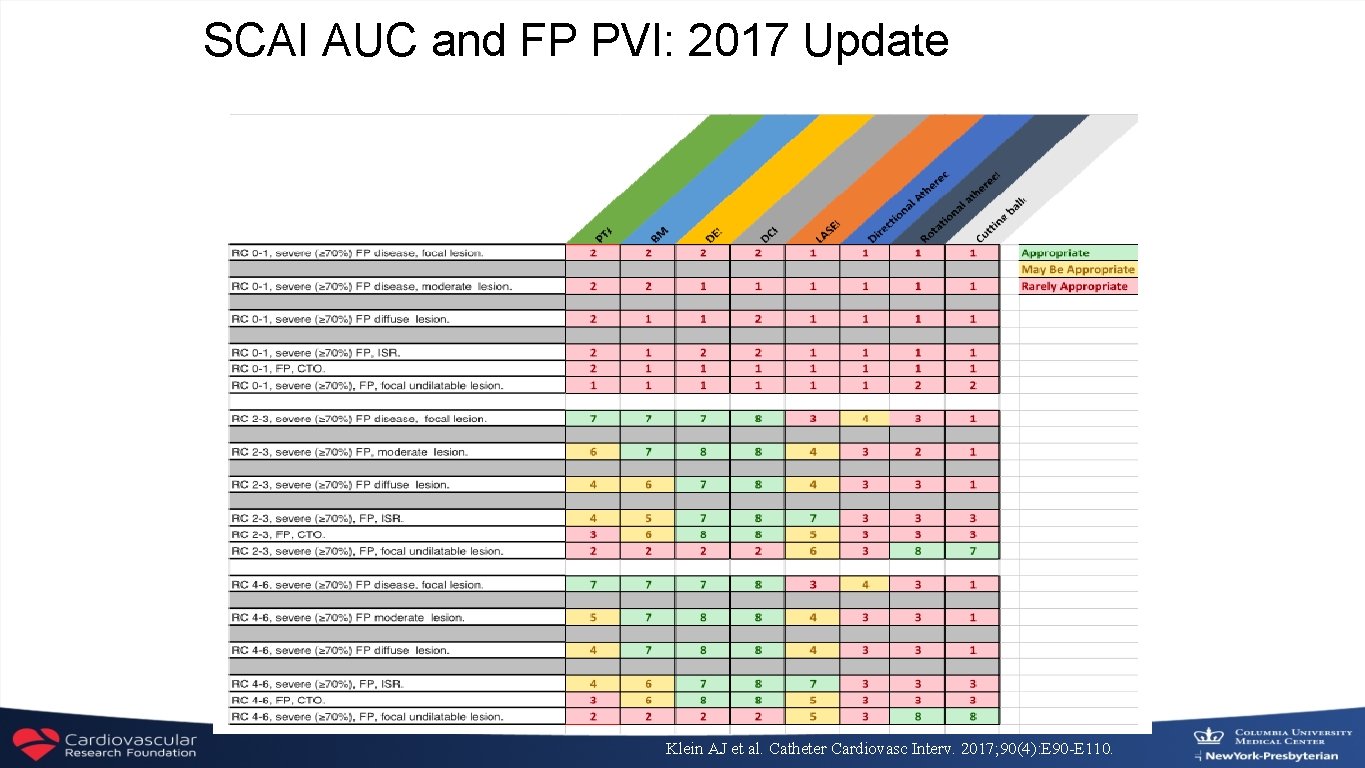
SCAI AUC and FP PVI: 2017 Update Klein AJ et al. Catheter Cardiovasc Interv. 2017; 90(4): E 90 -E 110.

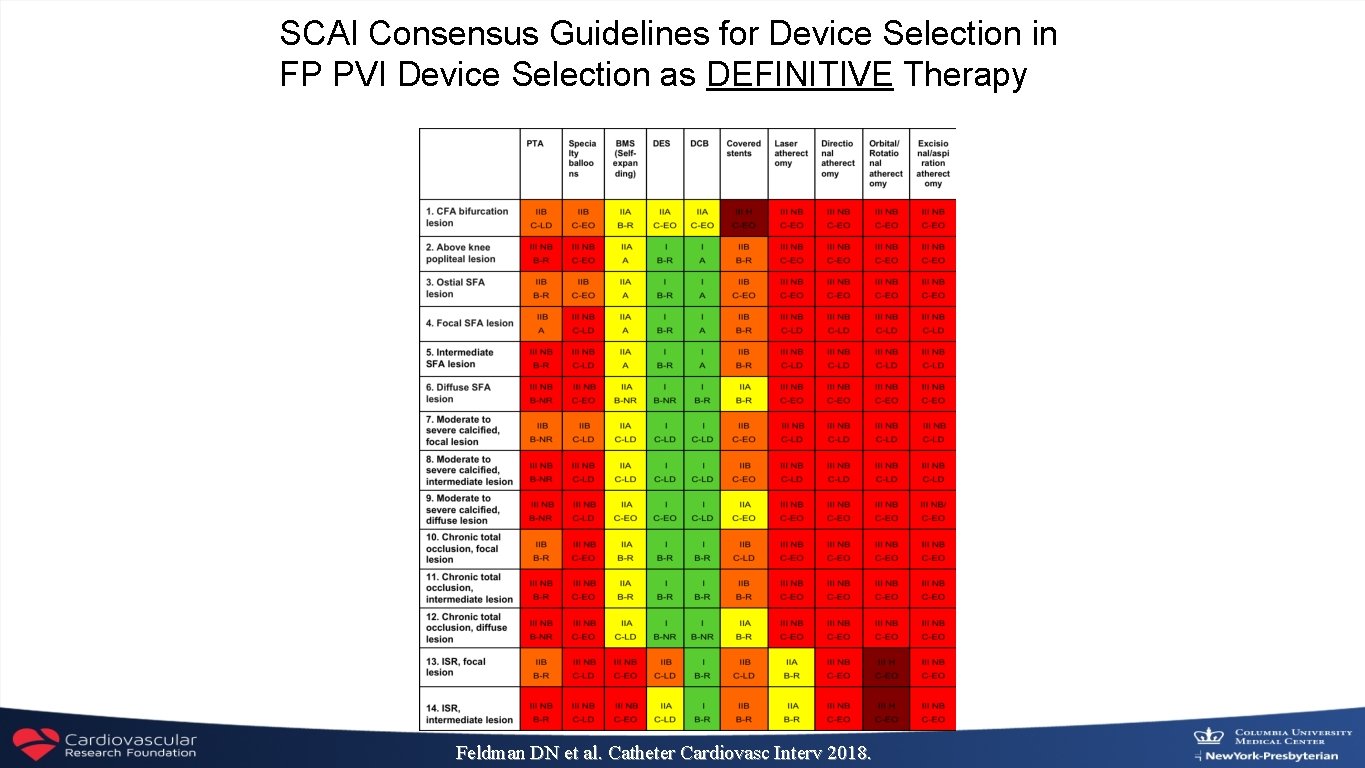
SCAI Consensus Guidelines for Device Selection in FP PVI Device Selection as DEFINITIVE Therapy Feldman DN et al. Catheter Cardiovasc Interv 2018.

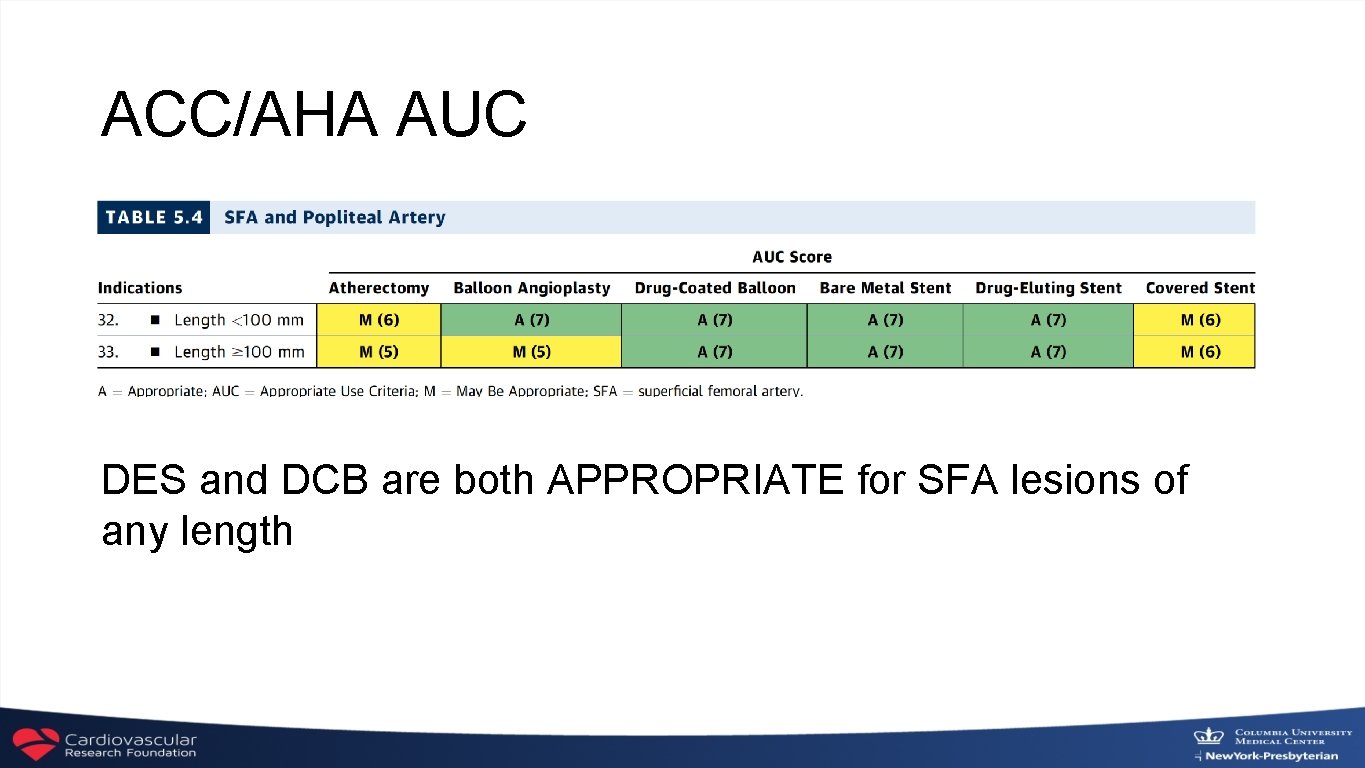
ACC/AHA AUC DES and DCB are both APPROPRIATE for SFA lesions of any length

