Sampling diatoms from running waters 1 Introduction Martyn














- Slides: 26

Sampling diatoms from running waters 1: Introduction Martyn Kelly and Marian Yallop

This slide has been inserted by the Environment Agency • NOTE: Staff should always undertake a dynamic risk assessment to assess health risks at time of sampling. Where necessary a supplementary risk assessment should be undertaken to reduce any additional risks. • In general, gloves should be worn when collecting diatom samples.

Introduction DARES is a project funded by the Environment Agency and SNIFFER to develop a diatom-based method for monitoring ecological status, as required for the WFD. These presentations are designed to show biologists how to collect diatom samples during routine GQA surveys. These samples will then be processed by the DARES team. Methods are very similar to those described in the TDI manual, which is itself based on a European standard method. However, there a few important differences between the DARES method and the TDI manual which need to be understood. This first presentation explains the principles of sample collection for DARES.

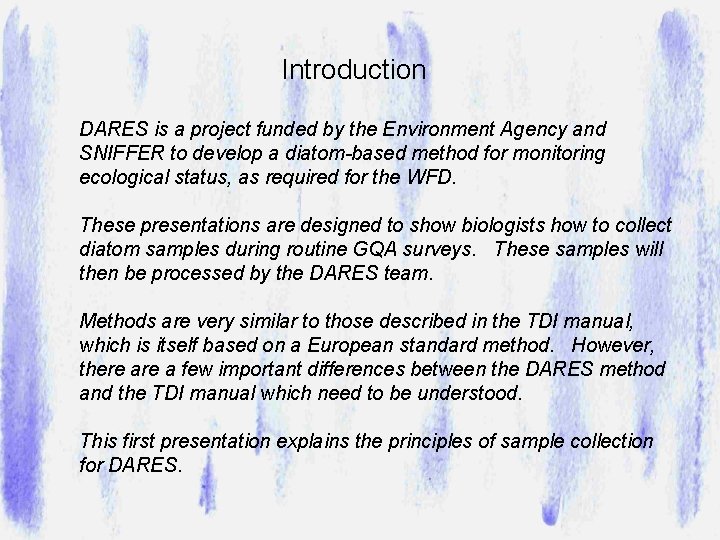
When we sample diatoms for water quality monitoring we assume that the distribution of taxa is influenced primarily by water quality variables … In this graph, we see Staurosirella (=Fragilaria) pinnata with a relatively low P optimum and Achnanthes conspicua with a much higher optimum.

However, as the following presentation will show, other factors can influence the composition of the diatom assemblage. For this reason it is important that we adhere to strict sampling protocols in order to minimise the impact that these have. The DARES protocols are based on those in the TDI Manual which are compliant with EN 13946 – the European Guidance Standard for diatom sampling.

Diatoms live in a thin, brown ‘biofilm’ which can be found on almost all surfaces in rivers This video clip shows a biofilm that has developed on a bottle found submerged in the River Avon in Hampshire.

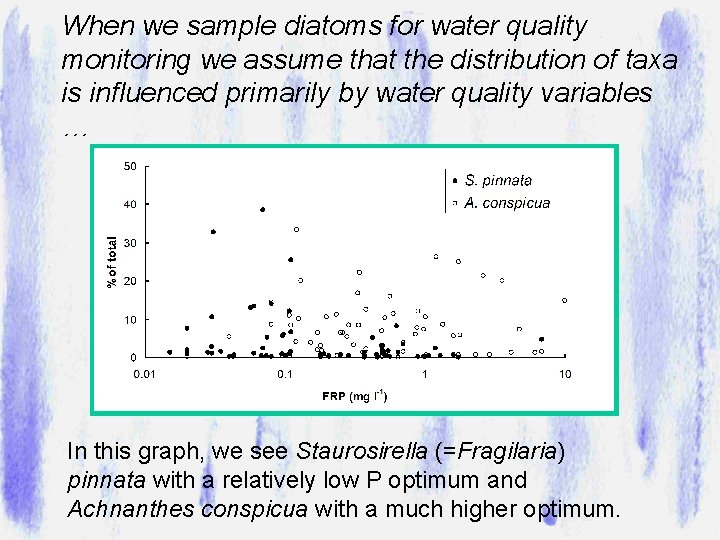
It might not look very exciting, but it is actually a complex 3 -dimensional community of which diatoms are typically the most abundant group of photosynthetic organisms exhibiting a range of ecological strategies. Polysaccharide matrix Plankton “in transit” 20 m Stalked taxa, e. g. Synedra Motile taxa, e. g. Nitzschia

