Endogenous DNA Adducts Key Events in the MOA



![Formation of [13 C 2]-EG in Hepatocyte DNA by Vinyl Chloride Formation of [13 C 2]-EG in Hepatocyte DNA by Vinyl Chloride](https://slidetodoc.com/presentation_image_h/0779f62810753dca33a2581321d38b86/image-5.jpg)

![in vivo data in rats exposed to [13 CD 2]-formaldehyde • • • Rats in vivo data in rats exposed to [13 CD 2]-formaldehyde • • • Rats](https://slidetodoc.com/presentation_image_h/0779f62810753dca33a2581321d38b86/image-13.jpg)
![Formaldehyde-induced monoadducts in tissues of rats exposed to 10 ppm [13 CD 2]-formaldehyde for Formaldehyde-induced monoadducts in tissues of rats exposed to 10 ppm [13 CD 2]-formaldehyde for](https://slidetodoc.com/presentation_image_h/0779f62810753dca33a2581321d38b86/image-16.jpg)
![Application to Risk Assessment • Because no [13 CD 2]-N 2 -Med. G adducts Application to Risk Assessment • Because no [13 CD 2]-N 2 -Med. G adducts](https://slidetodoc.com/presentation_image_h/0779f62810753dca33a2581321d38b86/image-24.jpg)



![Endogenous AP Sites in Rat Tissues and Human Liver [Nakamura and Swenberg, 1999] Endogenous AP Sites in Rat Tissues and Human Liver [Nakamura and Swenberg, 1999]](https://slidetodoc.com/presentation_image_h/0779f62810753dca33a2581321d38b86/image-32.jpg)


- Slides: 47

Endogenous DNA Adducts: Key Events in the MOA for Low Dose Risk Assessment James A. Swenberg, D. V. M. , Ph. D. University of North Carolina Chapel Hill, NC 27599

DNA Adducts: Biomarkers of Exposure • DNA adducts are biomarkers of exposure, not effect. • DNA adducts are not mutations, are repairable and have vastly different abilities to cause mutations • The molecular dose of DNA or protein adducts integrates our knowledge of metabolism, detoxication and DNA repair. Thus, it provides important information on species differences. • DNA adducts are expected to be linear at low doses. • An exception to this is when identical adducts are formed endogenously and methods used cannot discriminate between endogenous and exogenous adducts. • Many forms of endogenous DNA adducts have been quantified. These include direct oxidative adducts, exocyclic adducts, AP sites, ethylene oxide, formaldehyde, deamination products, etc. totaling >40, 000 per cell.

Vinyl Chloride • Vinyl chloride is a known human and animal carcinogen that induces hepatic angiosarcomas. • Carcinogenic response is associated with high exposure (>50 ppm). • To date, 197 VC workers have developed hepatic angiosarcomas. All of them started work prior to lowering the occupational exposure 1 ppm. • Issues related to low dose extrapolation are important, as vinyl chloride is present in many Superfund sites and some public drinking water in ppb amounts. • Identical endogenous adducts are present in rodent and human tissues.

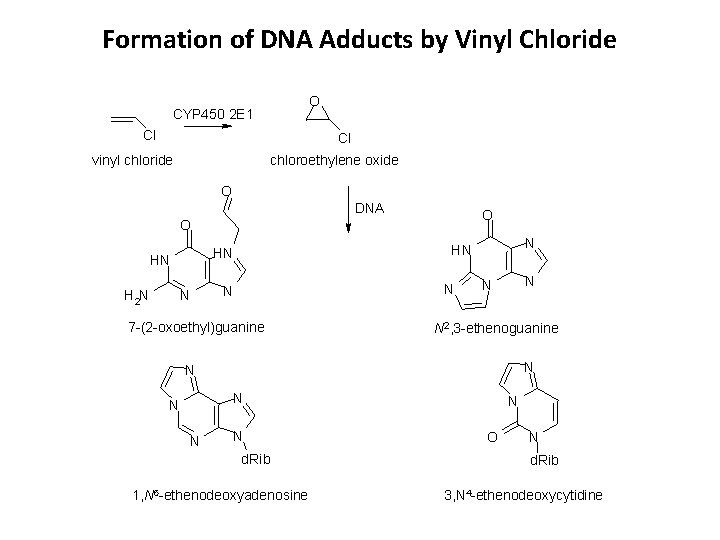
Formation of DNA Adducts by Vinyl Chloride O CYP 450 2 E 1 Cl Cl vinyl chloride chloroethylene oxide O DNA O O HN H 2 N N N HN HN N N 7 -(2 -oxoethyl)guanine N N N 2, 3 -ethenoguanine N N N d. Rib 1, N 6 -ethenodeoxyadenosine N O N d. Rib 3, N 4 -ethenodeoxycytidine
![Formation of 13 C 2EG in Hepatocyte DNA by Vinyl Chloride Formation of [13 C 2]-EG in Hepatocyte DNA by Vinyl Chloride](https://slidetodoc.com/presentation_image_h/0779f62810753dca33a2581321d38b86/image-5.jpg)
Formation of [13 C 2]-EG in Hepatocyte DNA by Vinyl Chloride

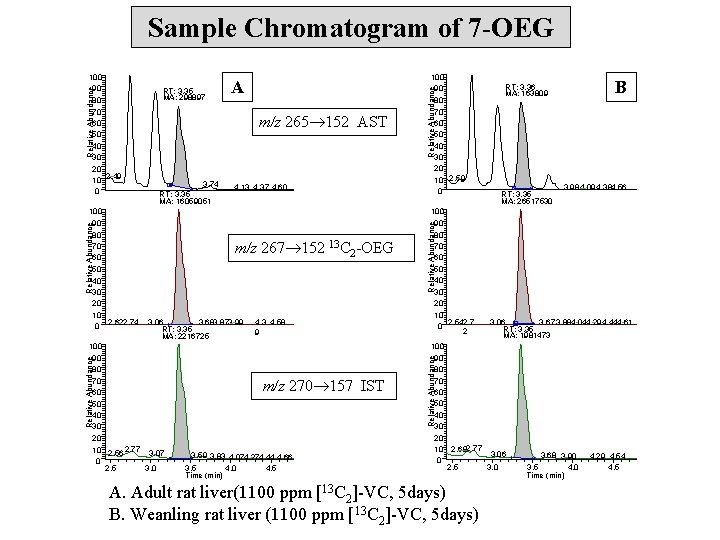
Sample Chromatogram of 7 -OEG 4. 13 4. 37 4. 60 m/z 267 152 13 C 2 -OEG 3. 06 3. 683. 873. 99 RT: 3. 35 MA: 2216725 4. 3 4. 58 9 m/z 270 157 IST 3. 59 3. 83 4. 074. 274. 44 4. 66 3. 5 4. 0 4. 5 Time (min) Relative Abundance 100 90 80 70 60 50 40 30 20 10 2. 542. 7 0 2 B RT: 3. 36 MA: 163809 RT: 3. 35 MA: 26517530 3. 984. 09 4. 384. 56 Relative Abundance 3. 74 RT: 3. 35 MA: 16059051 100 90 80 70 60 50 40 30 20 10 2. 56 2. 77 3. 07 0 2. 5 3. 0 Relative Abundance A m/z 265 152 AST Relative Abundance 100 90 80 70 60 50 40 30 20 10 0 2. 622. 74 RT: 3. 35 MA: 298897 100 90 80 70 60 50 40 30 20 10 2. 59 0 3. 06 3. 67 3. 884. 044. 29 4. 444. 61 RT: 3. 35 MA: 1981473 100 90 80 70 60 50 40 30 20 10 2. 682. 77 3. 06 0 2. 5 3. 0 Relative Abundance 100 90 80 70 60 50 40 30 20 2. 49 10 0 A. Adult rat liver(1100 ppm [13 C 2]-VC, 5 days) B. Weanling rat liver (1100 ppm [13 C 2]-VC, 5 days) 3. 68 3. 90 3. 5 4. 0 Time (min) 4. 29 4. 54 4. 5