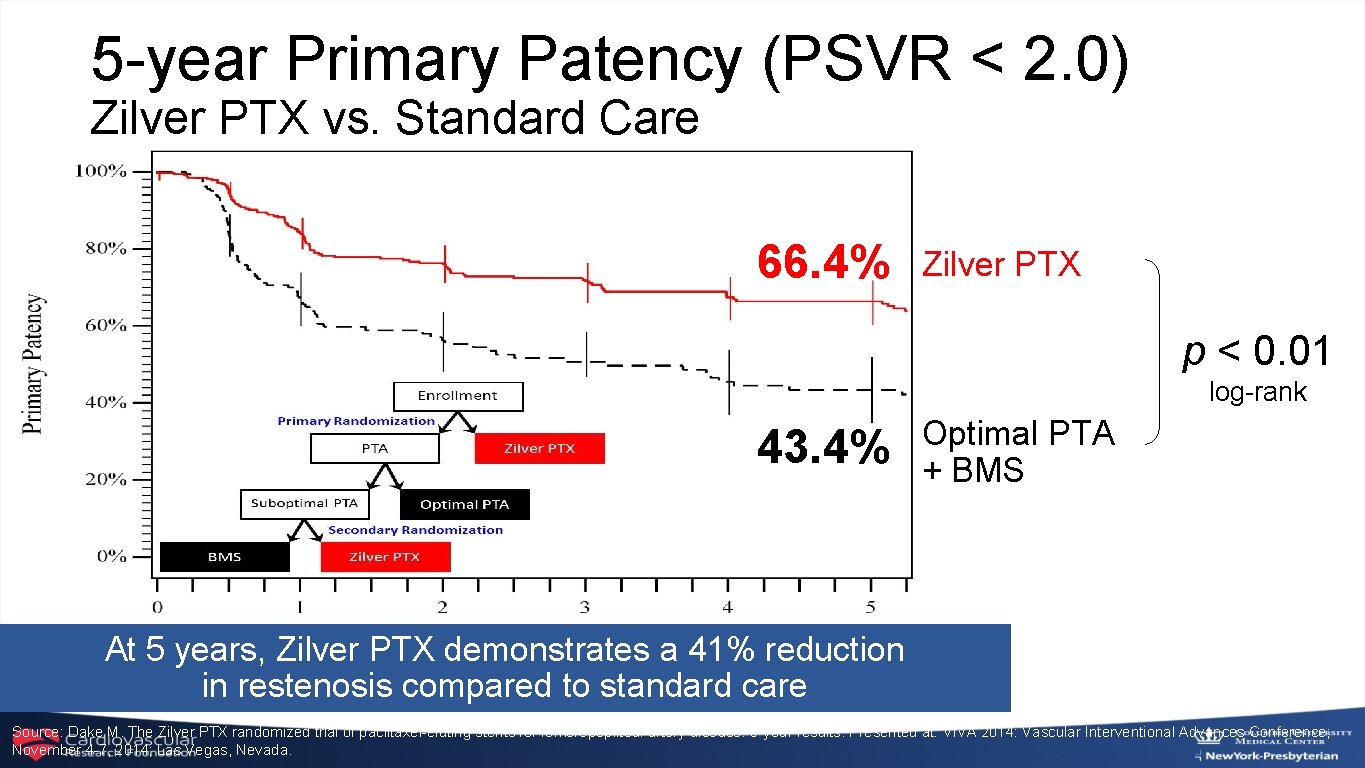
5 -year Primary Patency (PSVR < 2. 0) Zilver PTX vs. Standard Care 66. 4% Zilver PTX p < 0. 01 log-rank 43. 4% Optimal PTA + BMS At 5 years, Zilver PTX demonstrates a 41% reduction in restenosis compared to standard care Source: Dake M. The Zilver PTX randomized trial of paclitaxel-eluting stents for femoropopliteal artery disease: 5 -year results. Presented at: VIVA 2014: Vascular Interventional Advances Conference; November 4 -7, 2014; Las Vegas, Nevada.

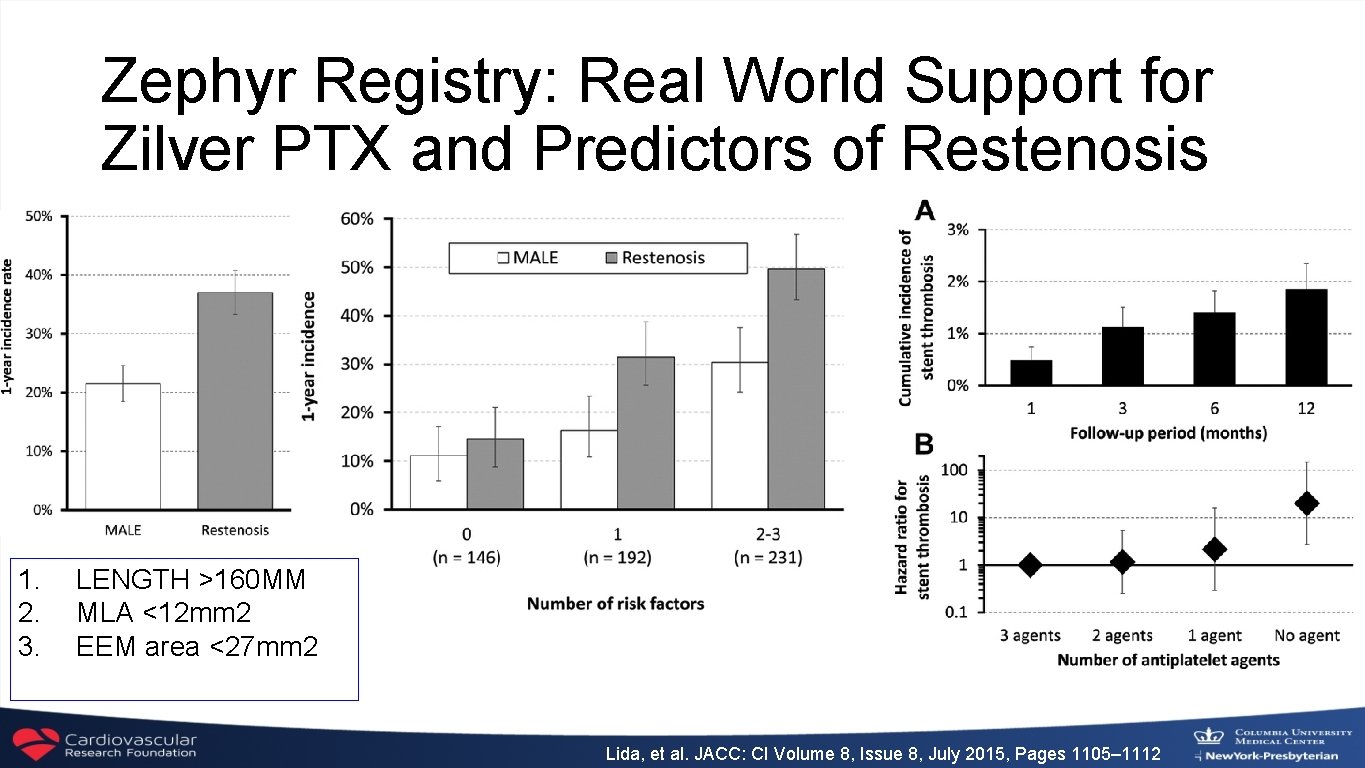
Zephyr Registry: Real World Support for Zilver PTX and Predictors of Restenosis 1. 2. 3. LENGTH >160 MM MLA <12 mm 2 EEM area <27 mm 2 Lida, et al. JACC: CI Volume 8, Issue 8, July 2015, Pages 1105– 1112

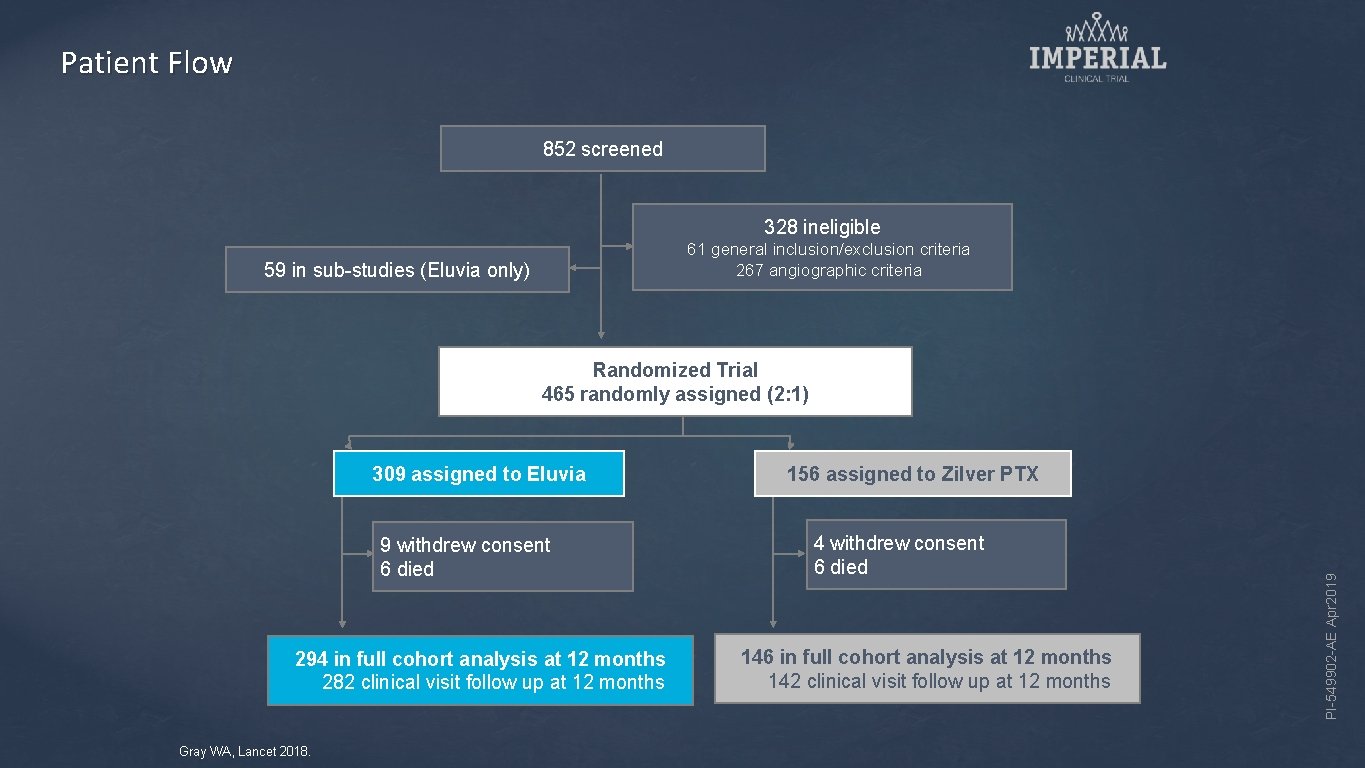
Patient Flow 852 screened 328 ineligible 61 general inclusion/exclusion criteria 267 angiographic criteria 59 in sub-studies (Eluvia only) Randomized Trial 465 randomly assigned (2: 1) 9 withdrew consent 6 died 294 in full cohort analysis at 12 months 282 clinical visit follow up at 12 months Gray WA, Lancet 2018. 156 assigned to Zilver PTX 4 withdrew consent 6 died 146 in full cohort analysis at 12 months 142 clinical visit follow up at 12 months PI-549902 -AE Apr 2019 309 assigned to Eluvia

