Rotational Spectra of Adducts of Formaldehyde with Freons



- Slides: 27

Rotational Spectra of Adducts of Formaldehyde with Freons Qian Gou, 1 Gang Feng, 1 Luca Evangelisti, 1 Montserrat Vallejo-López, 2 Alberto Lesarri, 2 Walther Caminati, 1 Emilio J. Cocinero 3 1 Dipartimento di Chimica “G. Ciamician” dell’Università, Via Selmi 2, I-40126 Bologna, Italy 2 Departamento de Química Física y Química Inorganica, Facultad de Ciencias, Universidad de Valladolid, E- 47011 Valladolid, Spain 3 Departamento de Quimica Fisica, Facultad de Ciencia y Tecnologia, Universidad del Pais Vasco, E-48080 Bilbao, Spain. 2013. 06. 18. Columbus

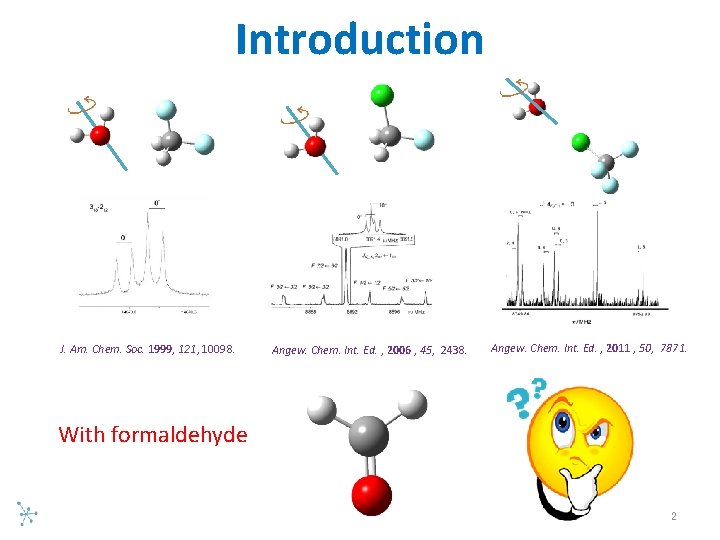
Introduction J. Am. Chem. Soc. 1999, 121, 10098. Angew. Chem. Int. Ed. , 2006 , 45, 2438. Angew. Chem. Int. Ed. , 2011 , 50, 7871. With formaldehyde 2

I. Difluoromethane − Formaldehyde CH 2 F 2 − H 2 CO Phys. Chem. Phys. , 2013, 15, 6714. 3

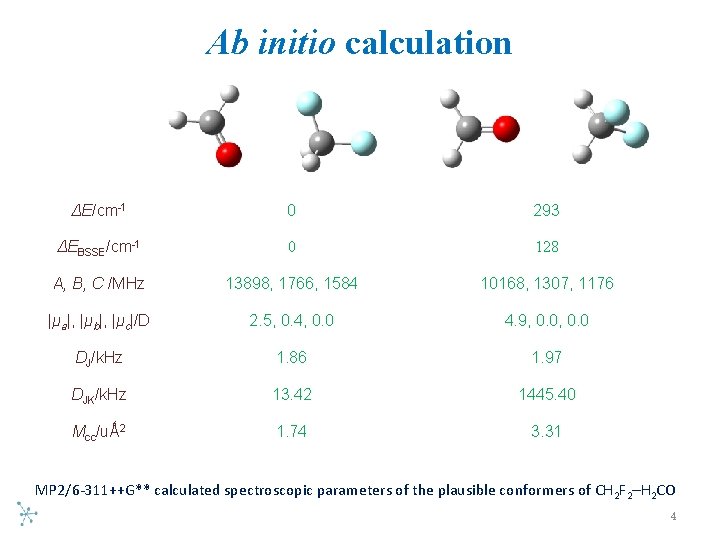
Ab initio calculation ΔE/cm-1 0 293 ΔEBSSE/cm-1 0 128 A, B, C /MHz 13898, 1766, 1584 10168, 1307, 1176 |μa|, |μb|, |μc|/D 2. 5, 0. 4, 0. 0 4. 9, 0. 0 DJ/k. Hz 1. 86 1. 97 DJK/k. Hz 13. 42 1445. 40 Mcc/uǺ 2 1. 74 3. 31 MP 2/6 311++G** calculated spectroscopic parameters of the plausible conformers of CH 2 F 2–H 2 CO 4

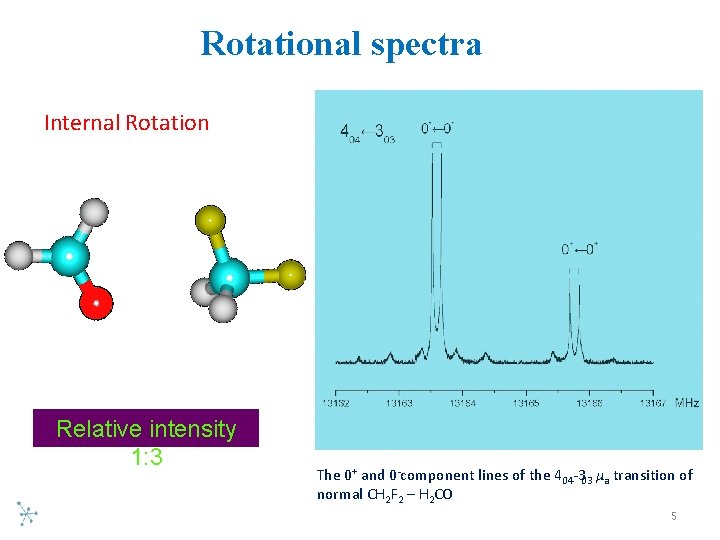
Rotational spectra Internal Rotation Relative intensity 1: 3 The 0+ and 0 component lines of the 404 303 μa transition of normal CH 2 F 2 – H 2 CO 5