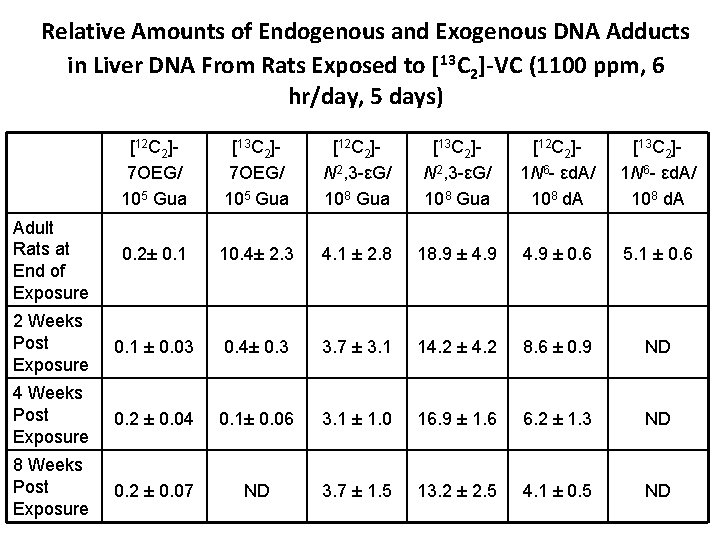
Relative Amounts of Endogenous and Exogenous DNA Adducts in Liver DNA From Rats Exposed to [13 C 2]-VC (1100 ppm, 6 hr/day, 5 days) [12 C 2]7 OEG/ 105 Gua [13 C 2]7 OEG/ 105 Gua [12 C 2]N 2, 3 -εG/ 108 Gua [13 C 2]N 2, 3 -εG/ 108 Gua [12 C 2]1 N 6 - εd. A/ 108 d. A [13 C 2]1 N 6 - εd. A/ 108 d. A Adult Rats at End of Exposure 0. 2± 0. 1 10. 4± 2. 3 4. 1 ± 2. 8 18. 9 ± 4. 9 ± 0. 6 5. 1 ± 0. 6 2 Weeks Post Exposure 0. 1 ± 0. 03 0. 4± 0. 3 3. 7 ± 3. 1 14. 2 ± 4. 2 8. 6 ± 0. 9 ND 4 Weeks Post Exposure 0. 2 ± 0. 04 0. 1± 0. 06 3. 1 ± 1. 0 16. 9 ± 1. 6 6. 2 ± 1. 3 ND 8 Weeks Post Exposure 0. 2 ± 0. 07 ND 3. 7 ± 1. 5 13. 2 ± 2. 5 4. 1 ± 0. 5 ND

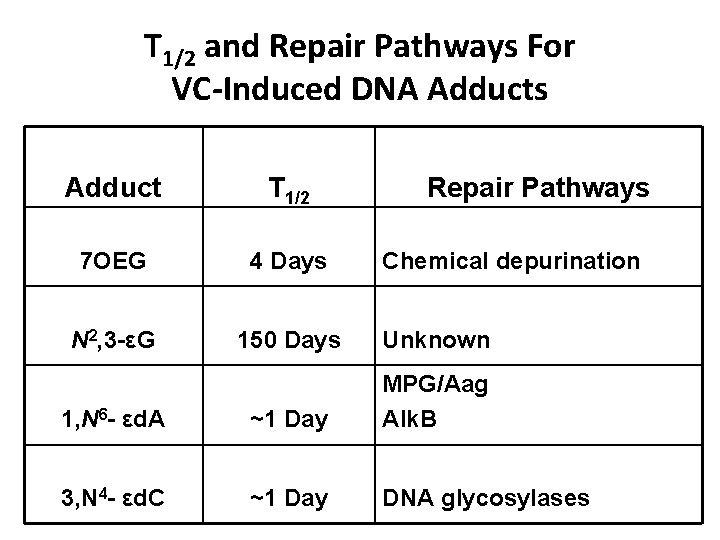
T 1/2 and Repair Pathways For VC-Induced DNA Adducts Adduct T 1/2 Repair Pathways 7 OEG 4 Days N 2, 3 -εG 150 Days Unknown 1, N 6 - εd. A ~1 Day MPG/Aag Alk. B 3, N 4 - εd. C ~1 Day DNA glycosylases Chemical depurination

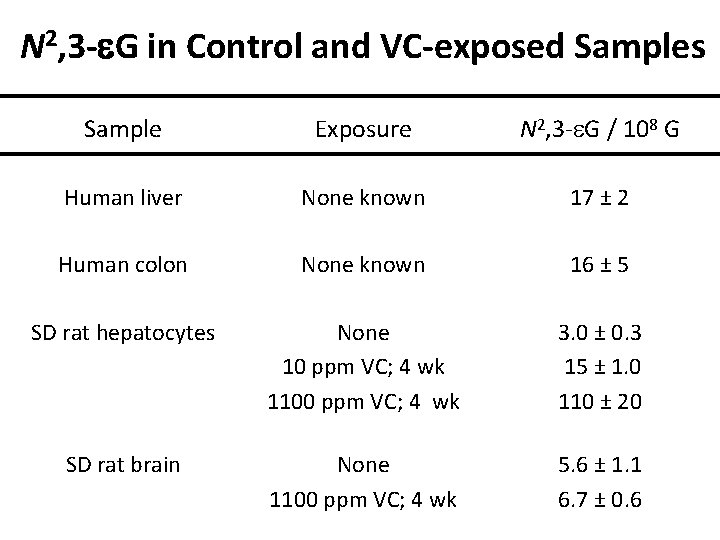
N 2, 3 -e. G in Control and VC-exposed Samples Sample Exposure N 2, 3 -e. G / 108 G Human liver None known 17 ± 2 Human colon None known 16 ± 5 SD rat hepatocytes None 10 ppm VC; 4 wk 1100 ppm VC; 4 wk 3. 0 ± 0. 3 15 ± 1. 0 110 ± 20 SD rat brain None 1100 ppm VC; 4 wk 5. 6 ± 1. 1 6. 7 ± 0. 6

Introduction • Formaldehyde is produced worldwide more than 20 million tons/year and used in a wide spectrum of applications. Therefore, formaldehyde exposures from environmental and occupational sources are quite common. • Formaldehyde is a known animal and human carcinogen, causing nasal cancer. FEMA trailers used after Hurricane Katrina 1. rats: 15 ppm formaldehyde induced 50% incidence of nasal carcinomas after 2 year-exposure (10 ppm formaldehyde caused 22% incidence). 2. humans: “sufficient epidemiological evidence that formaldehyde causes nasopharyngeal cancer in humans” according to IARC • Limited evidence to support formaldehyde inducing leukemia. 1. “strong but not sufficient evidence for a causal association between leukemia and occupational exposure to formaldehyde” based on IARC ( in 2006) 2. no mechanism of induction of leukemia in human has been identified 15 ppm 12 -month formaldehyde induced nasal tumor

