Progenitor Endothelial Cell Capturing with a Drug Eluting







- Slides: 13

Progenitor Endothelial Cell Capturing with a Drug Eluting Stent Stephen M Rowland, Ph. D VP R&D and Clinical Affairs, Orbus. Neich Medical Washington, DC 25 February, 2013

Disclosure • Employee of Orbus. Neich Medical, Inc.


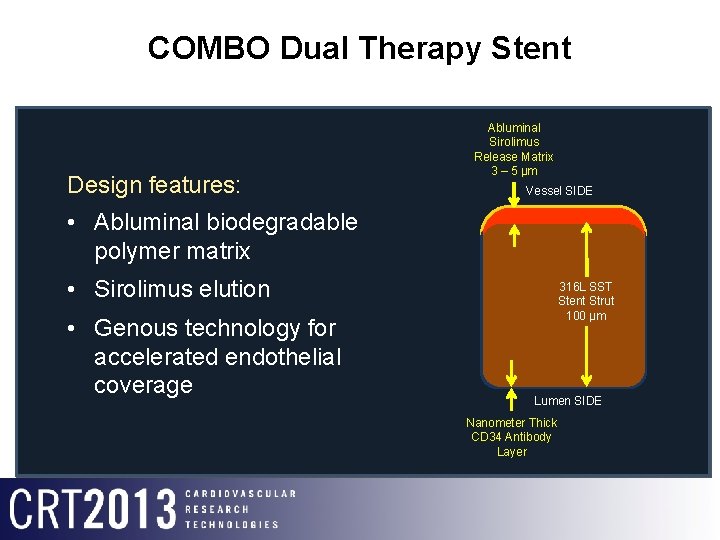
COMBO Dual Therapy Stent Design features: • Abluminal biodegradable polymer matrix • Sirolimus elution • Genous technology for accelerated endothelial coverage Abluminal Sirolimus Release Matrix 3 – 5 μm Vessel SIDE 316 L Stainless 316 L SST Steel Stent Strut Cross-section 100 μm Lumen SIDE Nanometer Thick CD 34 Antibody Layer

Clinical Performance • Conducted 3 studies with the Combo stent including multiple imaging modalities: – REMEDEE – EGO Combo – REMEDEE OCT stable angina patients (N=180) stable angina patients (N=61) STEMI & non-STEMI patients (N=60) • Shed light on 3 aspects of healing: – EARLY: Strut “coverage” – OCT @ 2 - 9 months – MID TERM: restenosis • NI formation & Late Loss – OCT @ 2 - 9 months – QCA @ 9 months – LONG TERM: Biostability • NI Characterization @ 9 months – OCT: Low Intensity Tissue & Micro Vessels in NI – IVUS-VH: Necrotic Core in NI

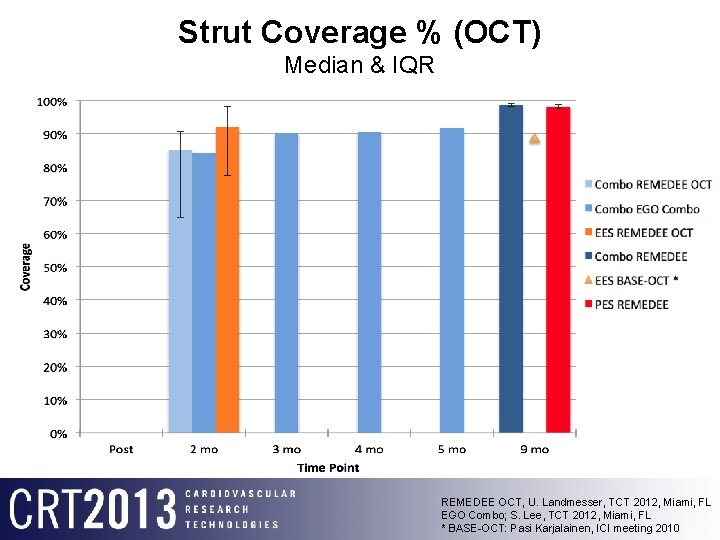
Strut Coverage % (OCT) Median & IQR REMEDEE OCT, U. Landmesser, TCT 2012, Miami, FL EGO Combo; S. Lee, TCT 2012, Miami, FL * BASE-OCT: Pasi Karjalainen, ICI meeting 2010

Cumulative Frequency Distribution of In-stent Late Lumen Loss * Haude M et al. , TCT 2012, Miami, FL

Histograms of In-stent Late Loss (QCA) Combo (REMEDEE, 9 mo) n=109 Taxus (REMEDEE, 9 mo) n=52 % In-Stent Late Loss [mm] Genous * (Healing IIb, 6 mo) n=86 % In-Stent Late Loss [mm] ISLL distributions show different patterns: • Combo slight tail • Taxus bi-modal appearance • Genous similar to BMS In-Stent Late Loss [mm] Haude M et al. , TCT 2012, Miami, FL