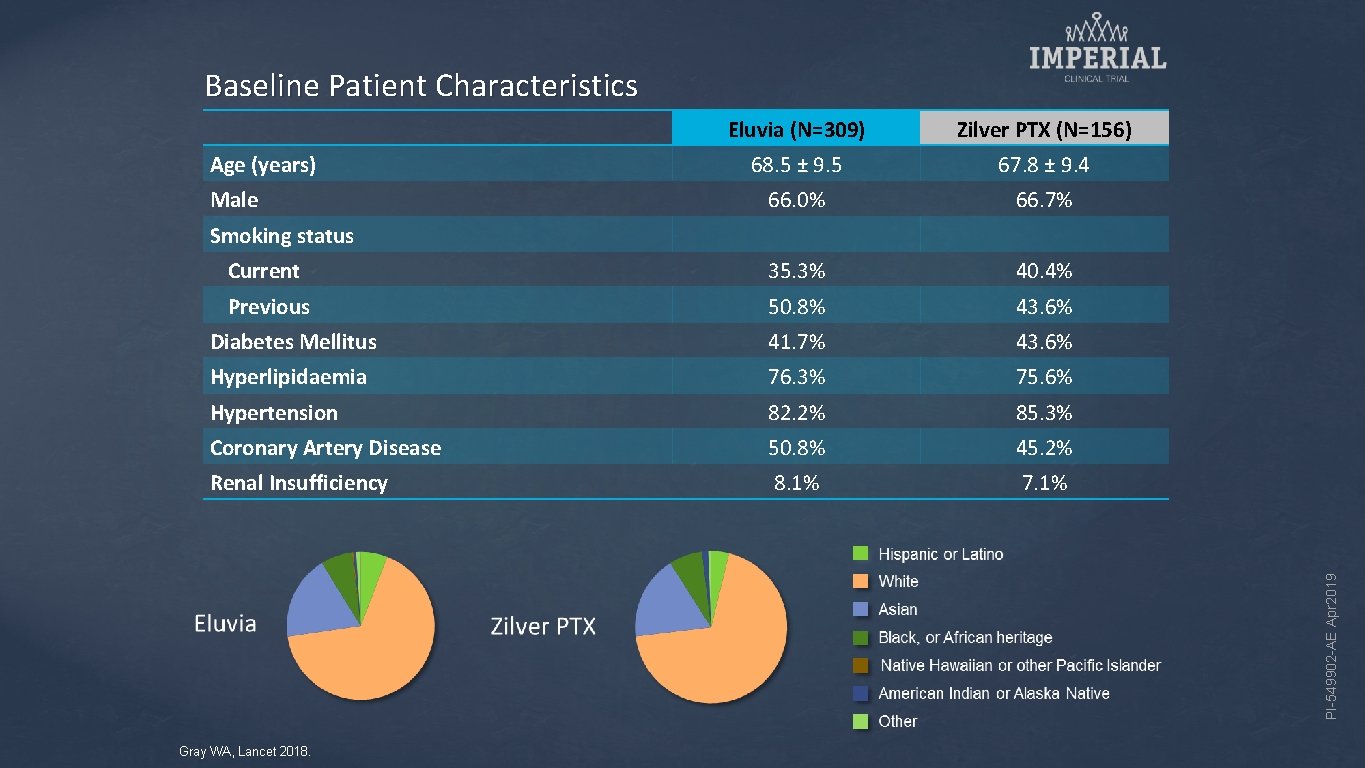
Baseline Patient Characteristics Eluvia (N=309) Zilver PTX (N=156) 68. 5 ± 9. 5 67. 8 ± 9. 4 66. 0% 66. 7% Current 35. 3% 40. 4% Previous 50. 8% 43. 6% Diabetes Mellitus 41. 7% 43. 6% Hyperlipidaemia 76. 3% 75. 6% Hypertension 82. 2% 85. 3% Coronary Artery Disease 50. 8% 45. 2% Renal Insufficiency 8. 1% 7. 1% Age (years) Male PI-549902 -AE Apr 2019 Smoking status Gray WA, Lancet 2018.

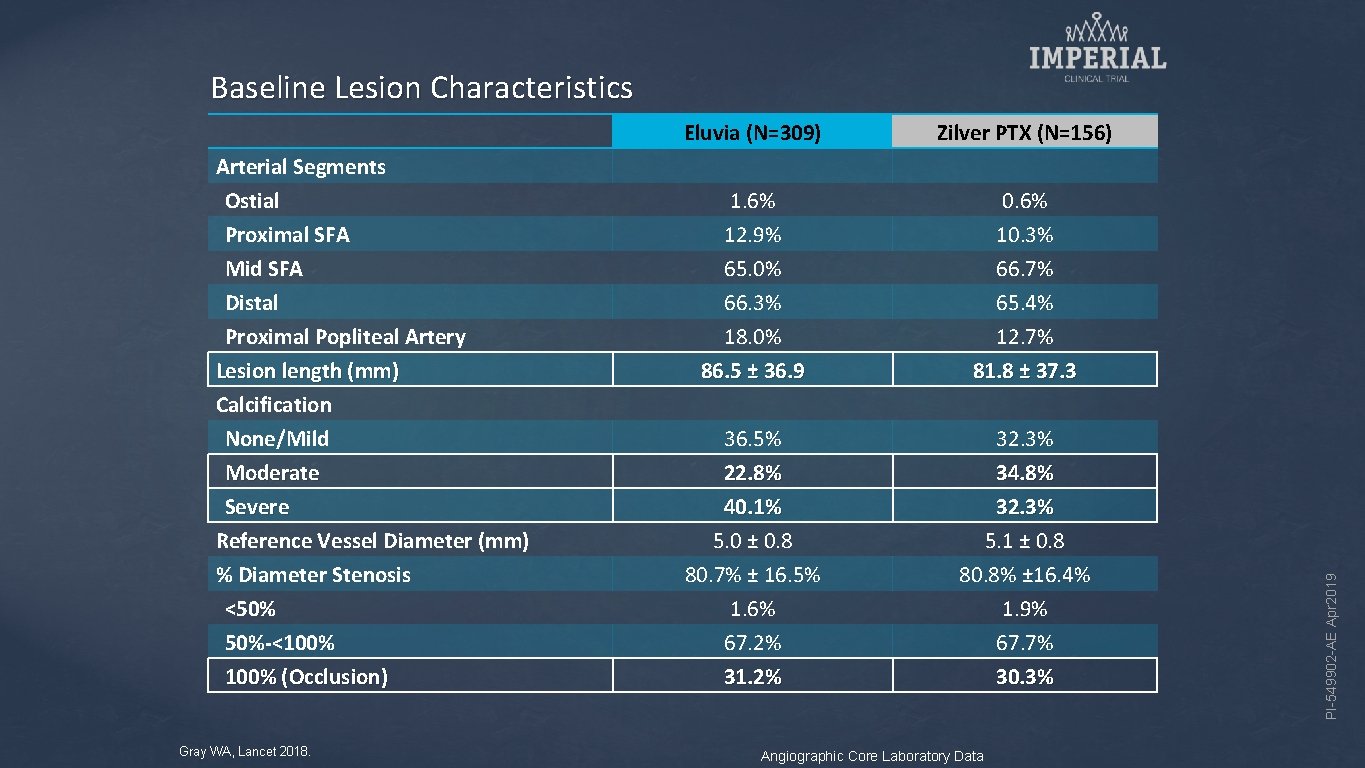
Arterial Segments Ostial Proximal SFA Mid SFA Distal Proximal Popliteal Artery Lesion length (mm) Calcification None/Mild Moderate Severe Reference Vessel Diameter (mm) % Diameter Stenosis <50% 50%-<100% (Occlusion) Gray WA, Lancet 2018. Eluvia (N=309) Zilver PTX (N=156) 1. 6% 12. 9% 65. 0% 66. 3% 18. 0% 86. 5 ± 36. 9 0. 6% 10. 3% 66. 7% 65. 4% 12. 7% 81. 8 ± 37. 3 36. 5% 22. 8% 40. 1% 5. 0 ± 0. 8 80. 7% ± 16. 5% 1. 6% 67. 2% 31. 2% 32. 3% 34. 8% 32. 3% 5. 1 ± 0. 8 80. 8% ± 16. 4% 1. 9% 67. 7% 30. 3% Angiographic Core Laboratory Data PI-549902 -AE Apr 2019 Baseline Lesion Characteristics

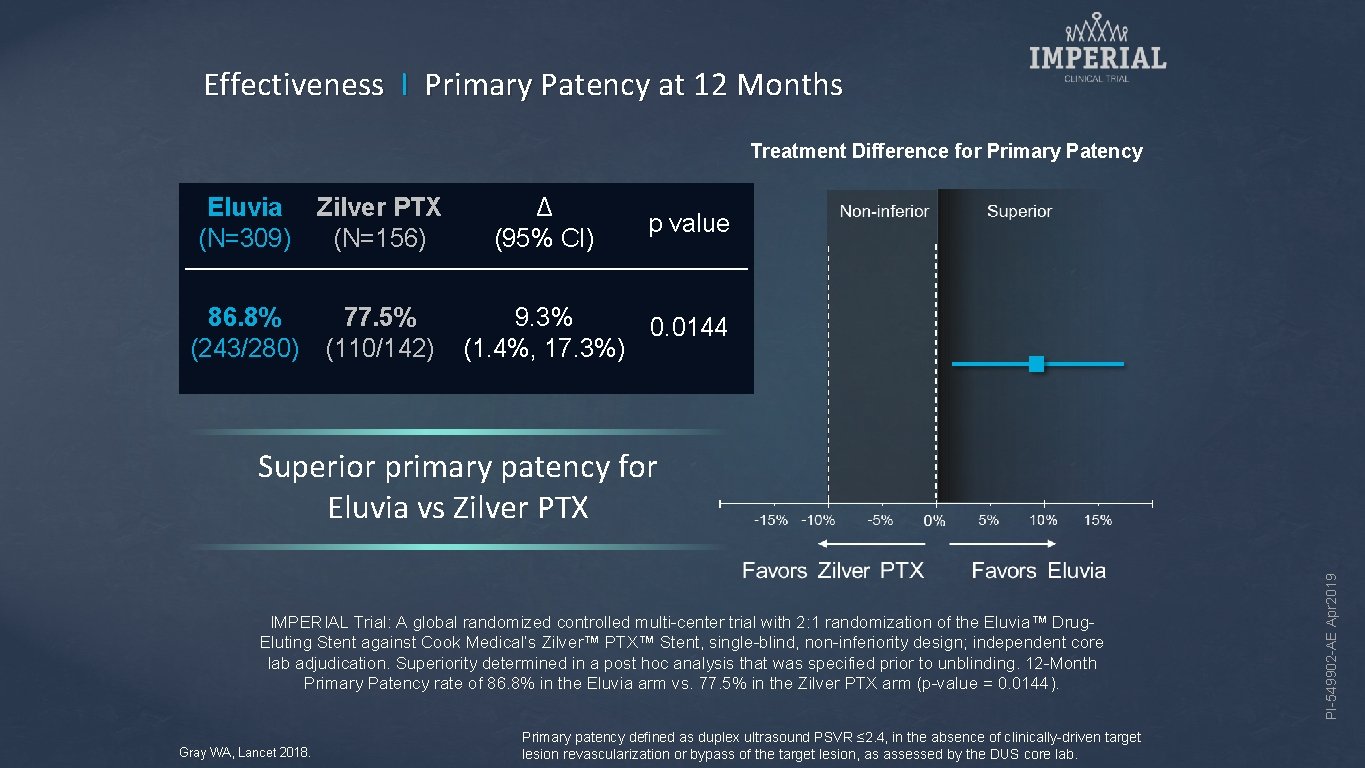
Effectiveness I Primary Patency at 12 Months Treatment Difference for Primary Patency Eluvia (N=309) Zilver PTX (N=156) 86. 8% (243/280) 77. 5% (110/142) Δ (95% CI) p value 9. 3% 0. 0144 (1. 4%, 17. 3%) IMPERIAL Trial: A global randomized controlled multi-center trial with 2: 1 randomization of the Eluvia™ Drug. Eluting Stent against Cook Medical’s Zilver™ PTX™ Stent, single-blind, non-inferiority design; independent core lab adjudication. Superiority determined in a post hoc analysis that was specified prior to unblinding. 12 -Month Primary Patency rate of 86. 8% in the Eluvia arm vs. 77. 5% in the Zilver PTX arm (p-value = 0. 0144). Gray WA, Lancet 2018. Primary patency defined as duplex ultrasound PSVR ≤ 2. 4, in the absence of clinically-driven target lesion revascularization or bypass of the target lesion, as assessed by the DUS core lab. PI-549902 -AE Apr 2019 Superior primary patency for Eluvia vs Zilver PTX