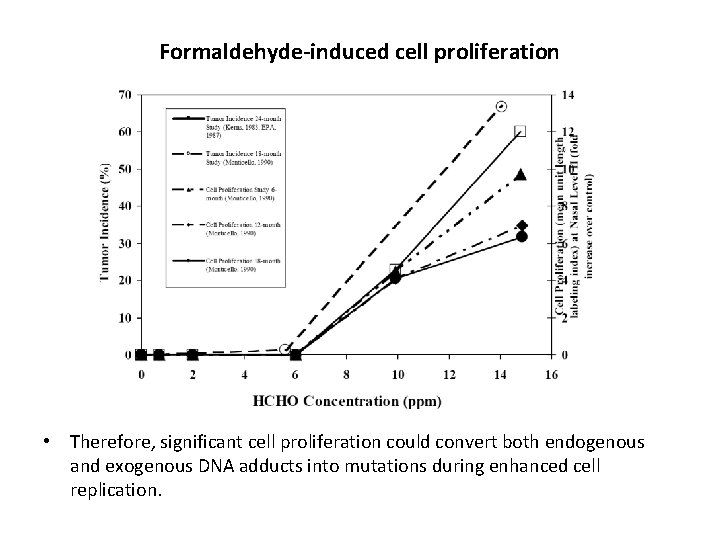
Formaldehyde-induced cell proliferation • Therefore, significant cell proliferation could convert both endogenous and exogenous DNA adducts into mutations during enhanced cell replication.

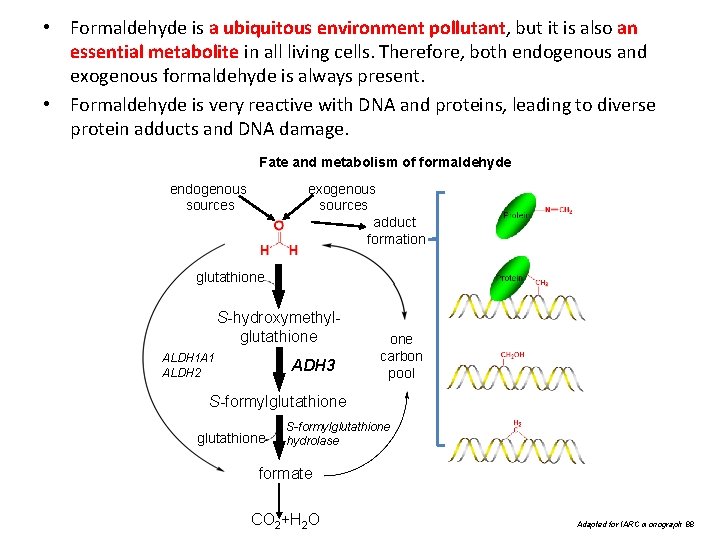
• Formaldehyde is a ubiquitous environment pollutant, but it is also an essential metabolite in all living cells. Therefore, both endogenous and exogenous formaldehyde is always present. • Formaldehyde is very reactive with DNA and proteins, leading to diverse protein adducts and DNA damage. Fate and metabolism of formaldehyde endogenous sources exogenous sources adduct formation glutathione S-hydroxymethylglutathione ALDH 1 A 1 ALDH 2 ADH 3 one carbon pool S-formylglutathione hydrolase formate CO 2+H 2 O Adapted for IARC monograph 88
![in vivo data in rats exposed to 13 CD 2formaldehyde Rats in vivo data in rats exposed to [13 CD 2]-formaldehyde • • • Rats](https://slidetodoc.com/presentation_image_h/0779f62810753dca33a2581321d38b86/image-13.jpg)
in vivo data in rats exposed to [13 CD 2]-formaldehyde • • • Rats were exposed to 10 ppm [13 CD 2]FA for 1 day (6 h/day) and 5 days. To determine whether or not formaldehyde has distant genotoxic effects. Both DNA monoadducts and DNADNA cross-links were measured. 30 -50µg DNA was used for nasal epithelium, while 200µg DNA was used for distant tissues. Digested DNA was separated by HPLC. All the adducts from DNA digestion was loaded on the column. Therefore, we have 5 times higher detecting capability for distant tissues. Lu, K. , Collins, L. B, Ru, H. Y. , Bermudez, E. , Swenberg, J. A. DNA Adducts Caused by Inhaled Formaldehyde Are Found in Nasal Epithelium, but not Bone Marrow. Toxicological Sciences 116: 441– 451 (2010).

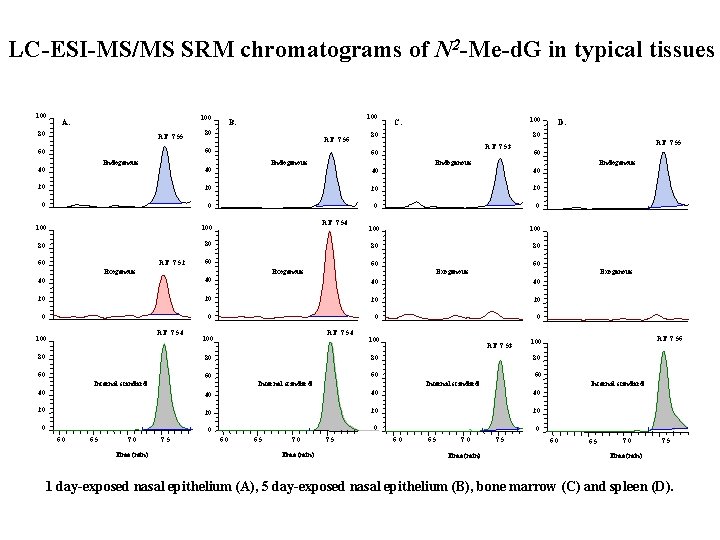
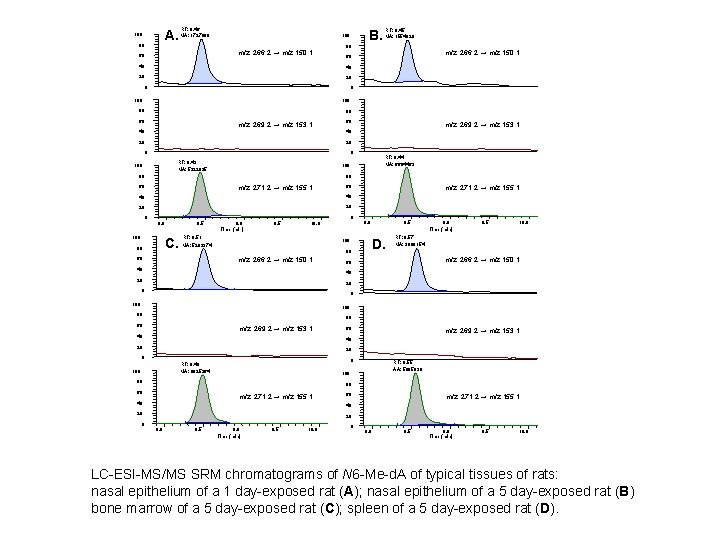
LC-ESI-MS/MS SRM chromatograms of N 2 -Me-d. G in typical tissues 100 A. 80 RT: 7. 55 100 B. 80 RT: 7. 56 60 60 Endogenous 40 100 C. RT: 7. 53 60 Endogenous 40 40 20 20 20 0 0 100 100 80 80 RT: 7. 52 RT: 7. 54 60 60 Exogenous 40 40 20 20 0 0 RT: 7. 54 100 80 RT: 7. 54 100 RT: 7. 53 80 80 60 60 Internal standard 40 40 20 20 0 0 0 6. 5 7. 0 Time (min) 7. 5 6. 0 6. 5 7. 0 Time (min) RT: 7. 56 100 80 60 60 Internal standard RT: 7. 55 60 20 60 D. 80 80 7. 5 0 6. 5 7. 0 Time (min) 7. 5 6. 0 6. 5 7. 0 7. 5 Time (min) 1 day-exposed nasal epithelium (A), 5 day-exposed nasal epithelium (B), bone marrow (C) and spleen (D).

