TimeResolved Raman Studies of the Electron Adducts of





- Slides: 26

Time-Resolved Raman Studies of the Electron Adducts of Benzoate Anion in Water Deanna O’Donnell University of Notre Dame Radiation Laboratory Department of Chemistry and Biochemistry June 16 th, 2008 1

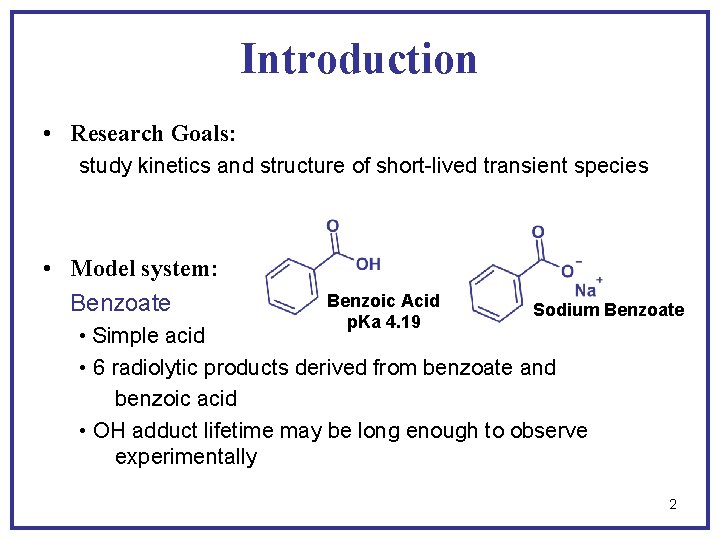
Introduction • Research Goals: study kinetics and structure of short-lived transient species • Model system: Benzoate • Simple acid Benzoic Acid p. Ka 4. 19 Sodium Benzoate • 6 radiolytic products derived from benzoate and benzoic acid • OH adduct lifetime may be long enough to observe experimentally 2

Problems with Benzoate • Preservative in Food Industry for over 80 years (E 211) • 1993 - Lawrence 1 provided mechanism for benzoate conversion to benzene • May 2006 - FDA released report 2 listing 100 soft drinks containing benzene – 10 exceeded the EPA drinking water legal limit • May 2008 - Coca Cola Company released a statement that they will phase out sodium benzoate in their soft drink in the UK 3 1 Lawrence, G. J. Agric. Food Chem. , 41, 1993, 693. FDA, 2006. "Data on Benzene in Soft Drinks and Other Beverages, " United States Food and Drug Administration. Accessed June 2 nd at: http: //www. cfsan. fda. gov/~dms/benzdata. html 3 3 http: //www. Beverage. Daily. com 2

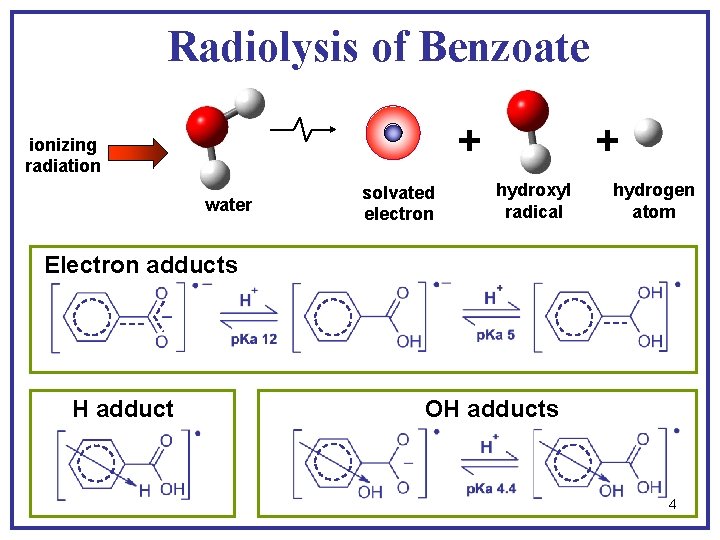
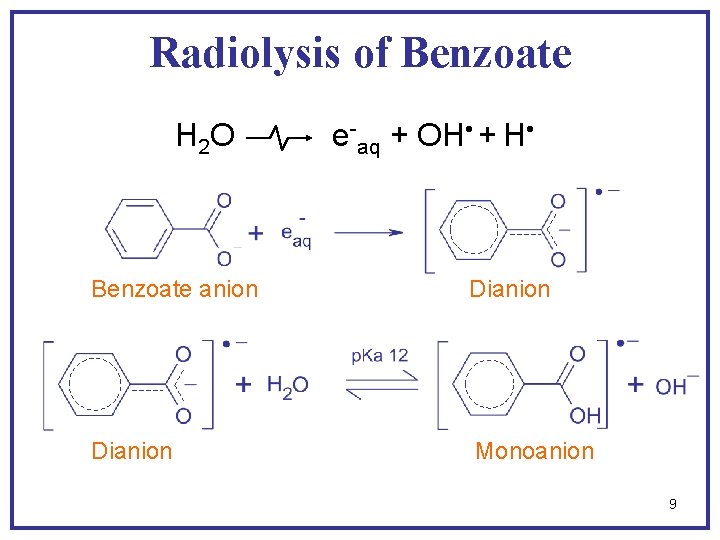
Radiolysis of Benzoate + ionizing radiation water solvated electron + hydroxyl radical hydrogen atom Electron adducts H adduct OH adducts 4