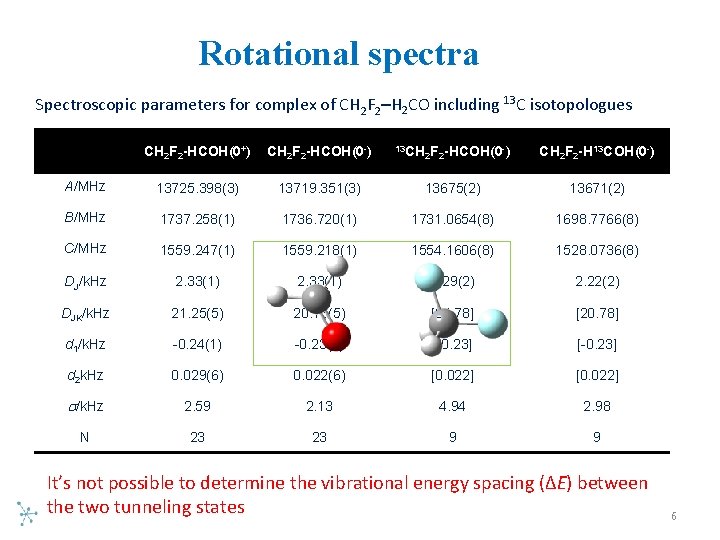
Rotational spectra Spectroscopic parameters for complex of CH 2 F 2–H 2 CO including 13 C isotopologues CH 2 F 2 -HCOH(0+) CH 2 F 2 -HCOH(0 -) 13 CH 2 F 2 -HCOH(0 A/MHz 13725. 398(3) 13719. 351(3) 13675(2) 13671(2) B/MHz 1737. 258(1) 1736. 720(1) 1731. 0654(8) 1698. 7766(8) C/MHz 1559. 247(1) 1559. 218(1) 1554. 1606(8) 1528. 0736(8) DJ/k. Hz 2. 33(1) 2. 29(2) 2. 22(2) DJK/k. Hz 21. 25(5) 20. 78(5) [20. 78] d 1/k. Hz -0. 24(1) -0. 23(1) [-0. 23] d 2 k. Hz 0. 029(6) 0. 022(6) [0. 022] /k. Hz 2. 59 2. 13 4. 94 2. 98 N 23 23 9 9 -) CH 2 F 2 -H 13 COH(0 -) It’s not possible to determine the vibrational energy spacing (∆E) between the two tunneling states 6

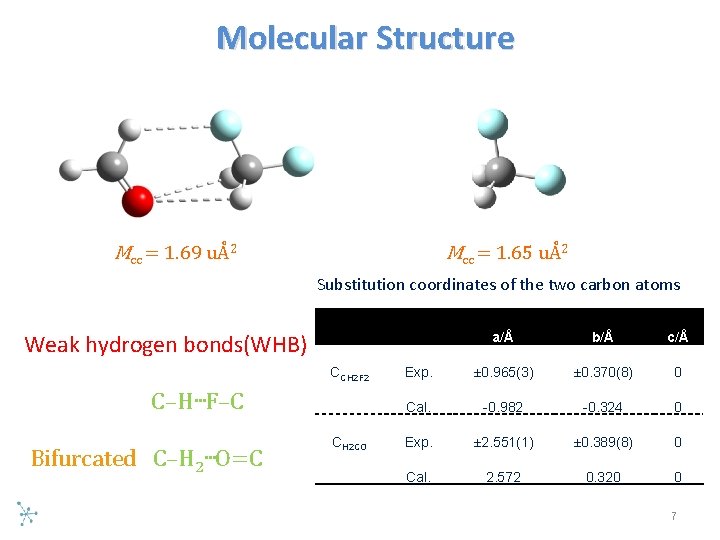
Molecular Structure Mcc = 1. 69 uÅ2 Mcc = 1. 65 uÅ2 Substitution coordinates of the two carbon atoms Weak hydrogen bonds(WHB) CCH 2 F 2 C–H…F–C Bifurcated C–H 2 …O=C CH 2 CO a/Å b/Å c/Å Exp. ± 0. 965(3) ± 0. 370(8) 0 Cal. -0. 982 -0. 324 0 Exp. ± 2. 551(1) ± 0. 389(8) 0 Cal. 2. 572 0. 320 0 7

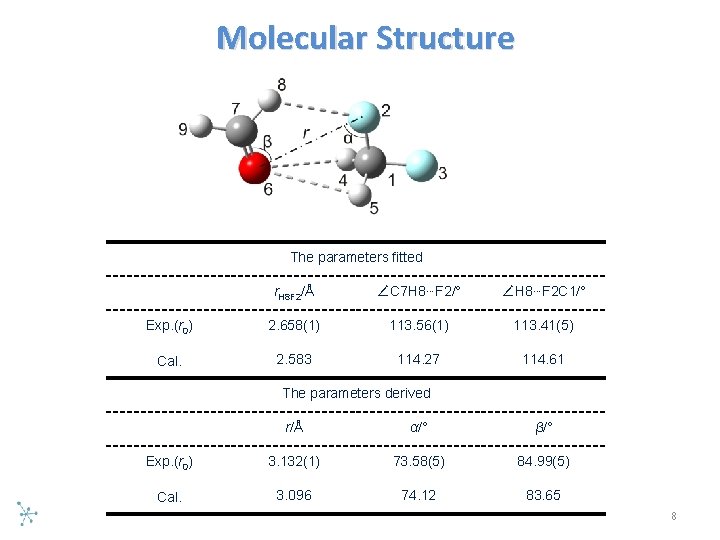
Molecular Structure The parameters fitted r. H 8 F 2/Å ∠C 7 H 8…F 2/° ∠H 8…F 2 C 1/° Exp. (r 0) 2. 658(1) 113. 56(1) 113. 41(5) Cal. 2. 583 114. 27 114. 61 The parameters derived r/Å α/° β/° Exp. (r 0) 3. 132(1) 73. 58(5) 84. 99(5) Cal. 3. 096 74. 12 83. 65 8