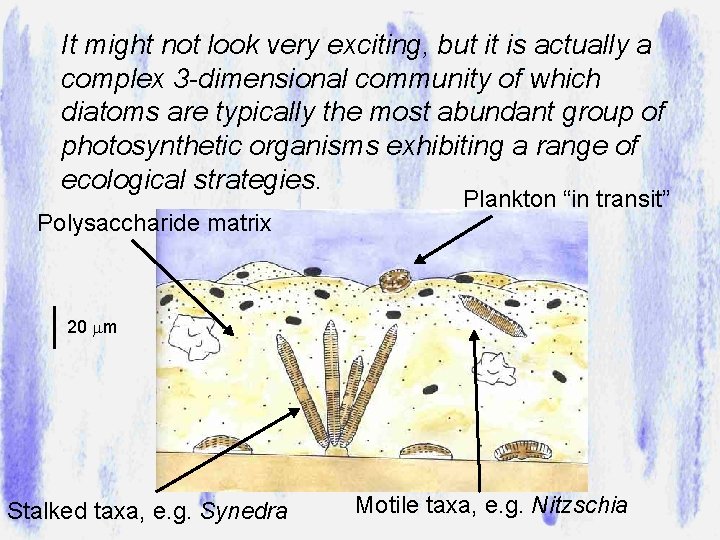
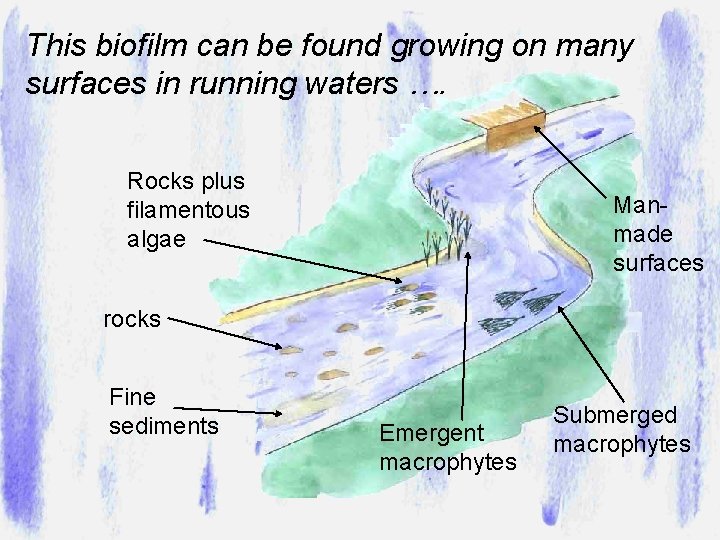
This biofilm can be found growing on many surfaces in running waters …. Rocks plus filamentous algae Manmade surfaces rocks Fine sediments Emergent macrophytes Submerged macrophytes