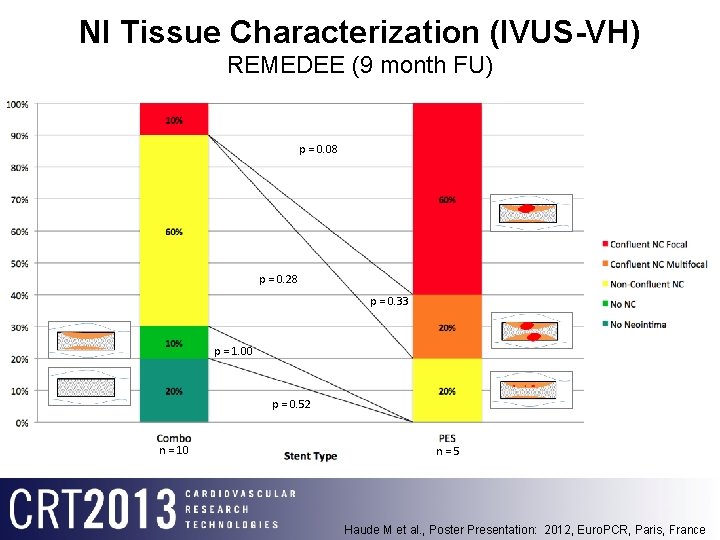
NI Tissue Characterization (IVUS-VH) REMEDEE (9 month FU) p = 0. 08 p = 0. 28 p = 0. 33 p = 1. 00 p = 0. 52 n = 10 n=5 Haude M et al. , Poster Presentation: 2012, Euro. PCR, Paris, France

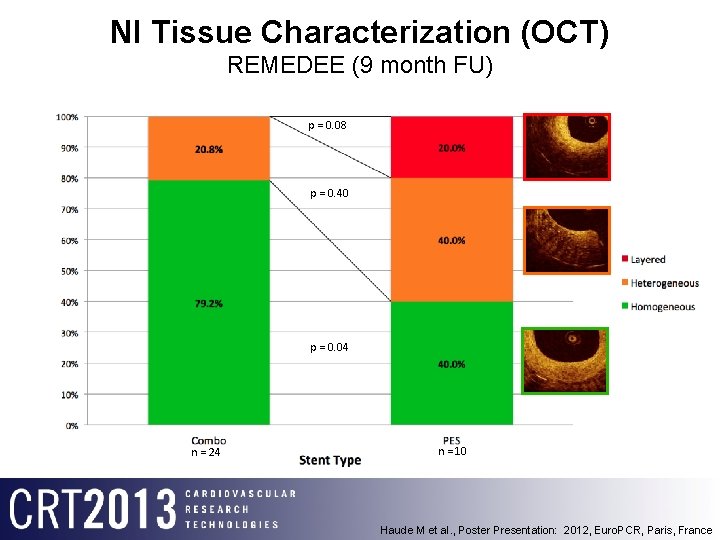
NI Tissue Characterization (OCT) REMEDEE (9 month FU) p = 0. 08 p = 0. 40 p = 0. 04 n = 24 n = 10 Haude M et al. , Poster Presentation: 2012, Euro. PCR, Paris, France

Lessons Learned • Signature of the Combo Stent reflects: – Abluminal drug delivery and – Luminal EPC capture • Translating into: – Early • Rapid strut coverage with endothelium – Mid Term • DES-like late loss (control of restenosis) – Long Term • Uniform homogeneous tissue with a reduced accumulation of newly formed necrotic core/neo-atherosclerosis

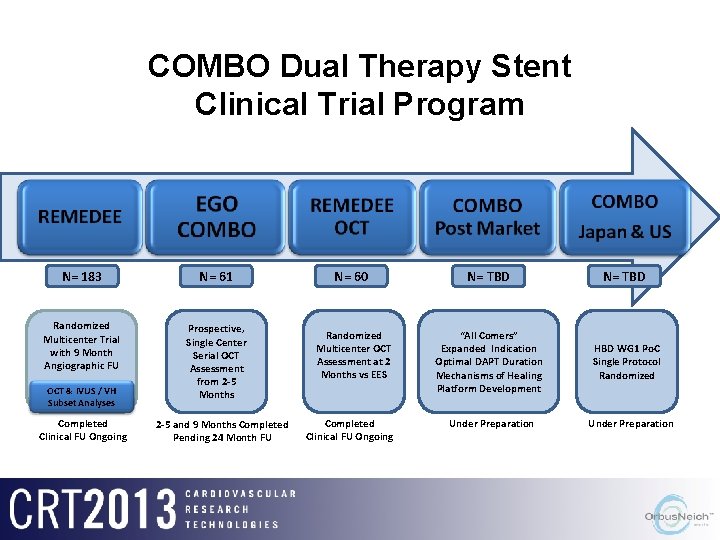
COMBO Dual Therapy Stent Clinical Trial Program N= 183 N= 61 Randomized Multicenter Trial with 9 Month Angiographic FU Prospective, Single Center Serial OCT Assessment from 2 -5 Months OCT & IVUS / VH Subset Analyses Completed Clinical FU Ongoing 2 -5 and 9 Months Completed Pending 24 Month FU N= 60 N= TBD Randomized Multicenter OCT Assessment at 2 Months vs EES “All Comers” Expanded Indication Optimal DAPT Duration Mechanisms of Healing Platform Development HBD WG 1 Po. C Single Protocol Randomized Completed Clinical FU Ongoing Under Preparation

Thank You