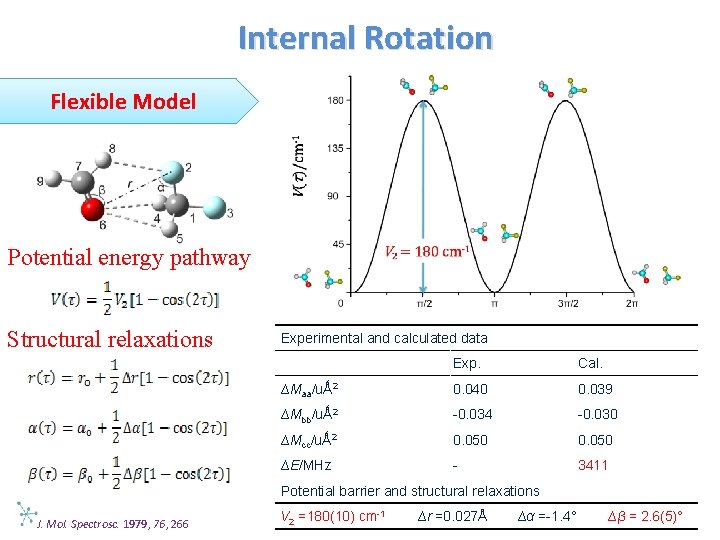
Internal Rotation Flexible Model Potential energy pathway Structural relaxations Experimental and calculated data Exp. Cal. ∆Maa/uǺ 2 0. 040 0. 039 ∆Mbb/uǺ 2 -0. 034 -0. 030 ∆Mcc/uǺ 2 0. 050 ∆E/MHz - 3411 Potential barrier and structural relaxations J. Mol. Spectrosc. 1979, 76, 266 V 2 =180(10) cm-1 ∆r =0. 027Å ∆α =-1. 4° ∆β = 2. 6(5)°

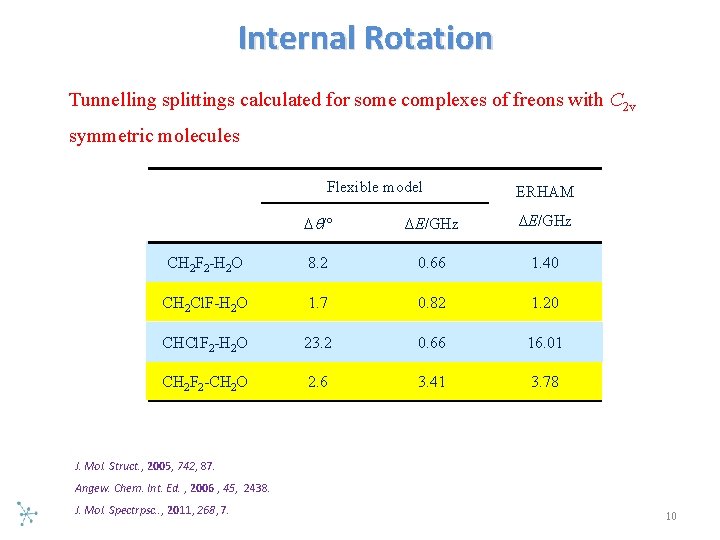
Internal Rotation Tunnelling splittings calculated for some complexes of freons with C 2 v symmetric molecules Flexible model ERHAM /° ΔE/GHz CH 2 F 2 -H 2 O 8. 2 0. 66 1. 40 CH 2 Cl. F-H 2 O 1. 7 0. 82 1. 20 CHCl. F 2 -H 2 O 23. 2 0. 66 16. 01 CH 2 F 2 -CH 2 O 2. 6 3. 41 3. 78 J. Mol. Struct. , 2005, 742, 87. Angew. Chem. Int. Ed. , 2006 , 45, 2438. J. Mol. Spectrpsc. . , 2011, 268, 7. 10

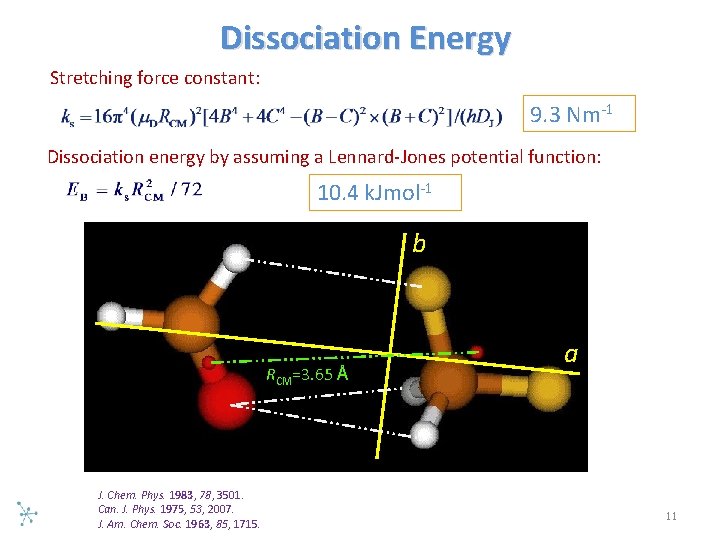
Dissociation Energy Stretching force constant: 9. 3 Nm 1 Dissociation energy by assuming a Lennard Jones potential function: 10. 4 k. Jmol 1 b RCM=3. 65 Å J. Chem. Phys. 1983, 78, 3501. Can. J. Phys. 1975, 53, 2007. J. Am. Chem. Soc. 1963, 85, 1715. a 11