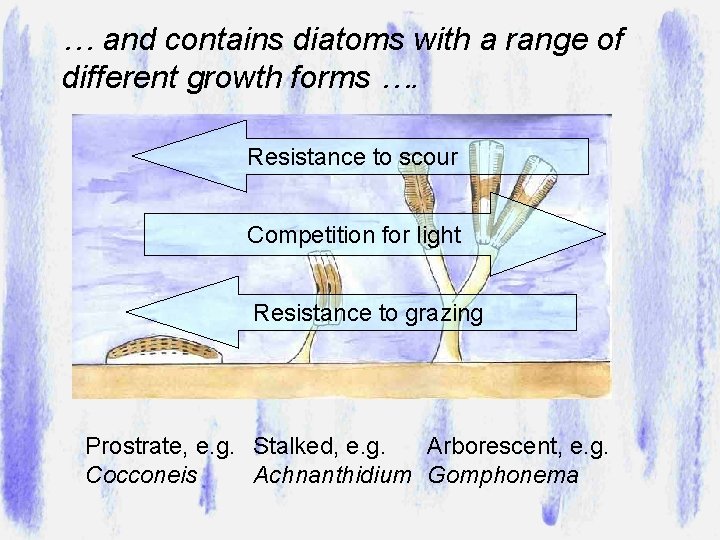
… and contains diatoms with a range of different growth forms …. Resistance to scour Competition for light Resistance to grazing Prostrate, e. g. Stalked, e. g. Arborescent, e. g. Cocconeis Achnanthidium Gomphonema

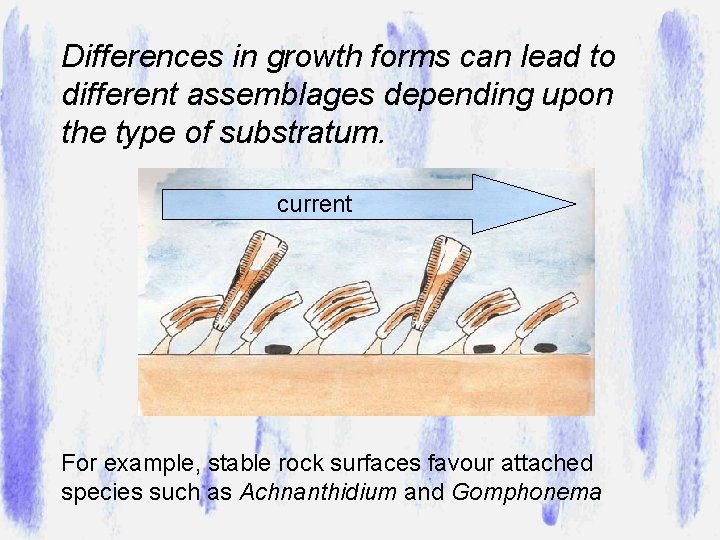
Differences in growth forms can lead to different assemblages depending upon the type of substratum. current For example, stable rock surfaces favour attached species such as Achnanthidium and Gomphonema

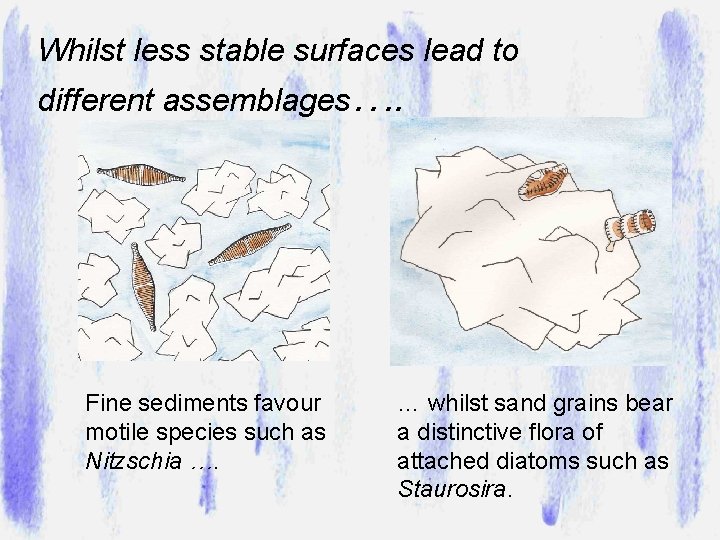
Whilst less stable surfaces lead to different assemblages…. Fine sediments favour motile species such as Nitzschia …. … whilst sand grains bear a distinctive flora of attached diatoms such as Staurosira.