A. 100 80 RT: 8. 46 MA: 1727880 B. 100 RT: 8. 45 MA: 1554929 80 m/z 266. 2 → m/z 150. 1 60 40 40 20 20 0 0 100 80 80 60 m/z 269. 2 → m/z 153. 1 60 40 40 20 20 0 0 RT: 8. 43 MA: 5322825 100 RT: 8. 44 MA: 6664463 100 80 80 60 m/z 271. 2 → m/z 155. 1 60 40 40 20 20 0 m/z 269. 2 → m/z 153. 1 0 8. 0 100 C. 80 8. 5 9. 0 Time (min) 9. 5 10. 0 RT: 8. 51 MA: 5282374 100 80 60 m/z 266. 2 → m/z 150. 1 40 m/z 271. 2 → m/z 155. 1 8. 0 8. 5 D. 9. 0 Time (min) 9. 5 10. 0 RT: 8. 57 MA: 3000154 m/z 266. 2 → m/z 150. 1 60 40 20 20 0 0 100 80 80 60 m/z 269. 2 → m/z 153. 1 40 60 20 20 0 0 RT: 8. 49 MA: 6825364 100 RT: 8. 55 AA: 5885930 100 80 80 60 m/z 271. 2 → m/z 155. 1 40 20 0 m/z 269. 2 → m/z 153. 1 40 20 8. 5 9. 0 Time (min) 9. 5 10. 0 LC-ESI-MS/MS SRM chromatograms of N 6 -Me-d. A of typical tissues of rats: nasal epithelium of a 1 day-exposed rat (A); nasal epithelium of a 5 day-exposed rat (B) bone marrow of a 5 day-exposed rat (C); spleen of a 5 day-exposed rat (D).
![Formaldehydeinduced monoadducts in tissues of rats exposed to 10 ppm 13 CD 2formaldehyde for Formaldehyde-induced monoadducts in tissues of rats exposed to 10 ppm [13 CD 2]-formaldehyde for](https://slidetodoc.com/presentation_image_h/0779f62810753dca33a2581321d38b86/image-16.jpg)
Formaldehyde-induced monoadducts in tissues of rats exposed to 10 ppm [13 CD 2]-formaldehyde for 1 day or 5 days N 2 -HOCH 2 -d. G (adducts/107 d. G) Exposure period N 6 -HOCH 2 -d. A (adducts/107 d. A) Tissues exogenous endogenous Nose 1. 28± 0. 49* 2. 63± 0. 73 n. d. 3. 95± 0. 26 Lung n. d. + 2. 39± 0. 16‡ n. d. 2. 62± 0. 24 Liver n. d. 2. 66± 0. 53 n. d. 2. 62± 0. 46 # Spleen n. d. 2. 35± 0. 31 n. d. 1. 85± 0. 19 Bone Marrow n. d. 1. 05± 0. 14 n. d. 2. 95± 1. 32 Thymus n. d. 2. 19± 0. 36 n. d. 2. 98± 1. 11 Nose 2. 43± 0. 78 2. 84± 1. 13 n. d. 3. 61± 0. 95 Lung n. d. 2. 61± 0. 35 n. d. 2. 47± 0. 55 Liver n. d. 3. 24± 0. 42 n. d. 2. 87± 0. 65 Spleen n. d. 2. 35± 0. 59 n. d. 2. 23± 0. 89 Bone Marrow n. d. 1. 17± 0. 35 n. d. 2. 99± 0. 08 Thymus n. d. 1. 99± 0. 30 n. d. 2. 48± 0. 11 1 day 5 day # Endogenous N 6 -HOCH 2 -d. A was present in control rat liver at 1. 96± 1. 86 adducts/107 d. A From Cheng et al. , Chem. Res. Toxicol. 21, 746 -751, 2008.

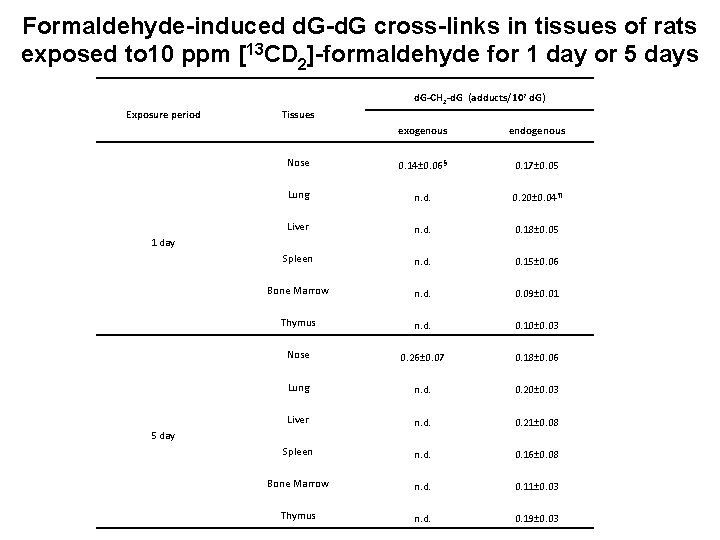
Formaldehyde-induced d. G-d. G cross-links in tissues of rats exposed to 10 ppm [13 CD 2]-formaldehyde for 1 day or 5 days d. G-CH 2 -d. G (adducts/107 d. G) Exposure period Tissues exogenous endogenous Nose 0. 14± 0. 06 § 0. 17± 0. 05 Lung n. d. 0. 20± 0. 04 ¶ Liver n. d. 0. 18± 0. 05 Spleen n. d. 0. 15± 0. 06 Bone Marrow n. d. 0. 09± 0. 01 Thymus n. d. 0. 10± 0. 03 Nose 0. 26± 0. 07 0. 18± 0. 06 Lung n. d. 0. 20± 0. 03 Liver n. d. 0. 21± 0. 08 Spleen n. d. 0. 16± 0. 08 Bone Marrow n. d. 0. 11± 0. 03 Thymus n. d. 0. 19± 0. 03 1 day 5 day