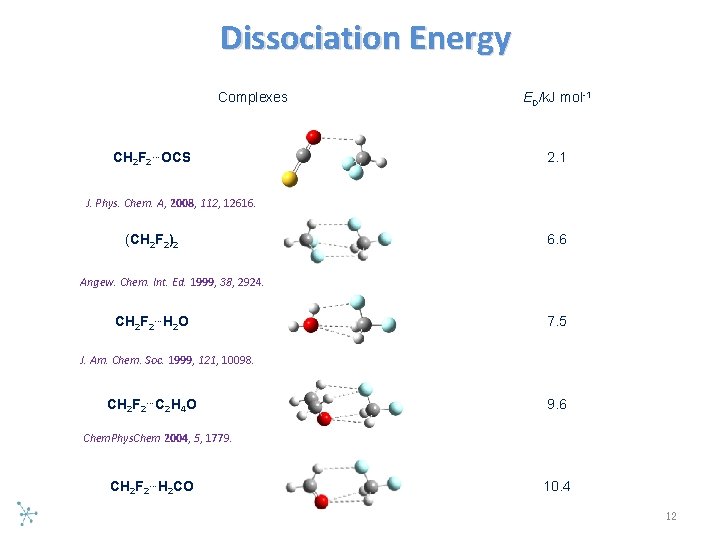
Dissociation Energy Complexes CH 2 F 2…OCS ED/k. J mol-1 2. 1 J. Phys. Chem. A, 2008, 112, 12616. (CH 2 F 2)2 6. 6 Angew. Chem. Int. Ed. 1999, 38, 2924. CH 2 F 2…H 2 O 7. 5 J. Am. Chem. Soc. 1999, 121, 10098. CH 2 F 2…C 2 H 4 O 9. 6 Chem. Phys. Chem 2004, 5, 1779. CH 2 F 2…H 2 CO 10. 4 12

II. Chlorofluoromethane− Formaldehyde CH 2 FCl − H 2 CO 13

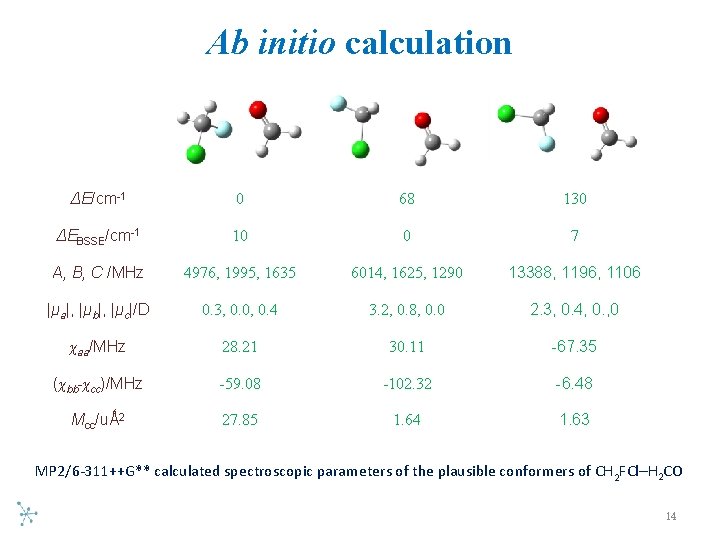
Ab initio calculation ΔE/cm-1 0 68 130 ΔEBSSE/cm-1 10 0 7 A, B, C /MHz 4976, 1995, 1635 6014, 1625, 1290 13388, 1196, 1106 |μa|, |μb|, |μc|/D 0. 3, 0. 0, 0. 4 3. 2, 0. 8, 0. 0 2. 3, 0. 4, 0. , 0 χaa/MHz 28. 21 30. 11 -67. 35 (χbb-χcc)/MHz -59. 08 -102. 32 -6. 48 Mcc/uǺ 2 27. 85 1. 64 1. 63 MP 2/6 311++G** calculated spectroscopic parameters of the plausible conformers of CH 2 FCl–H 2 CO 14

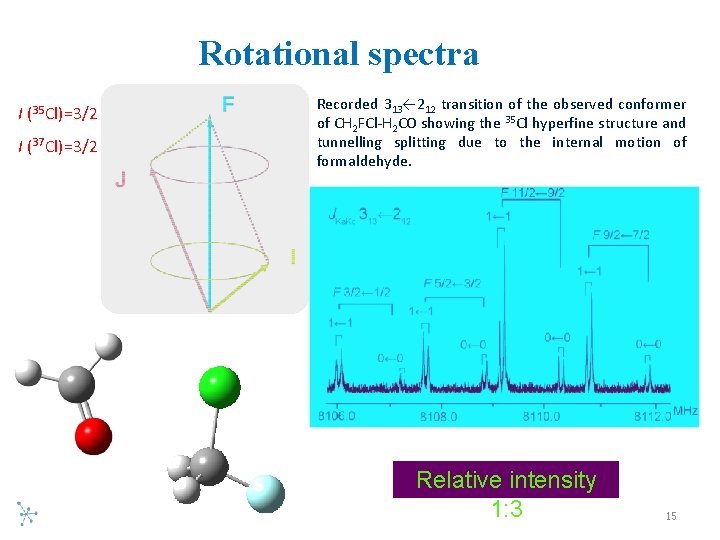
Rotational spectra I (35 Cl)=3/2 I (37 Cl)=3/2 Recorded 313← 212 transition of the observed conformer of CH 2 FCl H 2 CO showing the 35 Cl hyperfine structure and tunnelling splitting due to the internal motion of formaldehyde. Relative intensity 1: 3 15