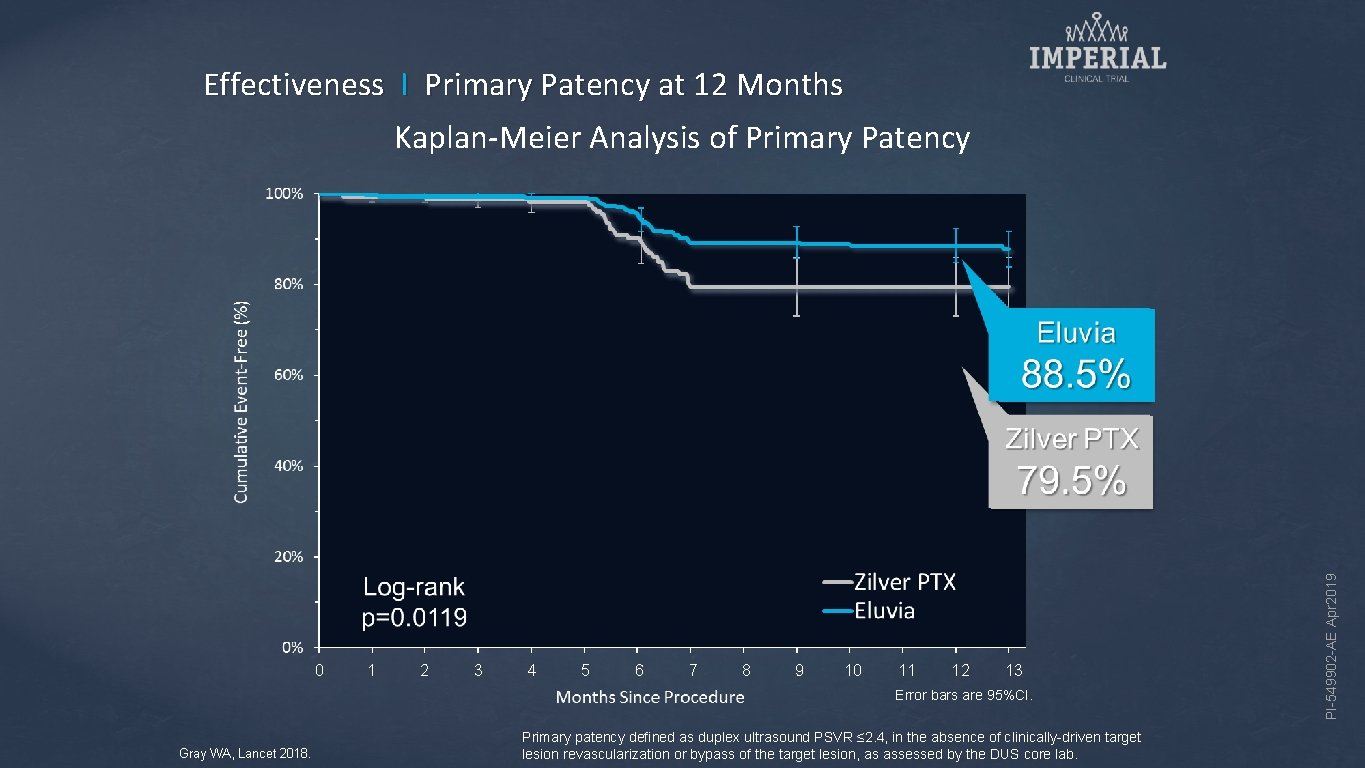
Effectiveness I Primary Patency at 12 Months 0 1 2 3 4 5 6 7 8 9 10 11 12 13 Error bars are 95%CI. Gray WA, Lancet 2018. Primary patency defined as duplex ultrasound PSVR ≤ 2. 4, in the absence of clinically-driven target lesion revascularization or bypass of the target lesion, as assessed by the DUS core lab. PI-549902 -AE Apr 2019 Kaplan-Meier Analysis of Primary Patency

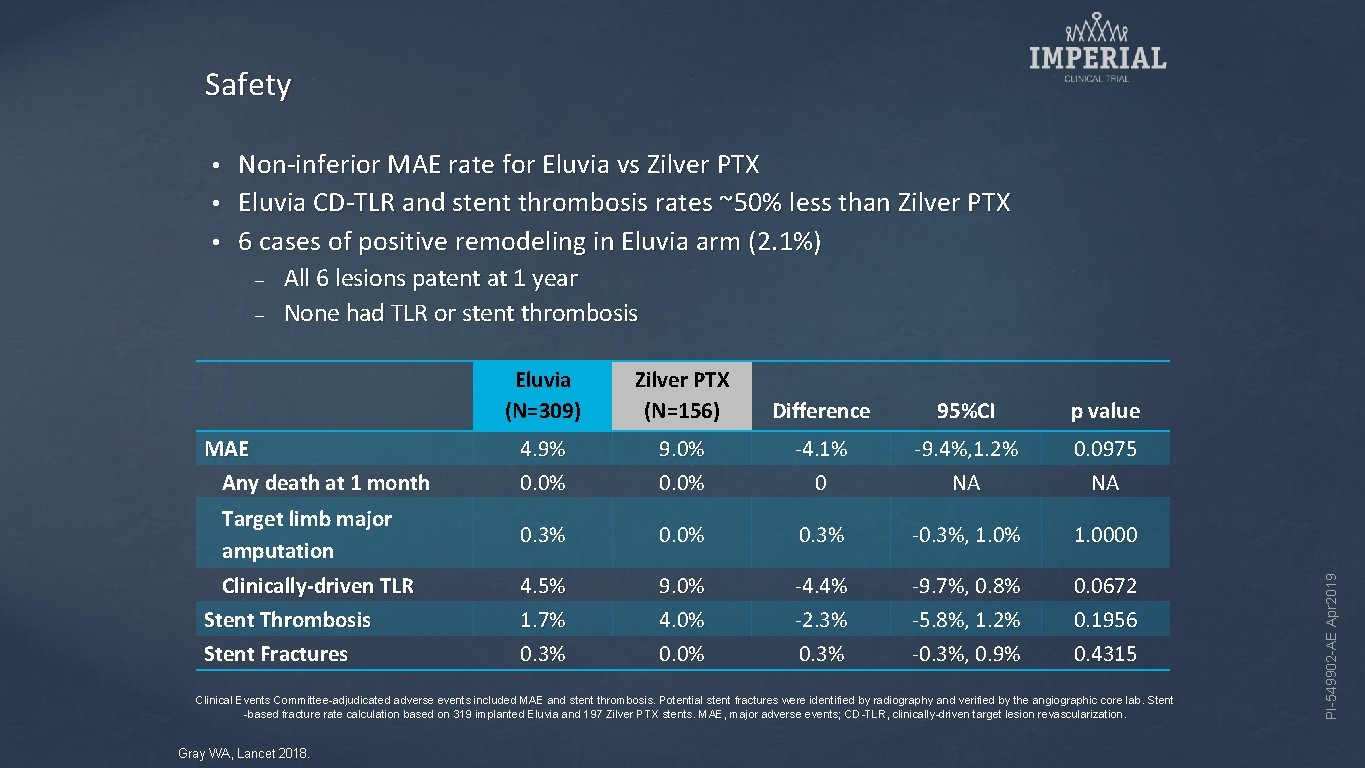
Safety Non-inferior MAE rate for Eluvia vs Zilver PTX • Eluvia CD-TLR and stent thrombosis rates ~50% less than Zilver PTX • 6 cases of positive remodeling in Eluvia arm (2. 1%) • – All 6 lesions patent at 1 year None had TLR or stent thrombosis Eluvia (N=309) Zilver PTX (N=156) Difference 95%CI p value 4. 9% 0. 0% 9. 0% 0. 0% -4. 1% 0 -9. 4%, 1. 2% NA 0. 0975 NA Target limb major amputation 0. 3% 0. 0% 0. 3% -0. 3%, 1. 0% 1. 0000 Clinically-driven TLR Stent Thrombosis Stent Fractures 4. 5% 1. 7% 0. 3% 9. 0% 4. 0% 0. 0% -4. 4% -2. 3% 0. 3% -9. 7%, 0. 8% -5. 8%, 1. 2% -0. 3%, 0. 9% 0. 0672 0. 1956 0. 4315 MAE Any death at 1 month Clinical Events Committee-adjudicated adverse events included MAE and stent thrombosis. Potential stent fractures were identified by radiography and verified by the angiographic core lab. Stent -based fracture rate calculation based on 319 implanted Eluvia and 197 Zilver PTX stents. MAE, major adverse events; CD-TLR, clinically-driven target lesion revascularization. Gray WA, Lancet 2018. PI-549902 -AE Apr 2019 –

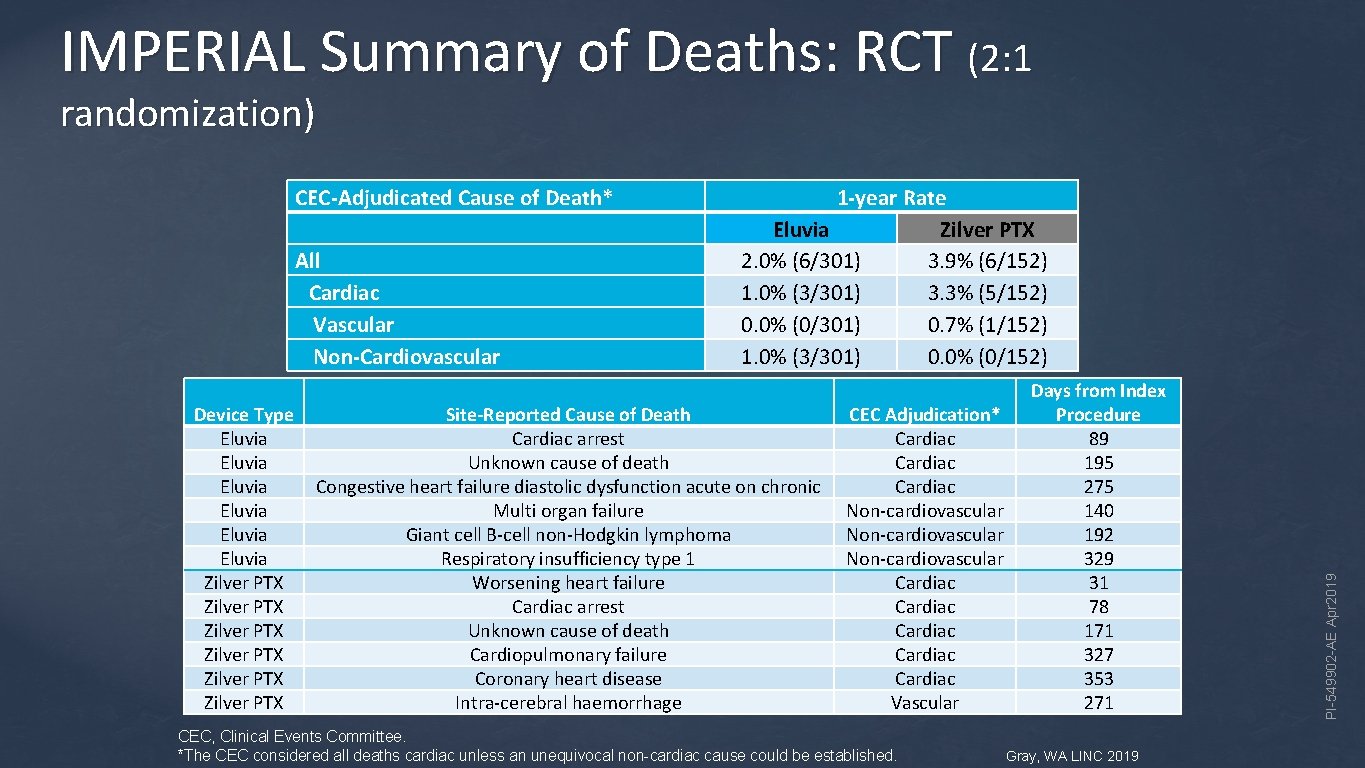
IMPERIAL Summary of Deaths: RCT (2: 1 randomization) All Cardiac Vascular Non-Cardiovascular Device Type Eluvia Eluvia Zilver PTX Zilver PTX 1 -year Rate Eluvia Zilver PTX 2. 0% (6/301) 3. 9% (6/152) 1. 0% (3/301) 3. 3% (5/152) 0. 0% (0/301) 0. 7% (1/152) 1. 0% (3/301) 0. 0% (0/152) Site-Reported Cause of Death Cardiac arrest Unknown cause of death Congestive heart failure diastolic dysfunction acute on chronic Multi organ failure Giant cell B-cell non-Hodgkin lymphoma Respiratory insufficiency type 1 Worsening heart failure Cardiac arrest Unknown cause of death Cardiopulmonary failure Coronary heart disease Intra-cerebral haemorrhage CEC Adjudication* Cardiac Non-cardiovascular Cardiac Cardiac Vascular CEC, Clinical Events Committee. *The CEC considered all deaths cardiac unless an unequivocal non-cardiac cause could be established. Days from Index Procedure 89 195 275 140 192 329 31 78 171 327 353 271 Gray, WA LINC 2019 PI-549902 -AE Apr 2019 CEC-Adjudicated Cause of Death*