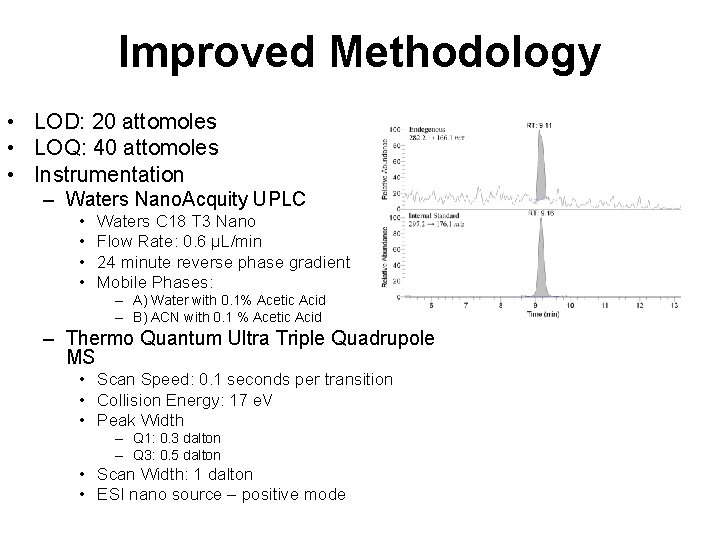
Improved Methodology • LOD: 20 attomoles • LOQ: 40 attomoles • Instrumentation – Waters Nano. Acquity UPLC • • Waters C 18 T 3 Nano Flow Rate: 0. 6 µL/min 24 minute reverse phase gradient Mobile Phases: – A) Water with 0. 1% Acetic Acid – B) ACN with 0. 1 % Acetic Acid – Thermo Quantum Ultra Triple Quadrupole MS • Scan Speed: 0. 1 seconds per transition • Collision Energy: 17 e. V • Peak Width – Q 1: 0. 3 dalton – Q 3: 0. 5 dalton • Scan Width: 1 dalton • ESI nano source – positive mode

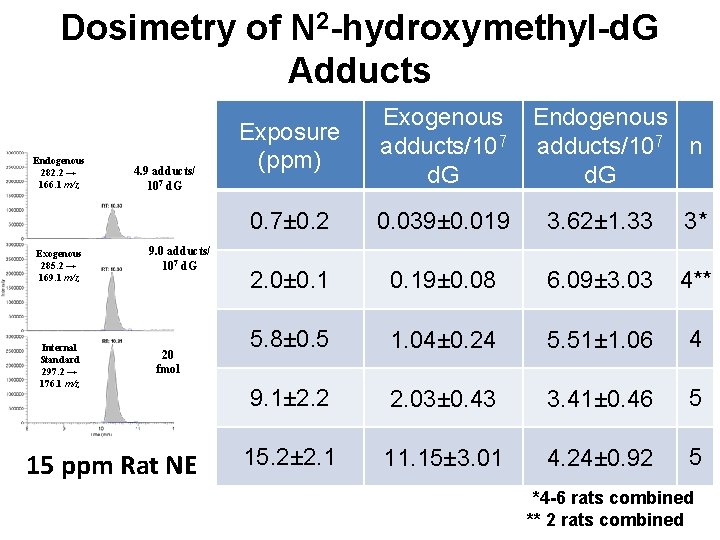
Dosimetry of N 2 -hydroxymethyl-d. G Adducts Endogenous 282. 2 → 166. 1 m/z Exogenous 285. 2 → 169. 1 m/z Internal Standard 297. 2 → 176. 1 m/z 4. 9 adducts/ 107 d. G 9. 0 adducts/ 107 d. G 20 fmol 15 ppm Rat NE Exposure (ppm) Exogenous adducts/107 d. G Endogenous adducts/107 n d. G 0. 7± 0. 2 0. 039± 0. 019 3. 62± 1. 33 3* 2. 0± 0. 19± 0. 08 6. 09± 3. 03 4** 5. 8± 0. 5 1. 04± 0. 24 5. 51± 1. 06 4 9. 1± 2. 2 2. 03± 0. 43 3. 41± 0. 46 5 15. 2± 2. 1 11. 15± 3. 01 4. 24± 0. 92 5 *4 -6 rats combined ** 2 rats combined

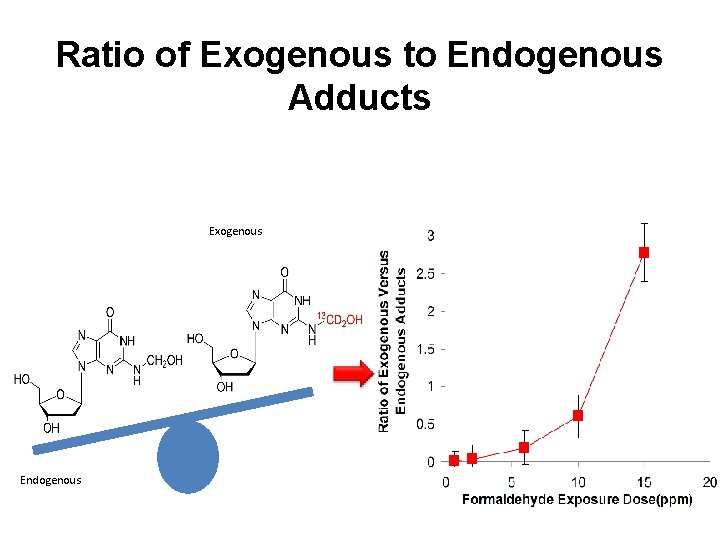
Ratio of Exogenous to Endogenous Adducts Exogenous Endogenous

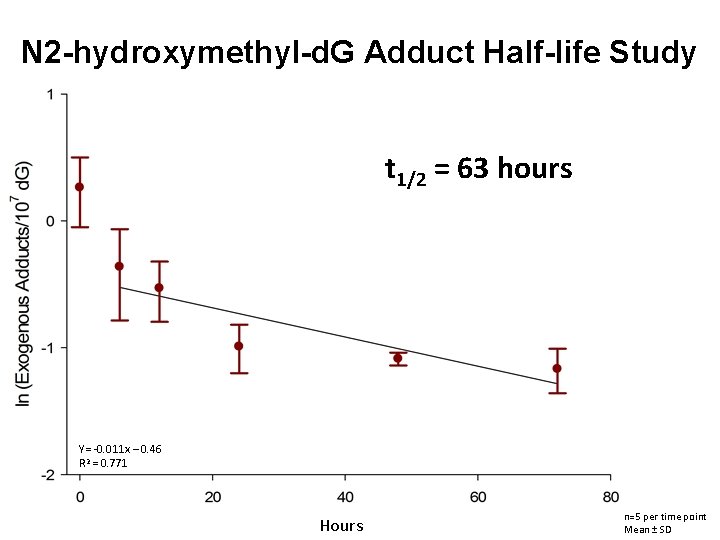
N 2 -hydroxymethyl-d. G Adduct Half-life Study t 1/2 = 63 hours Y= -0. 011 x – 0. 46 R 2 = 0. 771 Hours n=5 per time point Mean ± SD