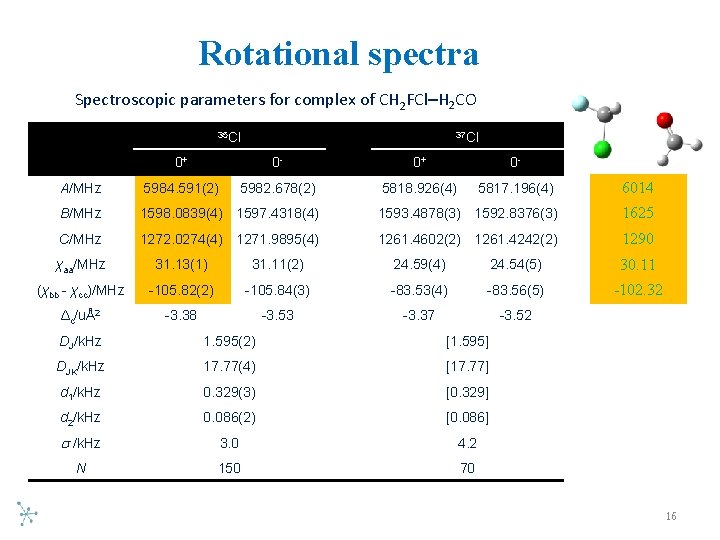
Rotational spectra Spectroscopic parameters for complex of CH 2 FCl–H 2 CO 35 Cl 37 Cl 0+ 0 - A/MHz 5984. 591(2) 5982. 678(2) 5818. 926(4) 5817. 196(4) 6014 B/MHz 1598. 0839(4) 1597. 4318(4) 1593. 4878(3) 1592. 8376(3) 1625 C/MHz 1272. 0274(4) 1271. 9895(4) 1261. 4602(2) 1261. 4242(2) 1290 χaa/MHz 31. 13(1) 31. 11(2) 24. 59(4) 24. 54(5) 30. 11 (χbb - χcc)/MHz -105. 82(2) -105. 84(3) -83. 53(4) -83. 56(5) -102. 32 Δc/uÅ2 -3. 38 -3. 53 -3. 37 -3. 52 DJ/k. Hz 1. 595(2) [1. 595] DJK/k. Hz 17. 77(4) [17. 77] d 1/k. Hz 0. 329(3) [0. 329] d 2/k. Hz 0. 086(2) [0. 086] /k. Hz 3. 0 4. 2 N 150 70 16

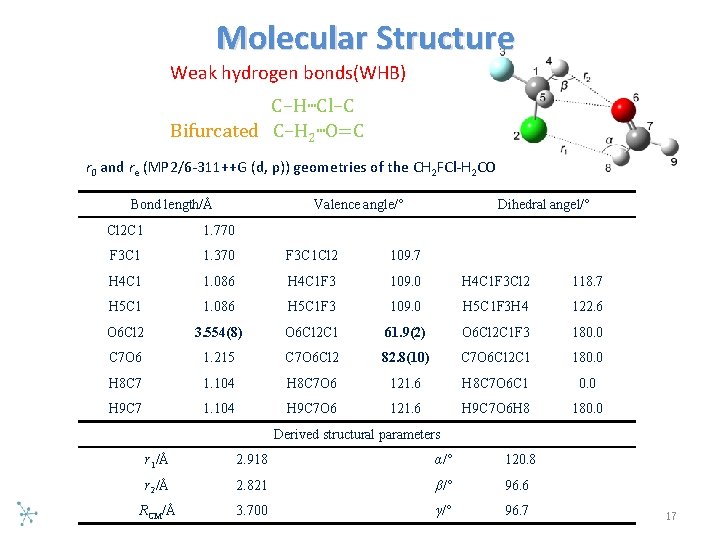
Molecular Structure Weak hydrogen bonds(WHB) C–H…Cl–C Bifurcated C–H 2…O=C r 0 and re (MP 2/6 311++G (d, p)) geometries of the CH 2 FCl H 2 CO Bond length/Å Valence angle/° Dihedral angel/° Cl 2 C 1 1. 770 F 3 C 1 1. 370 F 3 C 1 Cl 2 109. 7 H 4 C 1 1. 086 H 4 C 1 F 3 109. 0 H 4 C 1 F 3 Cl 2 118. 7 H 5 C 1 1. 086 H 5 C 1 F 3 109. 0 H 5 C 1 F 3 H 4 122. 6 O 6 Cl 2 3. 554(8) O 6 Cl 2 C 1 61. 9(2) O 6 Cl 2 C 1 F 3 180. 0 C 7 O 6 1. 215 C 7 O 6 Cl 2 82. 8(10) C 7 O 6 Cl 2 C 1 180. 0 H 8 C 7 1. 104 H 8 C 7 O 6 121. 6 H 8 C 7 O 6 C 1 0. 0 H 9 C 7 1. 104 H 9 C 7 O 6 121. 6 H 9 C 7 O 6 H 8 180. 0 Derived structural parameters r 1/Å 2. 918 α/° 120. 8 r 2/Å 2. 821 β/° 96. 6 RCM/Å 3. 700 γ/° 96. 7 17