Macrophytes and macroalgae also have distinctive diatom assemblages associated with them. Cladophora filaments, for example, often have distinctive epiphytes such as Rhoicosphenia (shown here) and Cocconeis. Photo: Marian Yallop

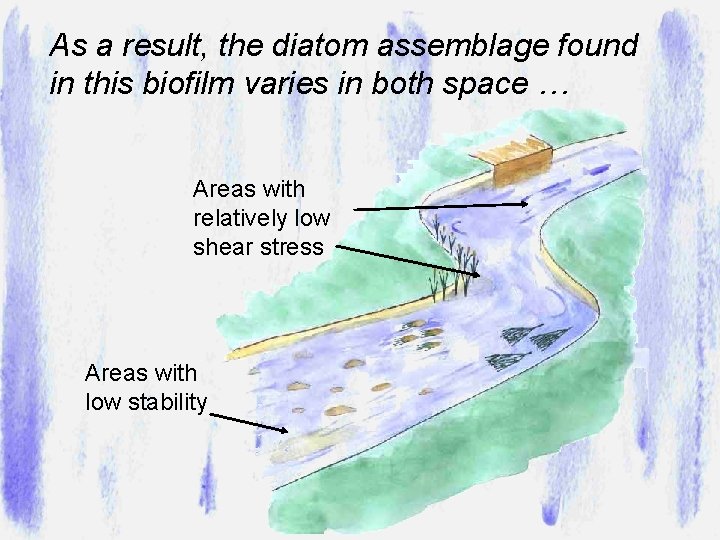
As a result, the diatom assemblage found in this biofilm varies in both space … Areas with relatively low shear stress Areas with low stability

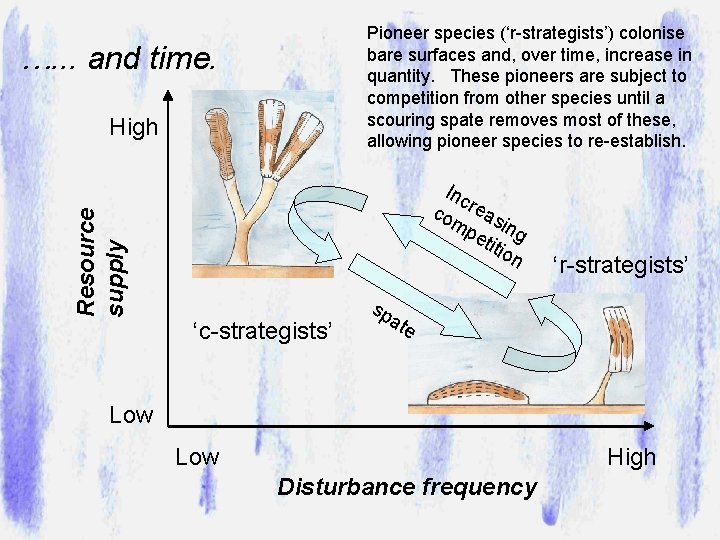
Pioneer species (‘r-strategists’) colonise bare surfaces and, over time, increase in quantity. These pioneers are subject to competition from other species until a scouring spate removes most of these, allowing pioneer species to re-establish. …. . . and time. High Resource supply Inc co reas mp in eti g tio n ‘c-strategists’ ‘r-strategists’ sp a te Low High Disturbance frequency

