Sampling diatoms from running waters 3 Sampling macrophytes








- Slides: 10

Sampling diatoms from running waters 3: Sampling macrophytes Martyn Kelly and Marian Yallop © The DARES consortium

This slide has been inserted by the Environment Agency • NOTE: Staff should always undertake a dynamic risk assessment to assess health risks at time of sampling. Where necessary a supplementary risk assessment should be undertaken to reduce any additional risks. • In general, gloves should be worn when collecting diatom samples.

• Because cobbles will not be found at all river types sampled for the DARES project, we need to consider alternative sampling strategies. • This presentation demonstrates how to sample emergent and submerged macrophytes. Photo: Tim Colborn Introduction

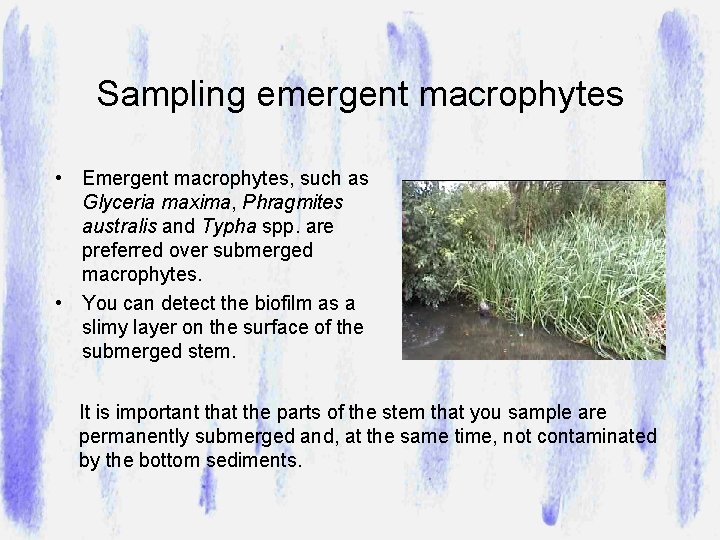
Sampling emergent macrophytes • Emergent macrophytes, such as Glyceria maxima, Phragmites australis and Typha spp. are preferred over submerged macrophytes. • You can detect the biofilm as a slimy layer on the surface of the submerged stem. It is important that the parts of the stem that you sample are permanently submerged and, at the same time, not contaminated by the bottom sediments.

Sampling emergent macrophytes (cont. ) To collect a sample, first remove the stem and leaves above water level, then place a sample bottle upside down over the stem. Finally, cut off the stem below the mouth of the bottle and remove the sample bottle plus stem. Repeat this for at least four more stems. This method reduces the risk of dislodging looselyattached diatoms from the stem.

Sampling emergent macrophytes (cont. ) Once you have collected the stems, you can remove the biofilm by scrubbing with a toothbrush. Remember to clean the toothbrush first, as shown in the previous presentation. When you have cleaned all the stems, decant the brown suspension into a labelled sample bottle.

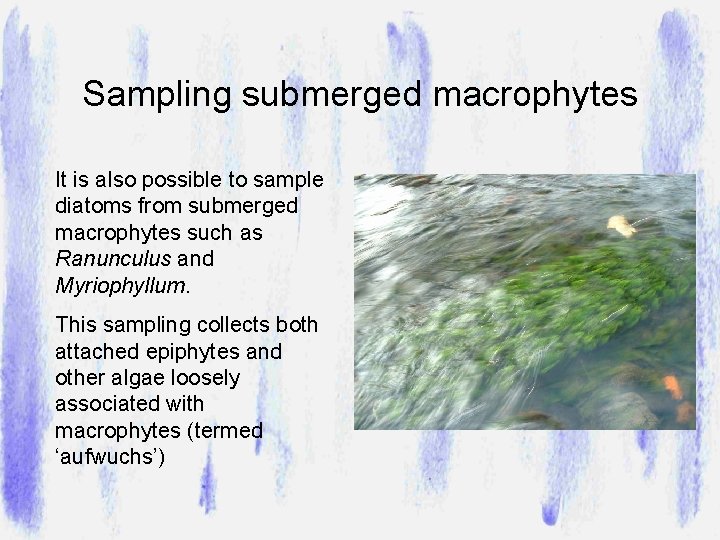
Sampling submerged macrophytes It is also possible to sample diatoms from submerged macrophytes such as Ranunculus and Myriophyllum. This sampling collects both attached epiphytes and other algae loosely associated with macrophytes (termed ‘aufwuchs’)

Sampling submerged macrophytes (cont. ) Collect about five healthy stems of the macrophyte from within the main flow of the river. Take care not to include parts of the stem contaminated with bottom sediments.

Sampling submerged macrophytes (cont. ) Transfer the stems to a plastic bag along with a little stream water, then shake the bag vigorously for about 10 seconds. If you prefer, you can use a bottle, instead of a bag. The grimaces are strictly optional

Sampling submerged macrophytes (cont. ) The result of this should be a brown suspension. We have poured it into a tray to illustrate this, but you can pour it straight into a sample bottle.