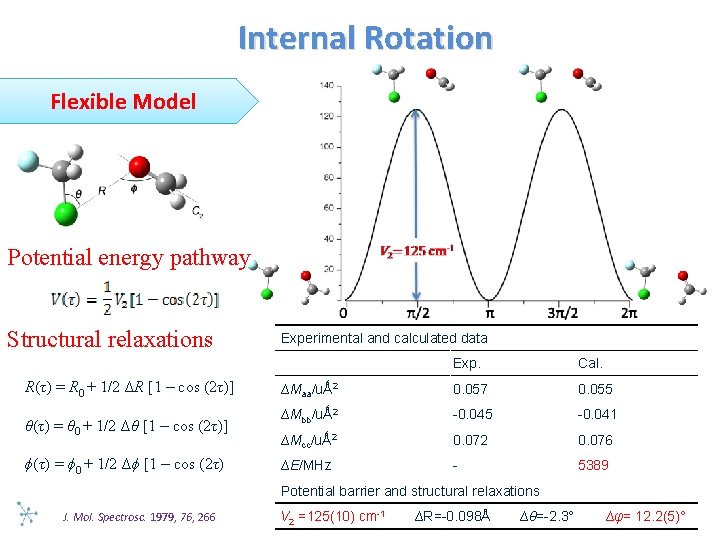
Internal Rotation Flexible Model Potential energy pathway Structural relaxations R(τ) = R 0 + 1/2 ΔR [1 – cos (2τ)] θ(τ) = θ 0 + 1/2 Δθ [1 – cos (2τ)] ϕ(τ) = ϕ 0 + 1/2 Δϕ [1 – cos (2τ) Experimental and calculated data Exp. Cal. ∆Maa/uǺ 2 0. 057 0. 055 ∆Mbb/uǺ 2 -0. 045 -0. 041 ∆Mcc/uǺ 2 0. 076 ∆E/MHz - 5389 Potential barrier and structural relaxations J. Mol. Spectrosc. 1979, 76, 266 V 2 =125(10) cm-1 ∆R=-0. 098Å ∆θ=-2. 3° ∆φ= 12. 2(5)°

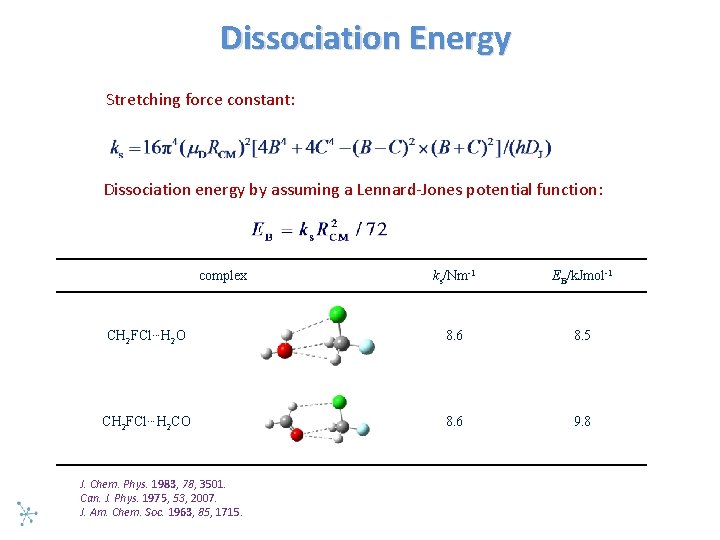
Dissociation Energy Stretching force constant: Dissociation energy by assuming a Lennard Jones potential function: complex ks/Nm-1 EB/k. Jmol-1 CH 2 FCl…H 2 O 8. 6 8. 5 CH 2 FCl…H 2 CO 8. 6 9. 8 J. Chem. Phys. 1983, 78, 3501. Can. J. Phys. 1975, 53, 2007. J. Am. Chem. Soc. 1963, 85, 1715.

III. Chlorotrifluoromethane − Formaldehyde CF 3 Cl − H 2 CO 20

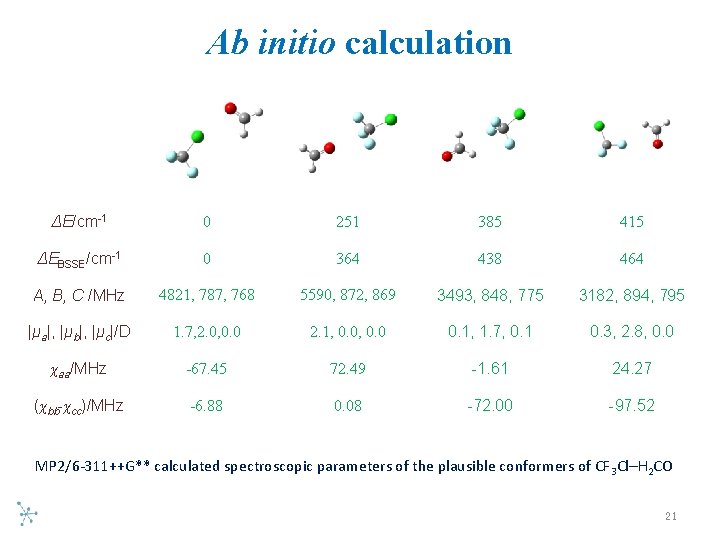
Ab initio calculation ΔE/cm-1 0 251 385 415 ΔEBSSE/cm-1 0 364 438 464 A, B, C /MHz 4821, 787, 768 5590, 872, 869 3493, 848, 775 3182, 894, 795 |μa|, |μb|, |μc|/D 1. 7, 2. 0, 0. 0 2. 1, 0. 0 0. 1, 1. 7, 0. 1 0. 3, 2. 8, 0. 0 χaa/MHz -67. 45 72. 49 -1. 61 24. 27 (χbb-χcc)/MHz -6. 88 0. 08 -72. 00 -97. 52 MP 2/6 311++G** calculated spectroscopic parameters of the plausible conformers of CF 3 Cl–H 2 CO 21