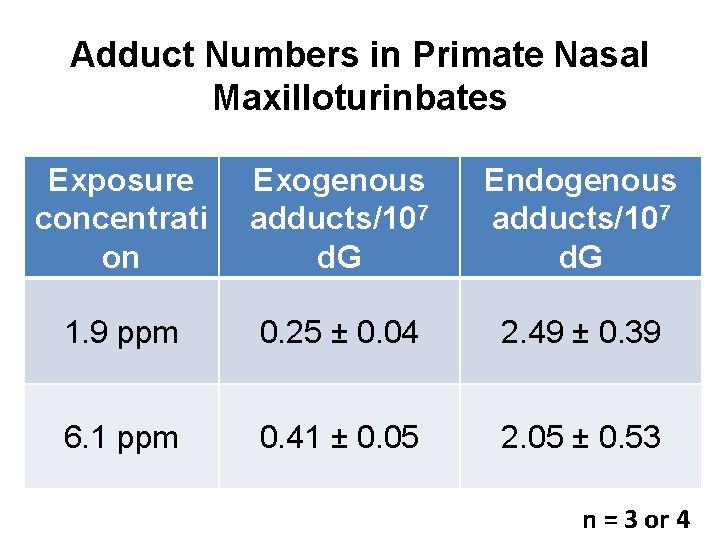
Adduct Numbers in Primate Nasal Maxilloturinbates Exposure concentrati on Exogenous adducts/107 d. G Endogenous adducts/107 d. G 1. 9 ppm 0. 25 ± 0. 04 2. 49 ± 0. 39 6. 1 ppm 0. 41 ± 0. 05 2. 05 ± 0. 53 n = 3 or 4

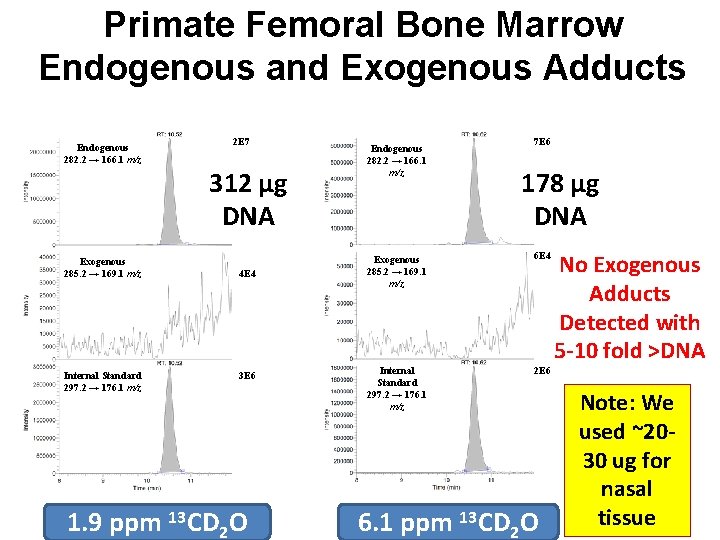
Primate Femoral Bone Marrow Endogenous and Exogenous Adducts Endogenous 282. 2 → 166. 1 m/z 2 E 7 312 µg DNA Exogenous 285. 2 → 169. 1 m/z Internal Standard 297. 2 → 176. 1 m/z 4 E 4 3 E 6 1. 9 ppm 13 CD 2 O Endogenous 282. 2 → 166. 1 m/z 7 E 6 178 µg DNA Exogenous 285. 2 → 169. 1 m/z 6 E 4 Internal Standard 297. 2 → 176. 1 m/z 2 E 6 6. 1 ppm 13 CD 2 O No Exogenous Adducts Detected with 5 -10 fold >DNA Note: We used ~2030 ug for nasal tissue
![Application to Risk Assessment Because no 13 CD 2N 2 Med G adducts Application to Risk Assessment • Because no [13 CD 2]-N 2 -Med. G adducts](https://slidetodoc.com/presentation_image_h/0779f62810753dca33a2581321d38b86/image-24.jpg)
Application to Risk Assessment • Because no [13 CD 2]-N 2 -Med. G adducts were detectable in primate bone marrow, we can state that they must be below the LOD. • Therefore, the LOD represents a worst case upper bound for the amount of DNA analyzed. • We have assumed that the relationship between airborne formaldehyde concentration and exogenous d. G adducts is linear through zero. • We calculated steady state concentrations based on the adduct half life and a 24/7 exposure. • Risk estimates were calculated for all data sets.

Risk Assessment Model is Public Health Conservative • Attributes all background risk to d. G adducts. • Only utilizes endogenous d. G adducts, even though endogenous d. A adducts are also present. • Risk model is linear. • Used lower 95% confidence bounds of measured endogenous adducts to generate upper 95% bounds on slopes. • Made conservative assumptions on kinetics and d. G adduct half-life data. • Used same scaling methods used by the USEPA.


Conclusions to date • Exposure-induced DNA monoadducts and cross-links only occur in nasal epithelial DNA in rats and primates. • Only d. G monoadducts and cross-links are formed following inhalation and in vitro exposures to formaldehyde. • d. A monoadducts arise from intracellular formation of formaldehyde secondary to intracellular metabolism. • Endogenous DNA monoadducts (d. G and d. A) are present in all cells and tissues. • Endogenous adducts are present in 2. 5 -3 -fold greater amounts than exogenous adducts following 10 ppm exposures to [13 CD 2]formaldehyde, but 100 -fold greater at 1 ppm. • These data strongly challenge the biological plausibility that formaldehyde causes leukemia.

Conclusions to date • Both cytotoxicity and genotoxicity are key events for the induction of nasal carcinoma. • The sustained increase in cell proliferation that results from formaldehyde cytotoxicity “fixes” both endogenous and exogenous DNA adducts into heritable mutations. • If a rat was placed in a FEMA trailer for 6 hours, only 91/100, 000 formaldehyde adducts would come from the exposure. The rest would be endogenous. • A 6 hr exposure of a rat to the USEPA proposed safe level of formaldehyde (0. 07 ppt) would induce 83/100, 000 adducts. • The lack of exogenous formaldehyde adduct formation in bone marrow and other distant sites does not support the biologic plausibility of leukemia.

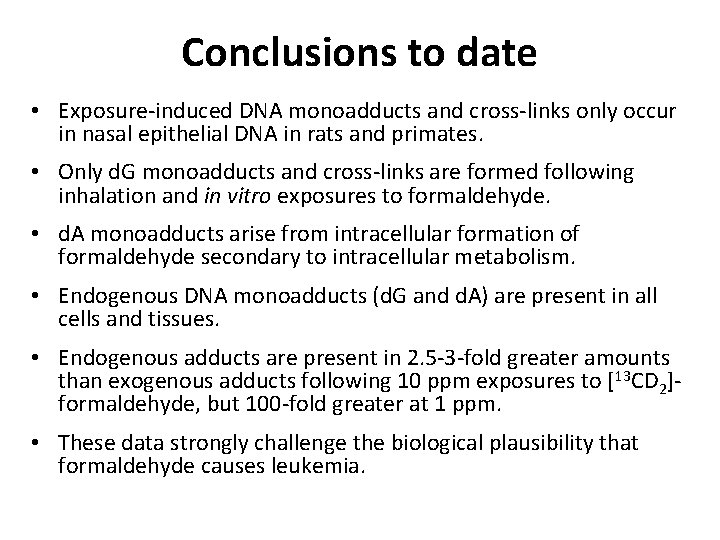
Ethylene Oxide • Ethylene oxide (EO) is a classified as a known human and animal carcinogen. • EO is genotoxic and mutagenic. • DNA and hemoglobin adducts of EO are present in all individuals and animals. • These arise from metabolism of ethylene to EO and from oxidative stress. • Everyone in this room is breathing off 0. 2 -2 ppm 24/7. • The draft IRIS risk assessment gives a virtually safe exposure as <1 ppt. This is ~1000 times less than our endogenous daily exposure.