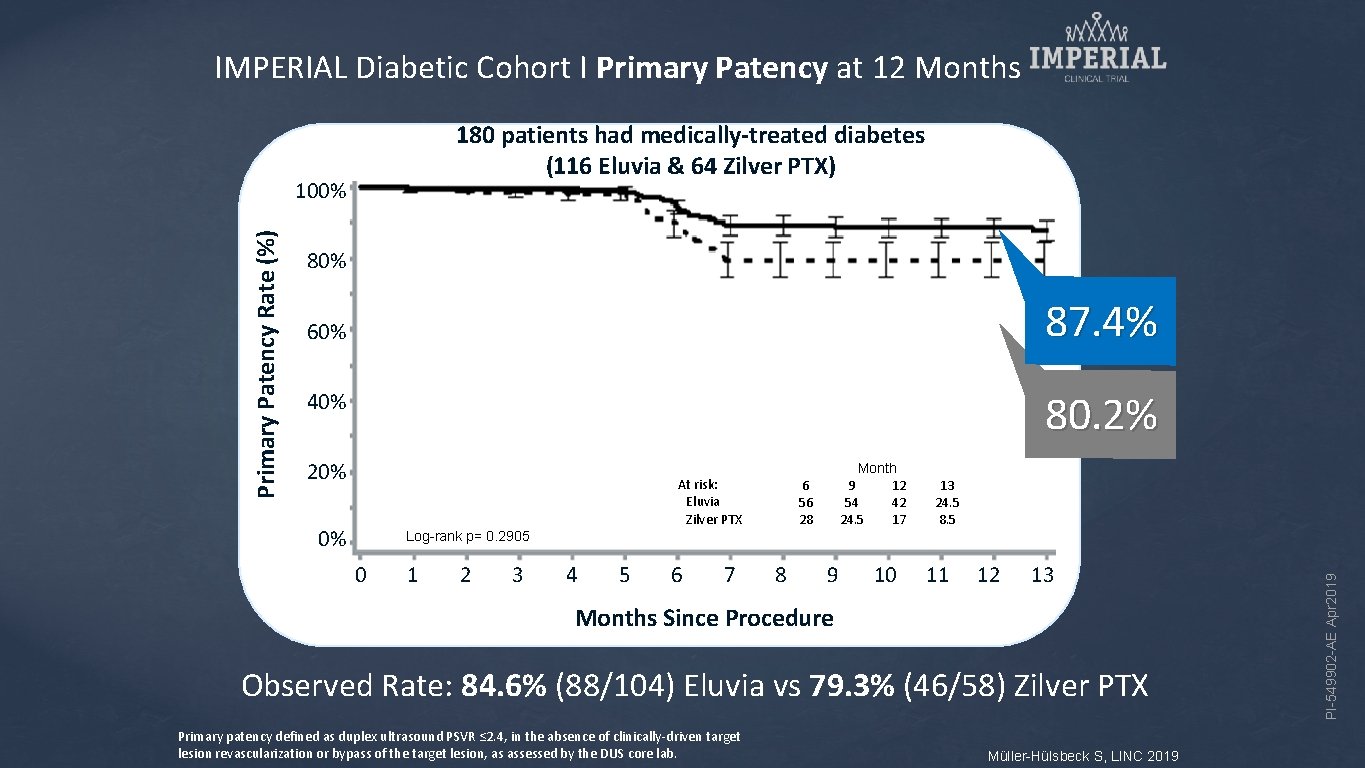
IMPERIAL Diabetic Cohort I Primary Patency at 12 Months 180 patients had medically-treated diabetes Kaplan-Meier Estimate (116 Eluvia & 64 Zilver PTX) 80% 87. 4% 60% 80. 2% 40% 20% At risk: Eluvia Zilver PTX 0% Month 9 12 54 42 24. 5 17 6 56 28 13 24. 5 8. 5 Log-rank p= 0. 2905 0 1 2 3 4 5 6 7 8 9 10 11 12 13 Months Since Procedure Observed Rate: 84. 6% (88/104) Eluvia vs 79. 3% (46/58) Zilver PTX Primary patency defined as duplex ultrasound PSVR ≤ 2. 4, in the absence of clinically-driven target lesion revascularization or bypass of the target lesion, as assessed by the DUS core lab. Müller-Hülsbeck S, LINC 2019 PI-549902 -AE Apr 2019 Primary Patency Rate (%) 100%

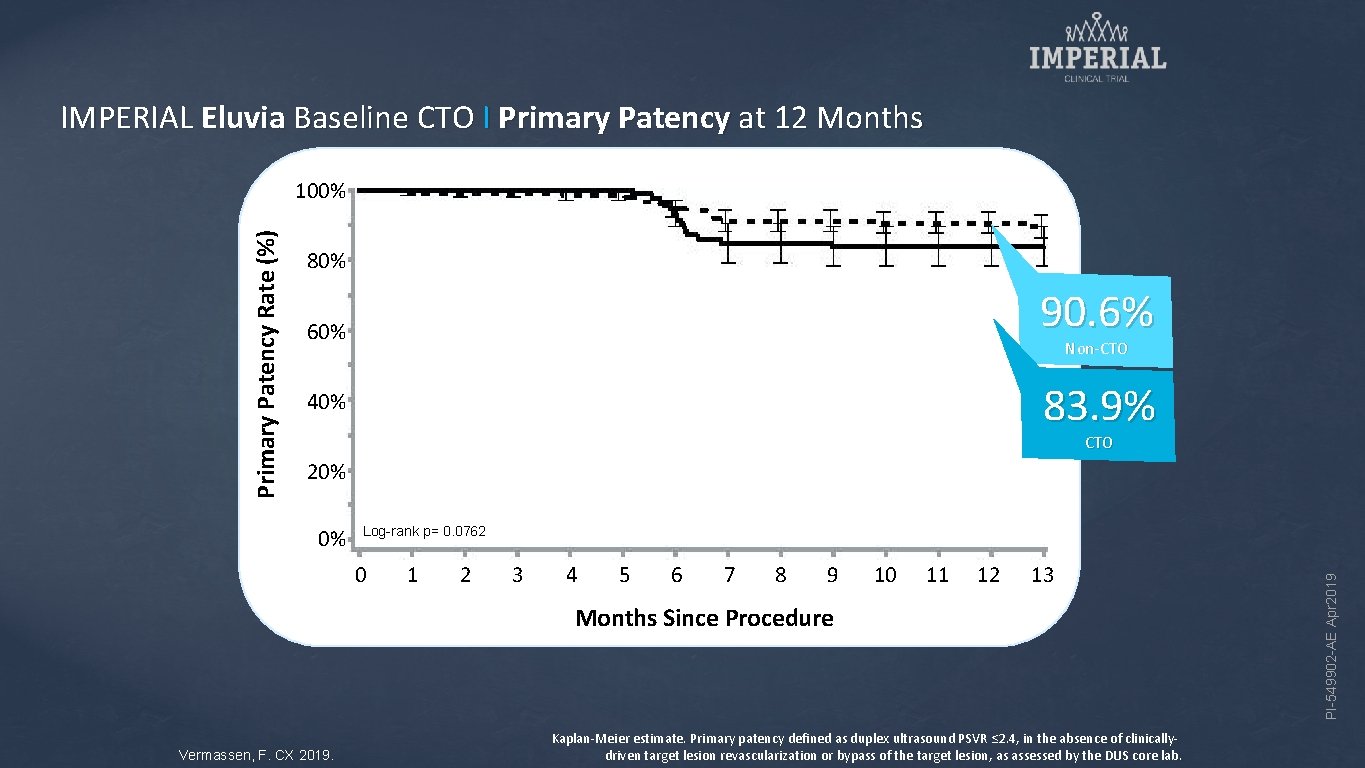
IMPERIAL Eluvia Baseline CTO I Primary Patency at 12 Months 80% 90. 6% 60% Non-CTO 83. 9% 40% CTO 20% 0% Log-rank p= 0. 0762 0 1 2 3 4 5 6 7 8 9 10 11 12 13 Months Since Procedure Vermassen, F. CX 2019. Kaplan-Meier estimate. Primary patency defined as duplex ultrasound PSVR ≤ 2. 4, in the absence of clinicallydriven target lesion revascularization or bypass of the target lesion, as assessed by the DUS core lab. PI-549902 -AE Apr 2019 Primary Patency Rate (%) 100%

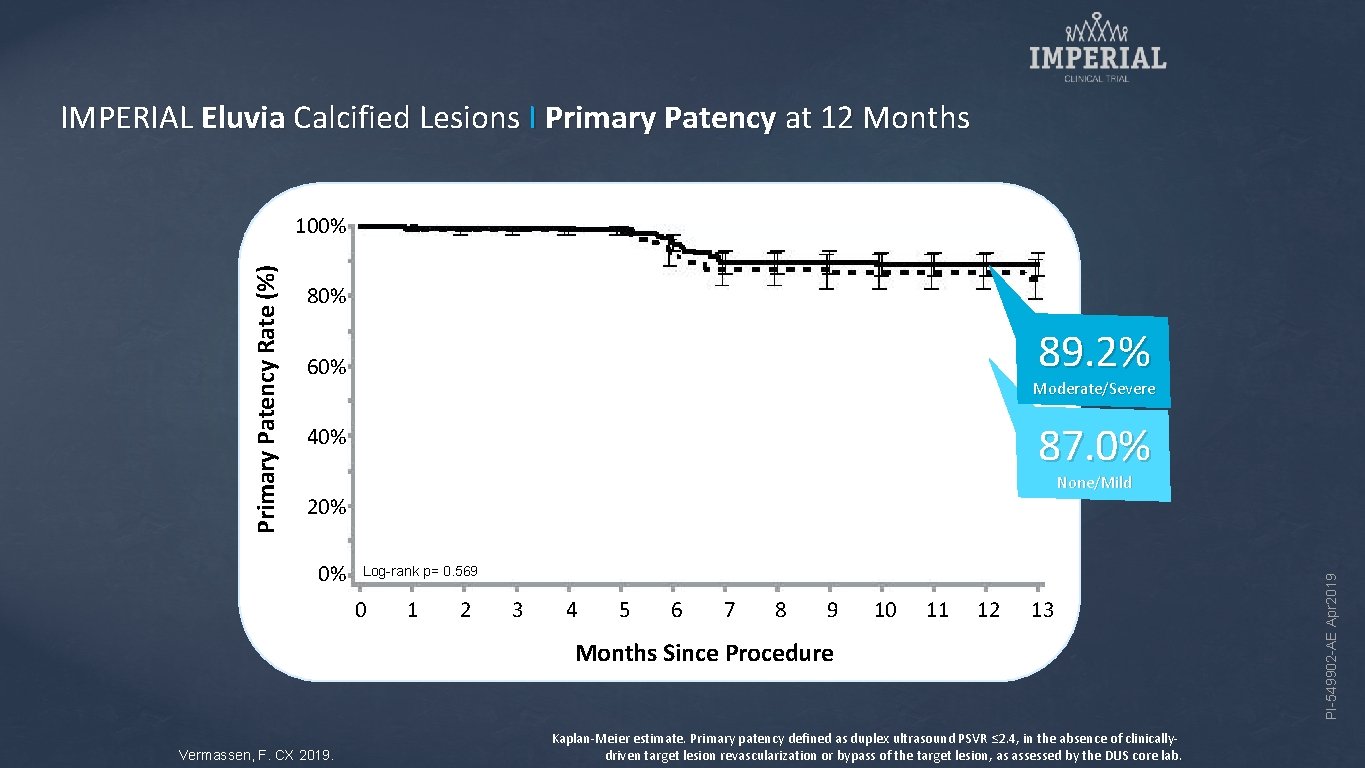
IMPERIAL Eluvia Calcified Lesions I Primary Patency at 12 Months 80% 89. 2% 60% Moderate/Severe 87. 0% 40% None/Mild 20% 0% Log-rank p= 0. 569 0 1 2 3 4 5 6 7 8 9 10 11 12 13 Months Since Procedure Vermassen, F. CX 2019. Kaplan-Meier estimate. Primary patency defined as duplex ultrasound PSVR ≤ 2. 4, in the absence of clinicallydriven target lesion revascularization or bypass of the target lesion, as assessed by the DUS core lab. PI-549902 -AE Apr 2019 Primary Patency Rate (%) 100%