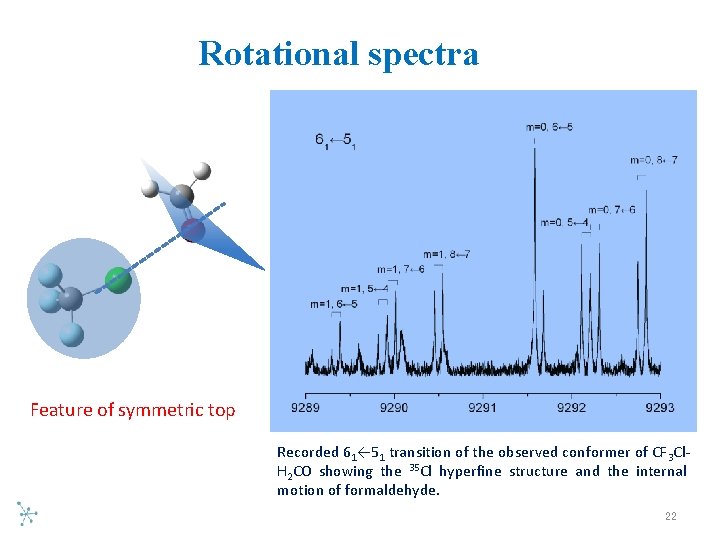
Rotational spectra Feature of symmetric top Recorded 61← 51 transition of the observed conformer of CF 3 Cl H 2 CO showing the 35 Cl hyperfine structure and the internal motion of formaldehyde. 22

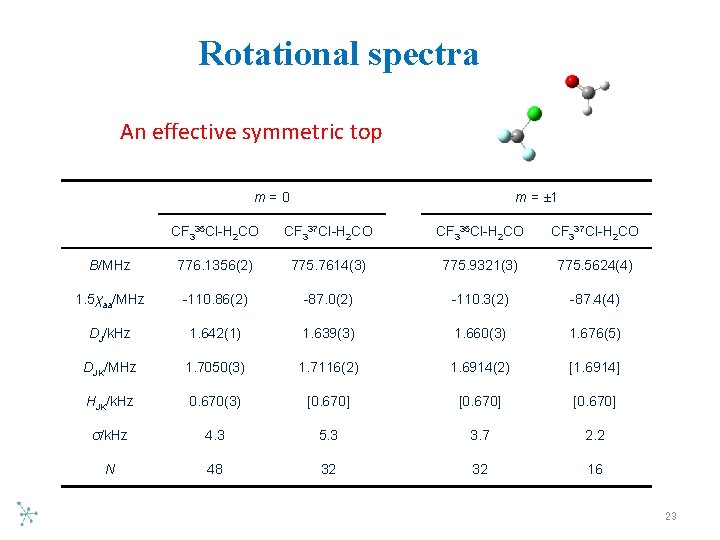
Rotational spectra An effective symmetric top m=0 m = ± 1 CF 335 Cl-H 2 CO CF 337 Cl-H 2 CO B/MHz 776. 1356(2) 775. 7614(3) 775. 9321(3) 775. 5624(4) 1. 5χaa/MHz -110. 86(2) -87. 0(2) -110. 3(2) -87. 4(4) DJ/k. Hz 1. 642(1) 1. 639(3) 1. 660(3) 1. 676(5) DJK/MHz 1. 7050(3) 1. 7116(2) 1. 6914(2) [1. 6914] HJK/k. Hz 0. 670(3) [0. 670] σ/k. Hz 4. 3 5. 3 3. 7 2. 2 N 48 32 32 16 23

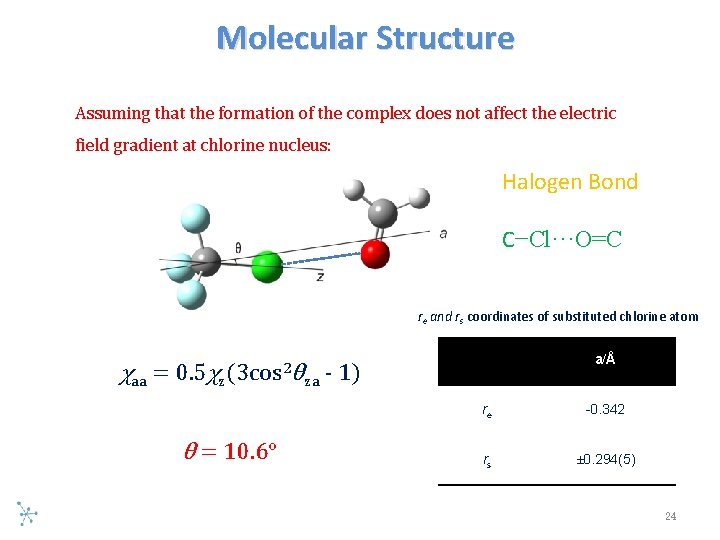
Molecular Structure Assuming that the formation of the complex does not affect the electric field gradient at chlorine nucleus: Halogen Bond C−Cl···O=C re and rs coordinates of substituted chlorine atom χaa = 0. 5χz (3 cos 2 θ = 10. 6° a/Å θza - 1) re -0. 342 rs ± 0. 294(5) 24