EO Low Dose Mutagenesis

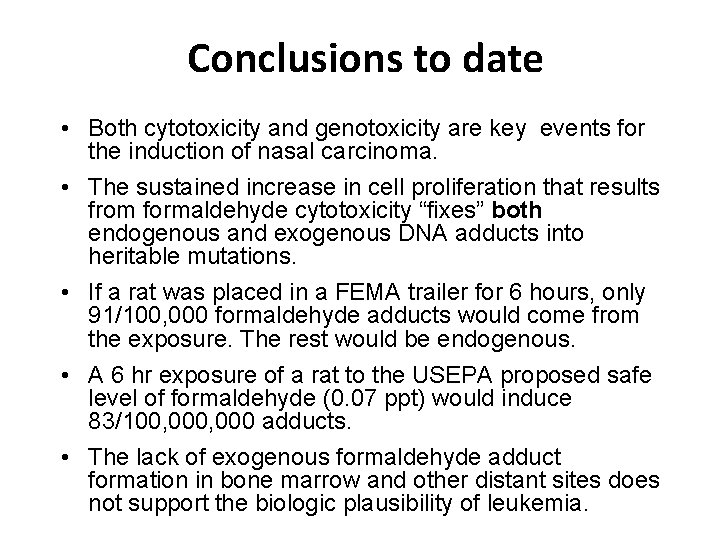
Oxidative Stress Induced-DNA Damage
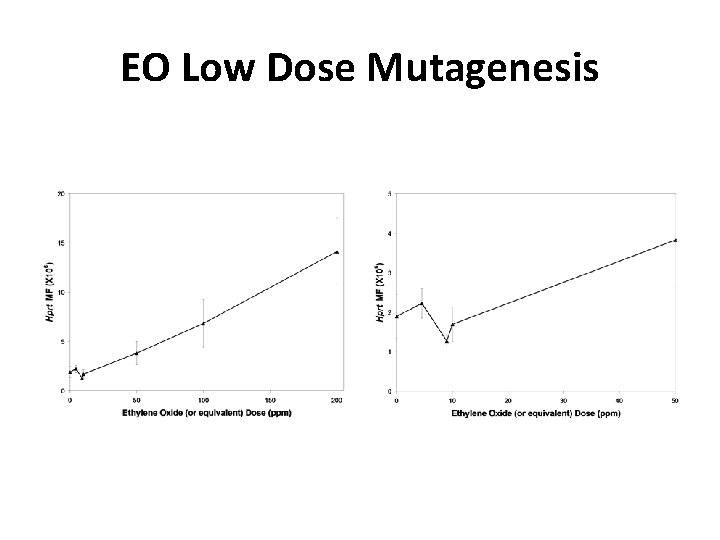
![Endogenous AP Sites in Rat Tissues and Human Liver Nakamura and Swenberg 1999 Endogenous AP Sites in Rat Tissues and Human Liver [Nakamura and Swenberg, 1999]](https://slidetodoc.com/presentation_image_h/0779f62810753dca33a2581321d38b86/image-32.jpg)
Endogenous AP Sites in Rat Tissues and Human Liver [Nakamura and Swenberg, 1999]

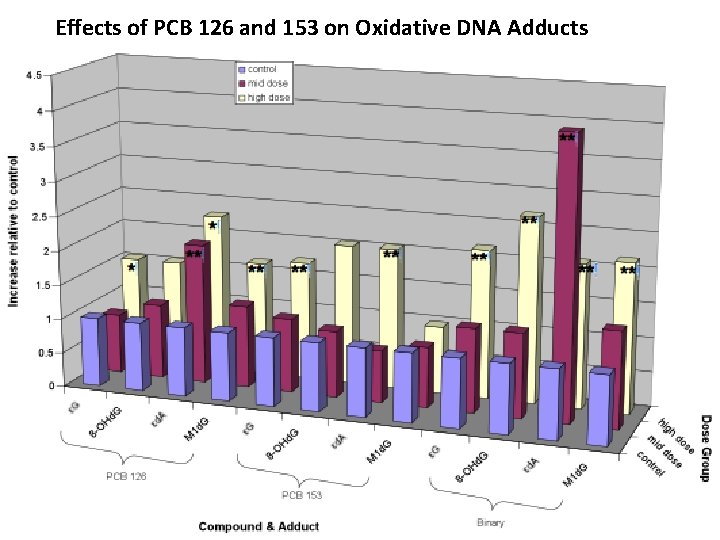
Effects of PCB 126 and 153 on Oxidative DNA Adducts

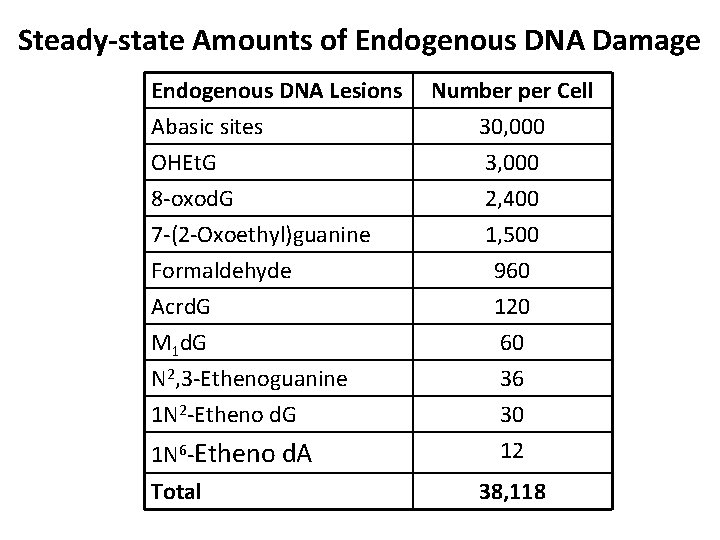
Steady-state Amounts of Endogenous DNA Damage Endogenous DNA Lesions Abasic sites OHEt. G Number per Cell 30, 000 3, 000 8 -oxod. G 2, 400 7 -(2 -Oxoethyl)guanine Formaldehyde Acrd. G M 1 d. G N 2, 3 -Ethenoguanine 1 N 2 -Etheno d. G 1 N 6 -Etheno d. A 1, 500 960 120 60 36 30 12 Total 38, 118

What are the Issues? • Linear extrapolations of cancer risks lead to highly conservative assessments of risk that represent science policy aimed at protecting the public’s health. – The accuracy of these estimates has a high degree of uncertainty – Genotoxicity issues drive a linear default • This presentation will examine our present understanding of the science involved in mutagenesis and carcinogenesis that should drive low dose risk assessment.

Mutations and Cancer • There is general consensus that multiple mutations are the primary mechanism involved in carcinogenesis (Hanahan and Weinberg, 2000). • The dose-response of this biomarker of effect has not received the same degree of consideration as have DNA adducts in cancer risk assessment, even though their use could be highly informative.