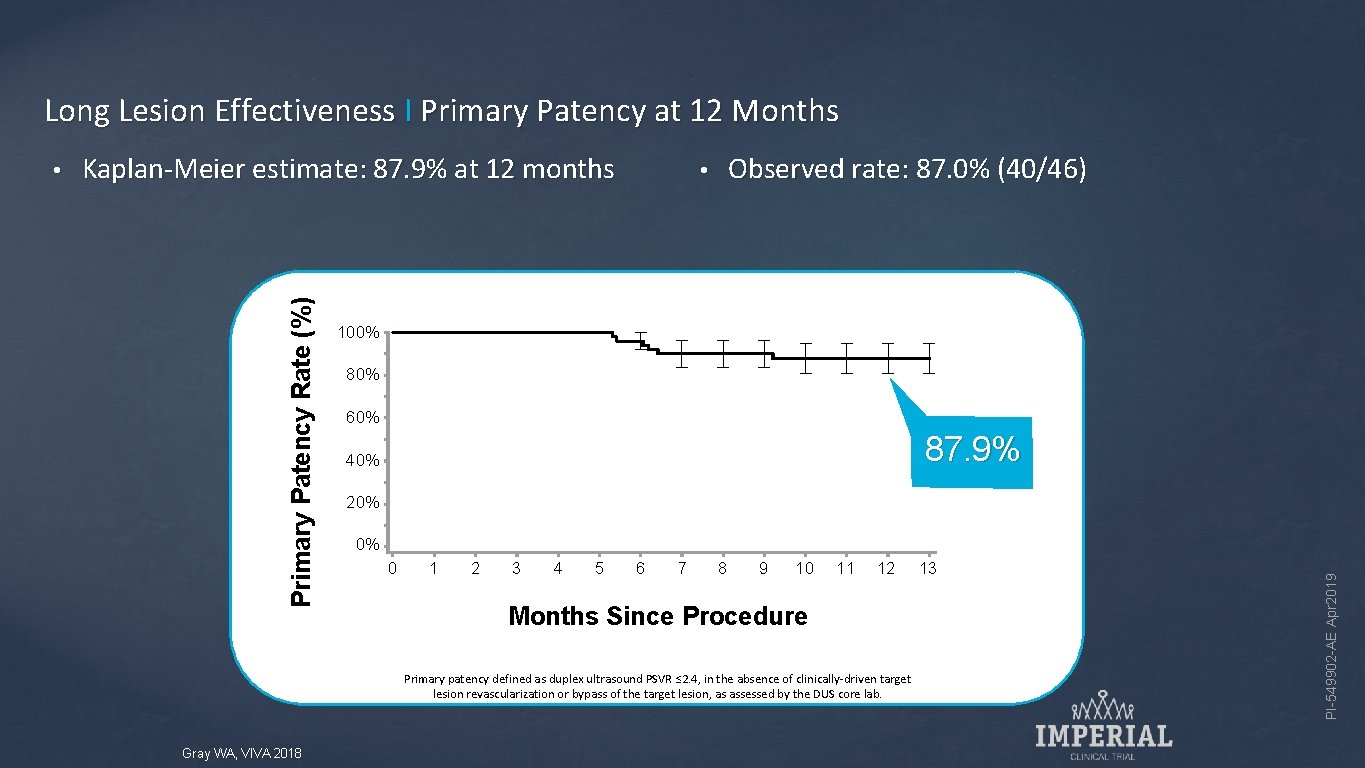
Long Lesion Effectiveness I Primary Patency at 12 Months Kaplan-Meier estimate: 87. 9% at 12 months Observed rate: 87. 0% (40/46) • 100% 80% 60% 87. 9% 40% 20% 0% 0 1 2 3 4 5 6 7 8 9 10 11 12 Months Since Procedure Primary patency defined as duplex ultrasound PSVR ≤ 2. 4, in the absence of clinically-driven target lesion revascularization or bypass of the target lesion, as assessed by the DUS core lab. Gray WA, VIVA 2018 13 PI-549902 -AE Apr 2019 Primary Patency Rate (%) •

IMPERIAL Pharmacokinetics Sub-study (N=13) All patients treated with Eluvia • Plasma paclitaxel unquantifiable (<1 ng/m. L) in all 13 patients at >10 minutes post-implantation • 1 -year mortality 0% PI-549902 -AE Apr 2019 • Plasma paclitaxel measured at 10 min, 30 min, and 1, 2, 3, 4, 6, 12, 24, 48 hours post-implant. Gray WA, Lancet 2018

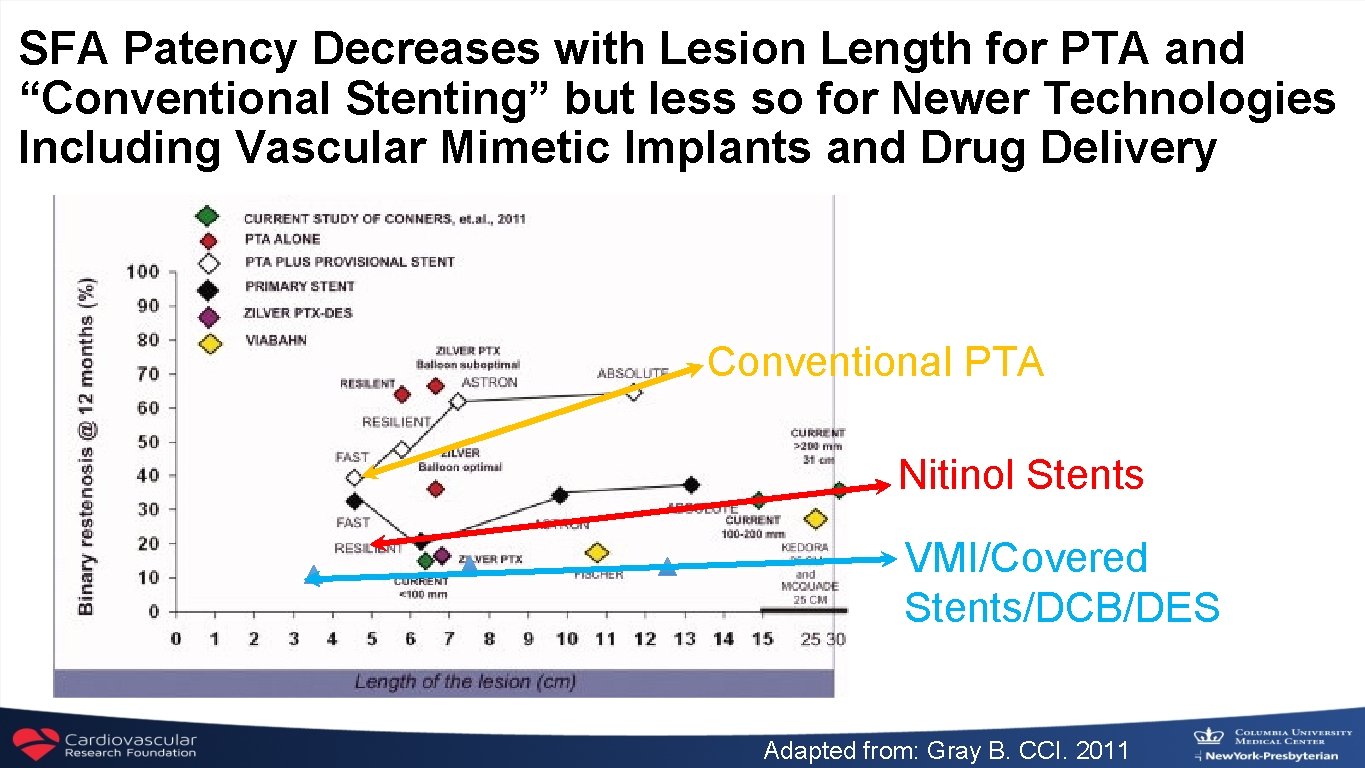
SFA Patency Decreases with Lesion Length for PTA and “Conventional Stenting” but less so for Newer Technologies Including Vascular Mimetic Implants and Drug Delivery Conventional PTA Nitinol Stents VMI/Covered Stents/DCB/DES Adapted from: Gray B. CCI. 2011