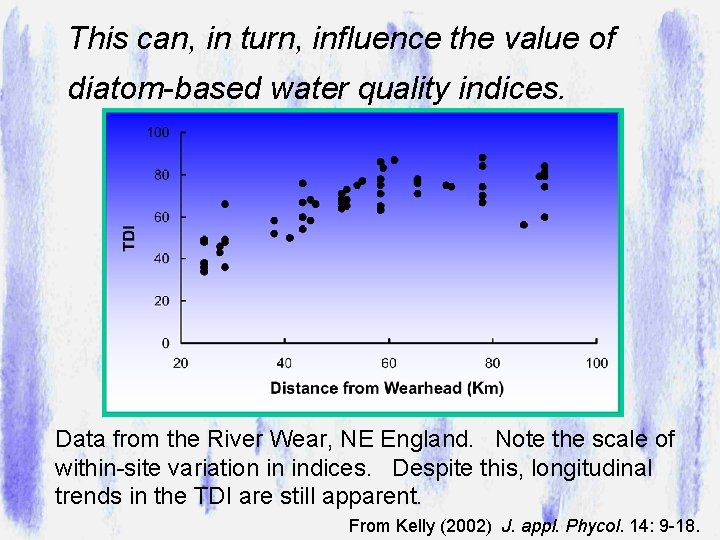
This can, in turn, influence the value of diatom-based water quality indices. Data from the River Wear, NE England. Note the scale of within-site variation in indices. Despite this, longitudinal trends in the TDI are still apparent. From Kelly (2002) J. appl. Phycol. 14: 9 -18.

Photo: Tim Colborn From theory to practice Now that we have some idea of the structure and composition of the biofilm, we need to think about how best to collect samples for monitoring ecological status. The second part of this presentation will deal with this.

The TDI method: diatoms for tactical monitoring • The TDI was developed in response to the Urban Wastewater Treatment Directive. • This required an assessment of point sources of nutrients on a case-by-case basis. • We asked the question ‘does this sewage works have a significant effect on this river? ’ • Methods needed to be robust but …. • …. allow flexibility so that biologists can “finetune” sampling to suit local conditions

UK strategy: UWWTD • The same substrate has to be sampled at all sites in a river system. • Cobbles and boulders are the preferred substrates • Artificial substrate (polypropylene rope) is also widely used.

Water Framework Directive • For this new Directive we need to compare the biota at a site with that expected in the absence of significant human impact. • This means that we need to use substrates that are ‘typical’ of the river type under investigation. • Cobbles and boulders are not characteristic of all river types, so we need to include other ‘natural’ substrates. • Assemblages found on an artificial substrata do not fulfil this criterion.

Sampling protocol • The sampling strategy outlined in the TDI manual has had to be modified for DARES in order to take account of the requirements of the WFD. • Cobbles and boulders are still the preferred substrate • However, if these are not found, then emergent macrophytes should be sampled. • If these are not found, then submerged macrophytes can be used.

Selection of sampling sites. • For DARES, samples will be collected from sites already used for GQA surveys. • Issues that were considered when selecting these sites include: – Location with respect to discharges / confluences etc. – Access from road – Location with respect to other monitoring records (chemical / macrophyte / invertebrate) • Glides, runs and riffles are all suitable for collecting diatom samples but most pools will be too slow-flowing.

However, once you have arrived at the site, you need to decide where in the survey reach to collect your samples. Aspects to consider include: • Current speed • Shade • Water depth • Selection of substrate

Factors to consider when selecting a sample site: 1. current speed • Sample from main flow of river – Not sidearms or backwaters • Avoid areas of very high current velocity for safety reasons • Areas of very slow flow (< 0. 1 m s-1) permit accumulation of loosely-attached species

Factors to consider when selecting a sample site: 2. depth • Two rules to follow: – If you can see the substrate, it is in the euphotic zone – If you can wade to it, then the water is not too deep • In addition, ensure that surfaces are permanently submerged – This will depend, to some extent, on local knowledge. – As a ‘rule of thumb’ ensure that the water depth is at least 10 cm.

Factors to consider when selecting a sample site: 3. light Photo: Martyn Kelly • Avoid heavy shade, unless this is characteristic of the system under study • Try to sample from sites with similar light regimes

Selection of substrate There are three options: Either cobbles … . . . or macrophytes Photo: Martyn Kelly Photo: Tim Colborn Emergent or submerged