MOA Key Events for Mutations Genotoxicity DNA Adducts Mutations in Reporter Genes Mutations in Cancer Genes (Bioindicators) Cancer

Mutations Do Not Go Through Zero • In contrast to most DNA adducts, mutations do not go through zero. • Rather, they reach a background level that reflects the summation of mutations arising from endogenous DNA damage and repair that occurs in cells. • The dose-response may be linear or nonlinear. • There may be an inflection point for a dose response curve where the number of mutations increases nonlinearly above the spontaneous level, or there may be a linear increase with data points that are not significantly different from controls at lower doses. • The point at which the mutations increase is where the exogenous DNA damage starts driving the biology that results in additional mutations.

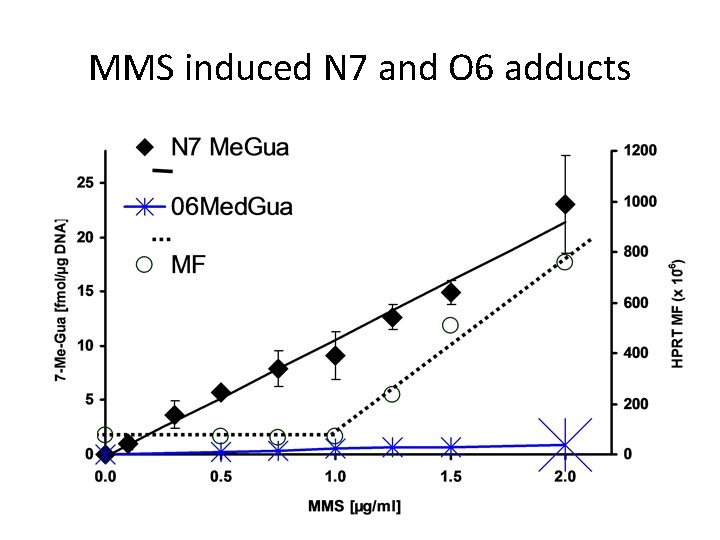
MMS induced N 7 and O 6 adducts

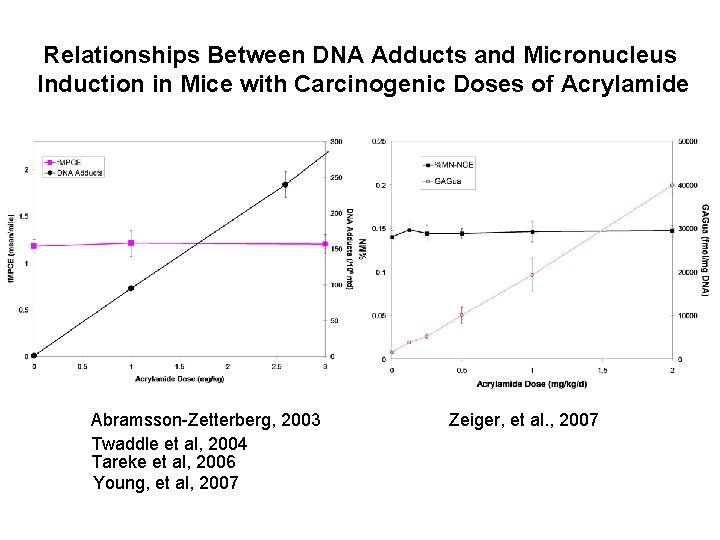
Relationships Between DNA Adducts and Micronucleus Induction in Mice with Carcinogenic Doses of Acrylamide Abramsson-Zetterberg, 2003 Twaddle et al, 2004 Tareke et al, 2006 Young, et al, 2007 Zeiger, et al. , 2007

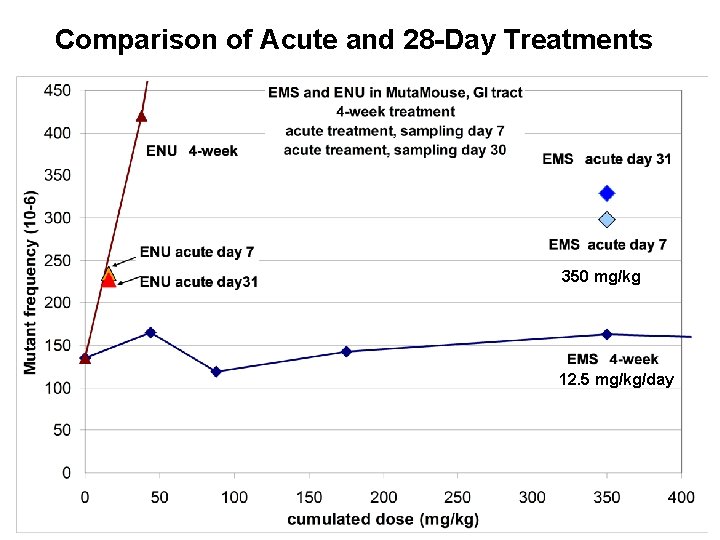
Comparison of Acute and 28 -Day Treatments 350 mg/kg 12. 5 mg/kg/day

ED 0. 01 Carcinogenicity Study • A carcinogenicity study has been conducted on Dibenzo[a, l]pyrene using 42, 000 rainbow trout. • This model is 50 times more sensitive than rodent bioassays due to the low background incidence of neoplasia. • The EPA linear risk model over estimated the actual observed liver cancer incidence by three orders of magnitude. Bailey et al, Chem. Res. Tox. , 2009

Conclusions • As our knowledge of carcinogenesis has expanded, concepts of “one molecule → cancer “ have little to no scientific support. • Mutations in genes controlling cell proliferation and cell death appear to play major roles in the induction of cancer. • While these genes are difficult to monitor in noncancer tissues, reporter gene mutations can be used to examine dose response relationships in cells, animals and humans.

Conclusions (cont. ) • New scientific approaches are needed to determine where in the dose response paradigm mutations are increased over background and where they are driven by endogenous biological processes. • The Bier Report (1980) Concluded “Use simple linear interpolation between the lowest reliable dose data and the spontaneous …rate. ” • Such data represent a critical need to support a Point of Departure for setting acceptable exposures. • This could be accomplished by using a Margin of Exposure approach to protect susceptible individuals.

Conclusions (cont. ) • All living cells have large amounts of endogenous DNA damage and this damage is responsible for background mutations. • Chemical exposures can cause DNA lesions that are unique or identical to endogenous DNA damage. • At high exposures, such as used in carcinogenicity bioassays, ROS-induced and exogenous DNA damage represent key factors driving mutagenesis. • At very low exposures, such as used in linear low dose extrapolations, the amount of endogenous DNA damage vastly exceeds chemical specific damage. • Focused research to increase our scientific knowledge related to the role of endogenous DNA adducts should play an important role in risk assessment.

Default • The word default first came into use in the 1200’s. – A failure to meet one’s obligation – A sin • The above concept is certainly applicable to risk assessment. – We have failed to meet our obligation to use the best science when we resort to defaults.

Collaborators and Sponsors • • • Jun Nakamura Paul Chastain Eric Morinello Esra Mutlu Lina Gao Kun Lu Gunnar Boysen Leonard Collins Patricia Upton Darrell Winsett Gary Hatch Ed Bermudez • Superfund Basic Research Program (P 42 -ES 5948) • NIEHS (P 30 ES 10126) • American Chemistry Council • Formaldehyde Council • Environmental Protection Agency • Hamner Institutes for Health Sciences