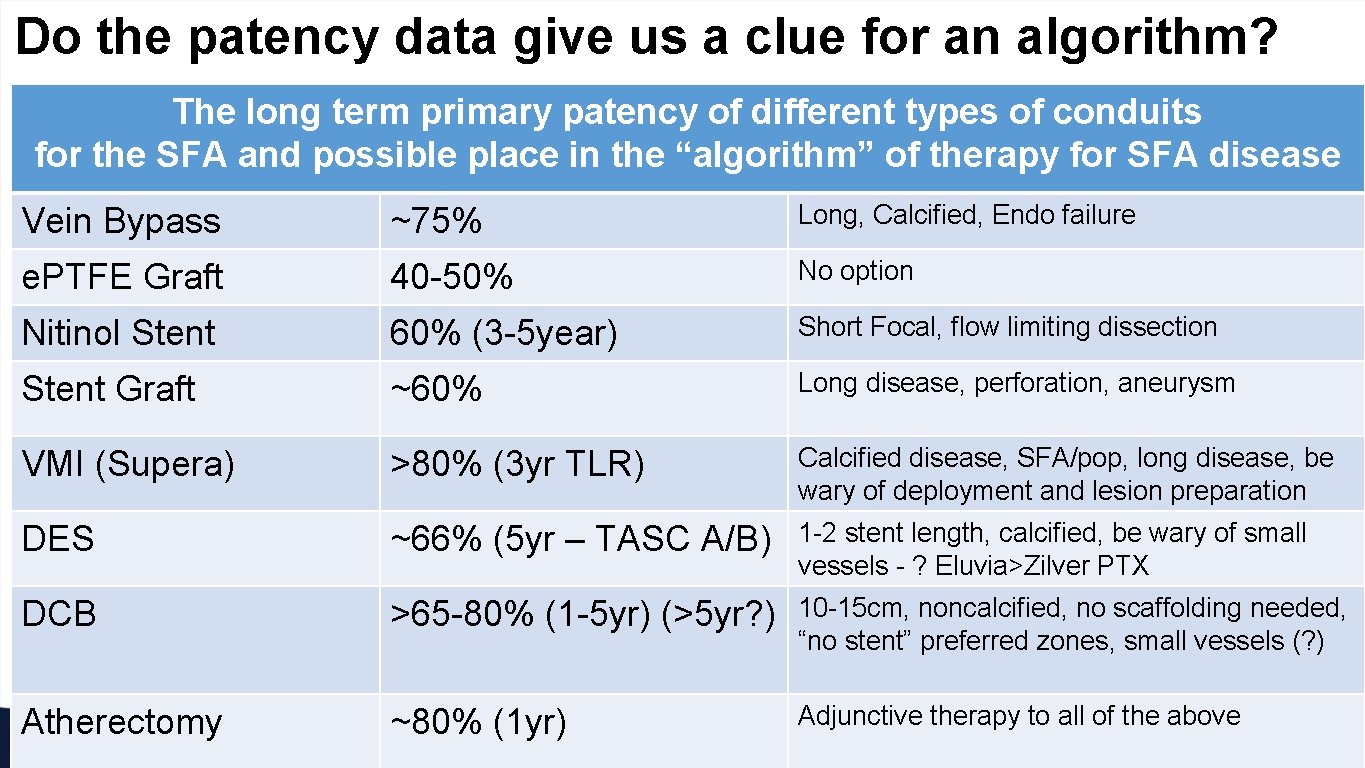
Do the patency data give us a clue for an algorithm? The long term primary patency of different types of conduits for the SFA and possible place in the “algorithm” of therapy for SFA disease Vein Bypass • ~75% PTA/stents Long, Calcified, Endo failure • Femoral-popliteal Nitinol Stent 40 -50% • Surgical reconstruction 60% (3 -5 year) • Femoral-popliteal 55 -75% No option Stent Graft • Femoral-tibial ~60% Long disease, perforation, aneurysm VMI (Supera) >80% (3 yr TLR) Calcified disease, SFA/pop, long disease, be wary of deployment and lesion preparation DES ~66% (5 yr – TASC A/B) 1 -2 stent length, calcified, be wary of small vessels - ? Eluvia>Zilver PTX DCB >65 -80% (1 -5 yr) (>5 yr? ) 10 -15 cm, noncalcified, no scaffolding needed, “no stent” preferred zones, small vessels (? ) Atherectomy ~80% (1 yr) Adjunctive therapy to all of the above e. PTFE Graft 60 -75% Short Focal, flow limiting dissection 65 -80%

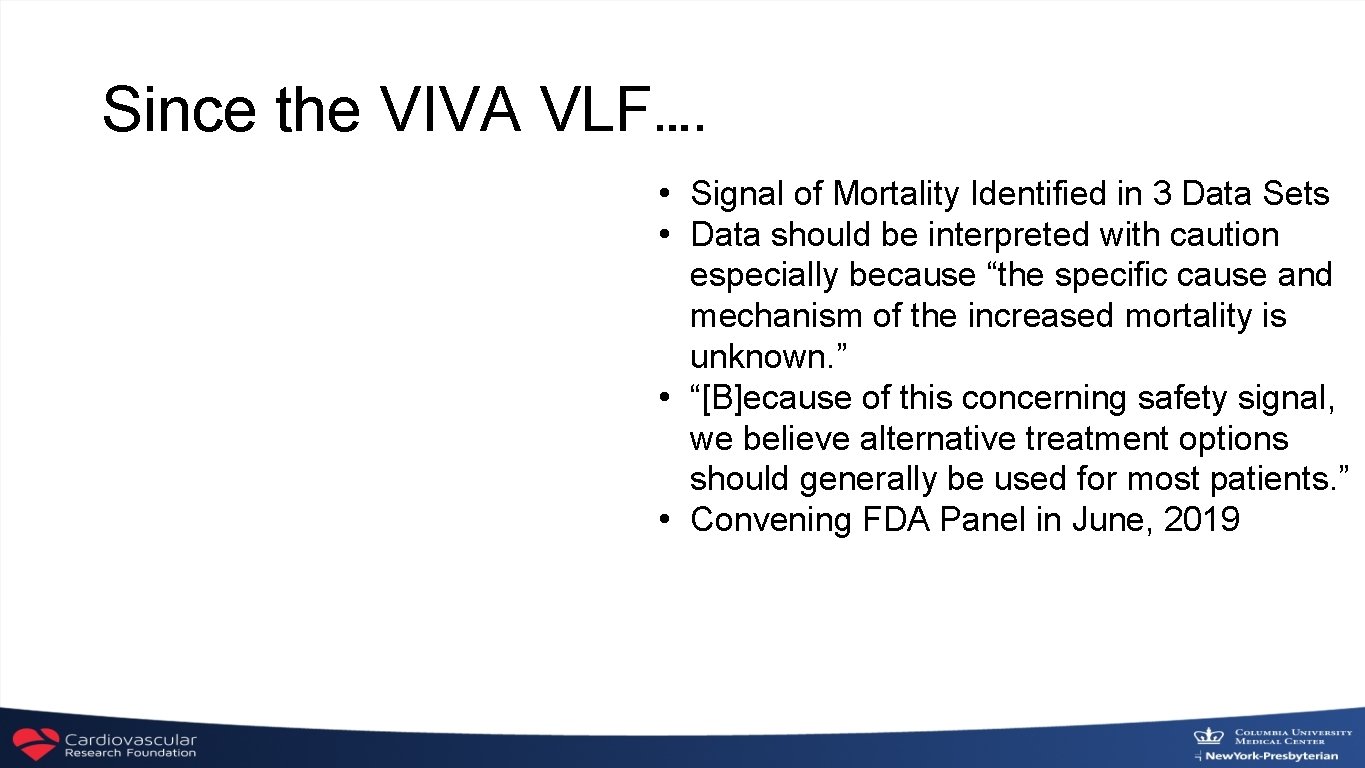
Since the VIVA VLF…. • Signal of Mortality Identified in 3 Data Sets • Data should be interpreted with caution especially because “the specific cause and mechanism of the increased mortality is unknown. ” • “[B]ecause of this concerning safety signal, we believe alternative treatment options should generally be used for most patients. ” • Convening FDA Panel in June, 2019

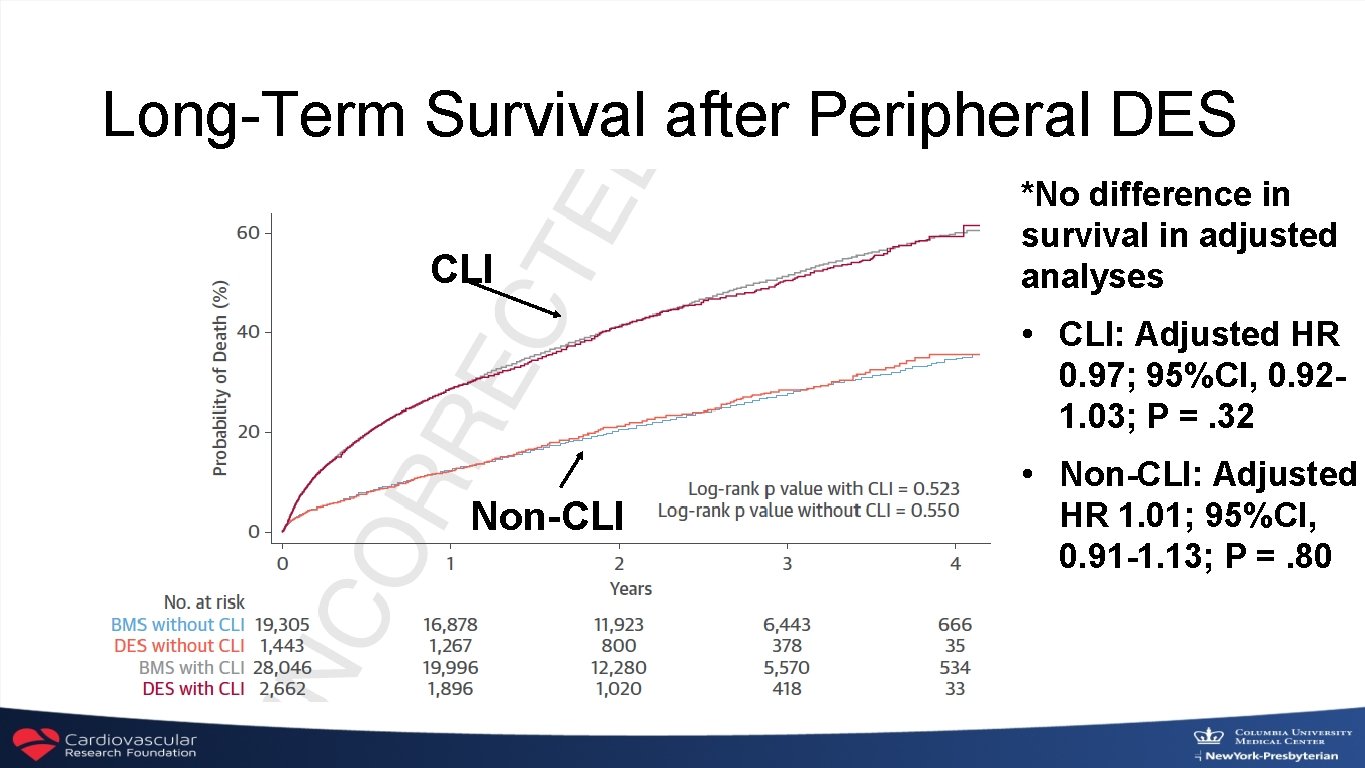
March 1, 2019 Drug-Eluting Stent Implantation and Long-Term Survival Following Peripheral Artery Revascularization Eric A. Secemsky, Harun Kundi, Ido Weinberg, Marc Schermerhorn, Joshua A. Beckman, Sahil A. Parikh, Michael R. Jaff, Jihad Mustapha, Kenneth Rosenfield and Robert W. Yeh http: //www. onlinejacc. org/content/early/recent

Long-Term Survival after Peripheral DES CLI *No difference in survival in adjusted analyses • CLI: Adjusted HR 0. 97; 95%CI, 0. 921. 03; P =. 32 Non-CLI • Non-CLI: Adjusted HR 1. 01; 95%CI, 0. 91 -1. 13; P =. 80