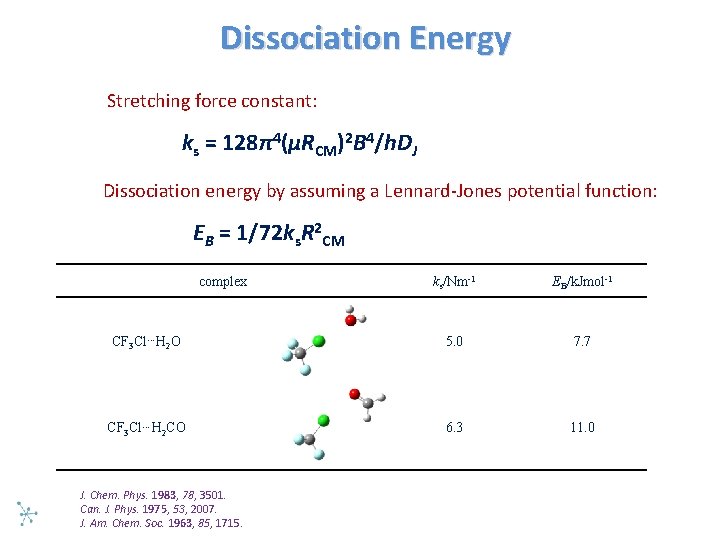
Dissociation Energy Stretching force constant: ks = 128π4(μRCM)2 B 4/h. DJ Dissociation energy by assuming a Lennard Jones potential function: EB = 1/72 ks. R 2 CM complex ks/Nm-1 EB/k. Jmol-1 CF 3 Cl…H 2 O 5. 0 7. 7 CF 3 Cl…H 2 CO 6. 3 11. 0 J. Chem. Phys. 1983, 78, 3501. Can. J. Phys. 1975, 53, 2007. J. Am. Chem. Soc. 1963, 85, 1715.

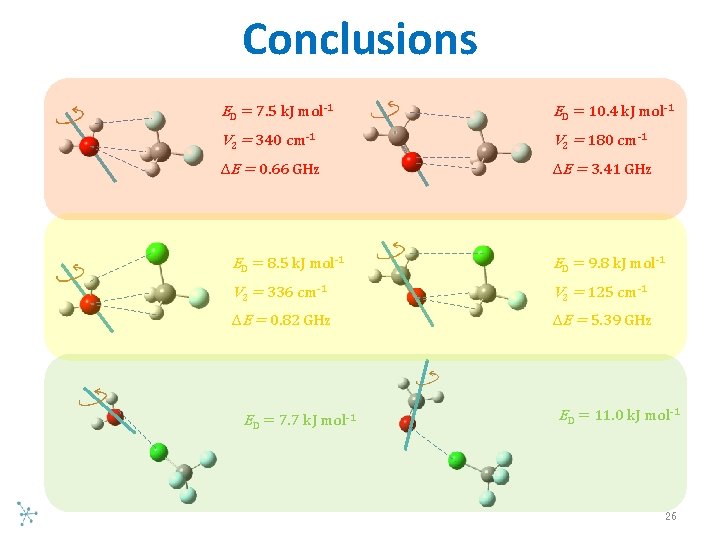
Conclusions ED = 7. 5 k. J mol-1 ED = 10. 4 k. J mol-1 V 2 = 340 cm-1 V 2 = 180 cm-1 ∆E = 0. 66 GHz ∆E = 3. 41 GHz ED = 8. 5 k. J mol-1 ED = 9. 8 k. J mol-1 V 2 = 336 cm-1 V 2 = 125 cm-1 ∆E = 0. 82 GHz ∆E = 5. 39 GHz ED = 7. 7 k. J mol-1 ED = 11. 0 k. J mol-1 26

Acknowledgement Prof. W. Caminati Dr. L. B. Favero Prof. S. Melandri Dr. A. Maris Dr. L. Evangelisti Thanks for attention! Dr. F. Gang Ms. C. Calabrese Mr. L. Spada 27
 Temporalis
Temporalis Second condition of equilibrium
Second condition of equilibrium Rotational equilibrium and rotational dynamics
Rotational equilibrium and rotational dynamics Formaldehyde
Formaldehyde Chlorahydrate
Chlorahydrate D-p orbital mixing
D-p orbital mixing Formaldehyde ppe
Formaldehyde ppe Formaldehyde is also called
Formaldehyde is also called Chocl lewis structure
Chocl lewis structure Condensed structural formula of formaldehyde
Condensed structural formula of formaldehyde Melamine formaldehyde advantages and disadvantages
Melamine formaldehyde advantages and disadvantages Formaldehyde ppt
Formaldehyde ppt Where is formaldehyde found
Where is formaldehyde found Dimethyl ether dipole dipole
Dimethyl ether dipole dipole Phenol formaldehyde uses
Phenol formaldehyde uses What are the bond angles for octahedral structure
What are the bond angles for octahedral structure Formaldehyde polar
Formaldehyde polar The empirical formula of styrene is ch
The empirical formula of styrene is ch Where is formaldehyde found
Where is formaldehyde found Electro technic products inc
Electro technic products inc Electronic spectra of coordination compounds
Electronic spectra of coordination compounds Electronic spectra of polyatomic molecules
Electronic spectra of polyatomic molecules Atomic emission spectra and the quantum mechanical model
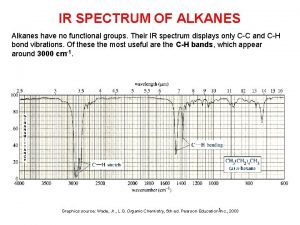
Atomic emission spectra and the quantum mechanical model Alkanes ir spectrum
Alkanes ir spectrum Spectra shropshire
Spectra shropshire Ir spectroscopy table
Ir spectroscopy table Emission line spectra
Emission line spectra Supernova spectra
Supernova spectra