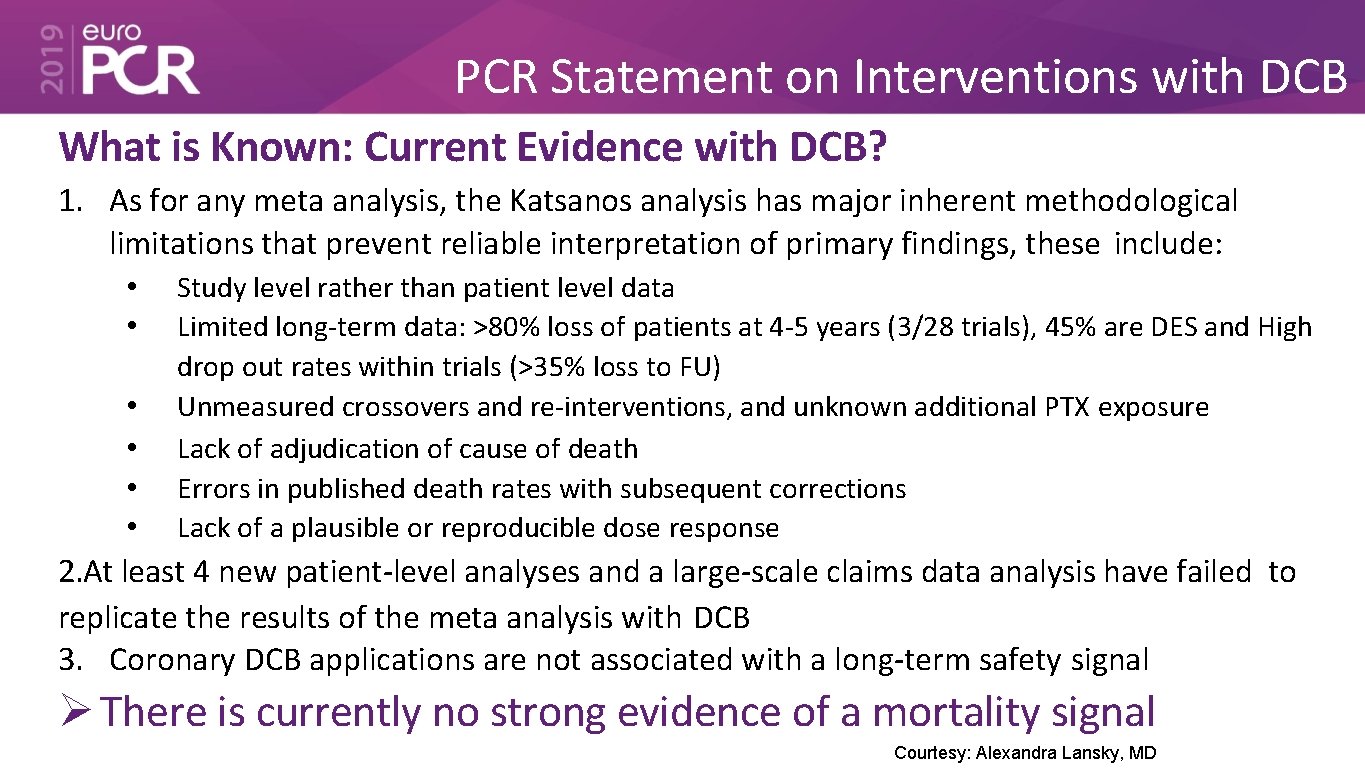
PCR Statement on Interventions with DCB What is Known: Current Evidence with DCB? 1. As for any meta analysis, the Katsanos analysis has major inherent methodological limitations that prevent reliable interpretation of primary findings, these include: • • • Study level rather than patient level data Limited long-term data: >80% loss of patients at 4 -5 years (3/28 trials), 45% are DES and High drop out rates within trials (>35% loss to FU) Unmeasured crossovers and re-interventions, and unknown additional PTX exposure Lack of adjudication of cause of death Errors in published death rates with subsequent corrections Lack of a plausible or reproducible dose response 2. At least 4 new patient-level analyses and a large-scale claims data analysis have failed to replicate the results of the meta analysis with DCB 3. Coronary DCB applications are not associated with a long-term safety signal There is currently no strong evidence of a mortality signal Courtesy: Alexandra Lansky, MD

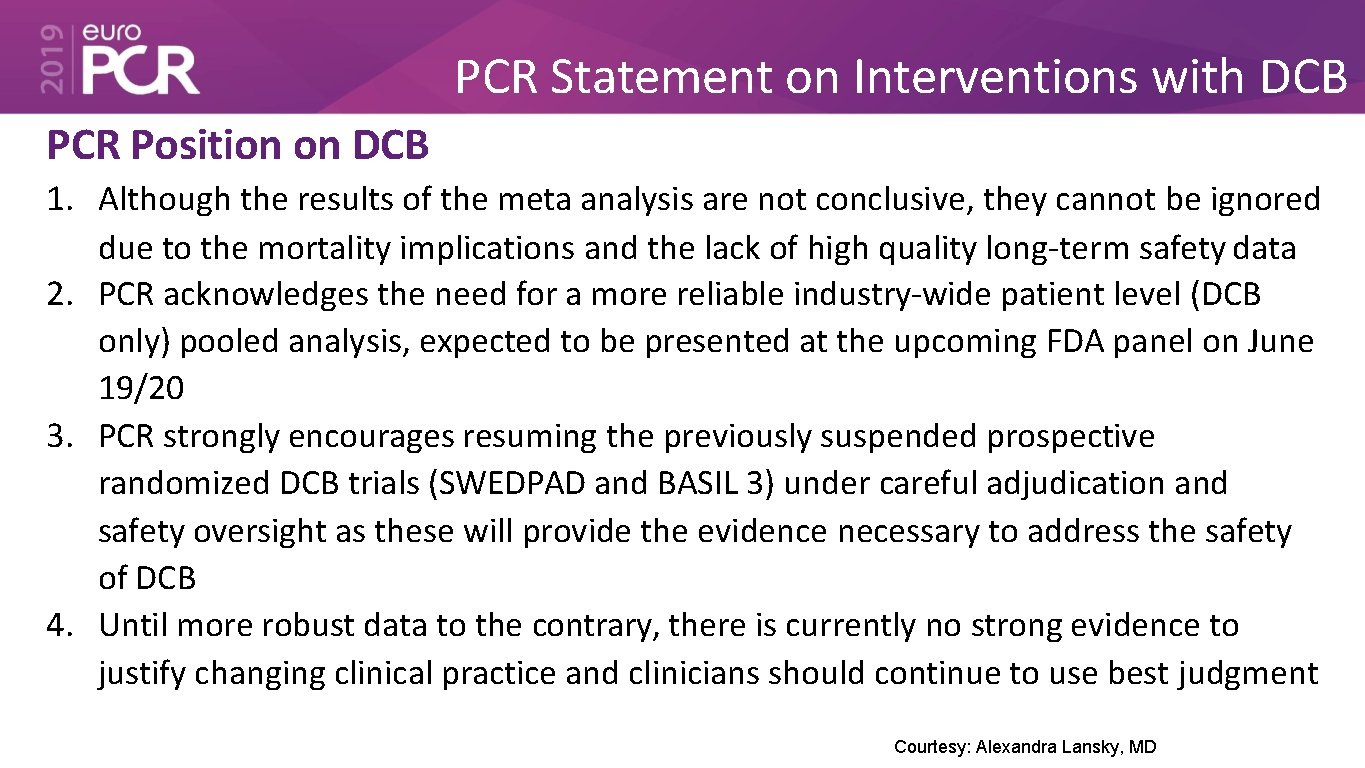
PCR Statement on Interventions with DCB PCR Position on DCB 1. Although the results of the meta analysis are not conclusive, they cannot be ignored due to the mortality implications and the lack of high quality long-term safety data 2. PCR acknowledges the need for a more reliable industry-wide patient level (DCB only) pooled analysis, expected to be presented at the upcoming FDA panel on June 19/20 3. PCR strongly encourages resuming the previously suspended prospective randomized DCB trials (SWEDPAD and BASIL 3) under careful adjudication and safety oversight as these will provide the evidence necessary to address the safety of DCB 4. Until more robust data to the contrary, there is currently no strong evidence to justify changing clinical practice and clinicians should continue to use best judgment Courtesy: Alexandra Lansky, MD

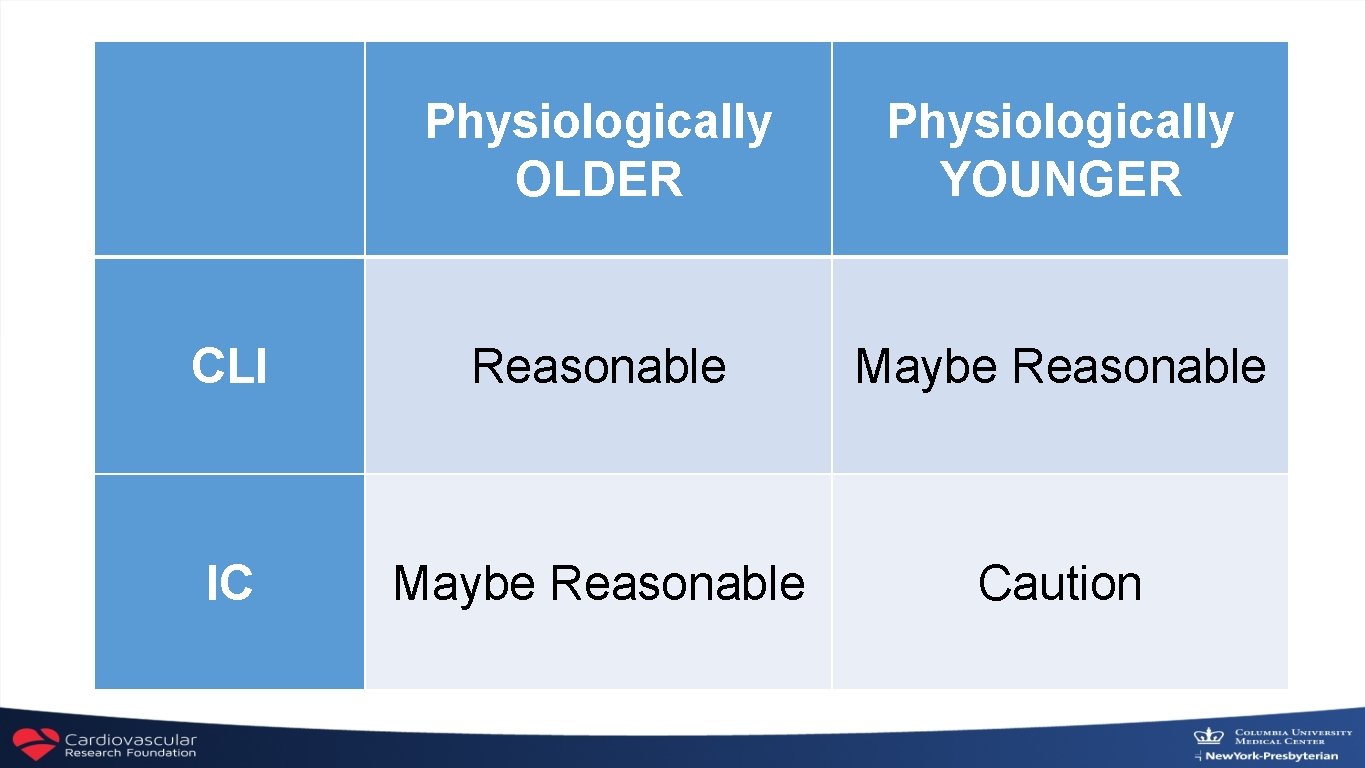
Physiologically OLDER Physiologically YOUNGER CLI Reasonable Maybe Reasonable IC Maybe Reasonable Caution
 Mikael ferm
Mikael ferm Aksi scai
Aksi scai Scai bowls
Scai bowls Scai cardiogenic shock
Scai cardiogenic shock Accidental adulteration definition
Accidental adulteration definition Hci patterns may or may not include code for implementation
Hci patterns may or may not include code for implementation Ar 140-185 table 2-3
Ar 140-185 table 2-3 History ia mark bands
History ia mark bands Objectives of drug abuse
Objectives of drug abuse Clark's rule formula
Clark's rule formula Nida principles of drug addiction treatment
Nida principles of drug addiction treatment Non parenteral route of drug administration
Non parenteral route of drug administration Drug
Drug Rate of elimination of drug
Rate of elimination of drug Tennessee drug court programs
Tennessee drug court programs Drug pooling
Drug pooling Computer aided drug design lecture notes
Computer aided drug design lecture notes Centre for drug evaluation and research
Centre for drug evaluation and research Medicated solution meant for rinsing a body cavity
Medicated solution meant for rinsing a body cavity Types of adulteration in pharmacognosy
Types of adulteration in pharmacognosy Sistem penghantaran obat melalui mata
Sistem penghantaran obat melalui mata Factor affecting drug metabolism
Factor affecting drug metabolism Drug impairment training
Drug impairment training Tremors and seizures
Tremors and seizures Drug seekers scd
Drug seekers scd Alabama prescription drug monitoring program
Alabama prescription drug monitoring program Parenteral medication examples
Parenteral medication examples Drug review
Drug review Drug and alcohol jeopardy
Drug and alcohol jeopardy Chapter 22 lesson 1 the health risks of drug use
Chapter 22 lesson 1 the health risks of drug use