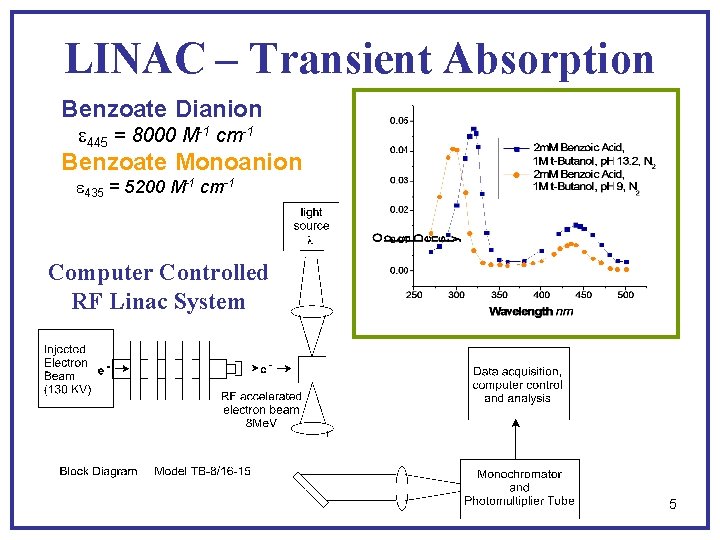
LINAC – Transient Absorption Benzoate Dianion e 445 = 8000 M-1 cm-1 Benzoate Monoanion e 435 = 5200 M-1 cm-1 Computer Controlled RF Linac System 5

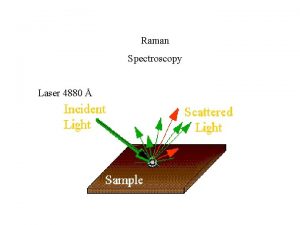
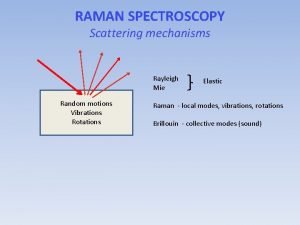
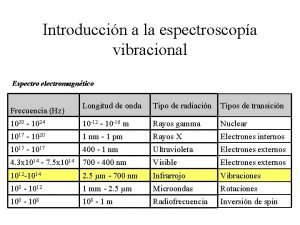
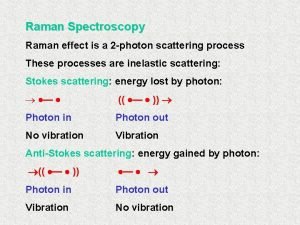
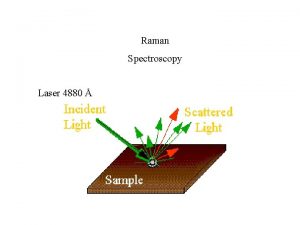
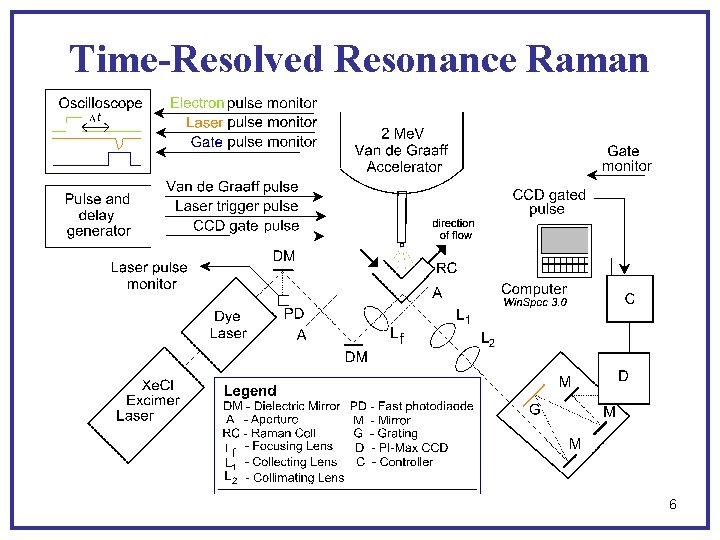
Time-Resolved Resonance Raman 6

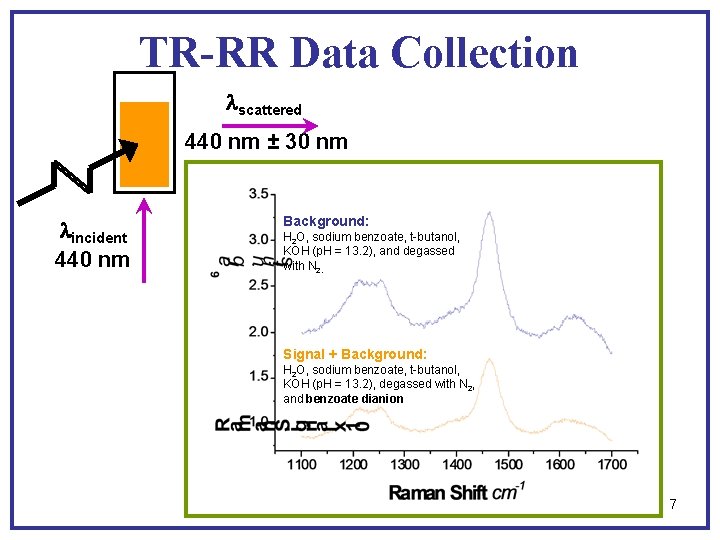
TR-RR Data Collection lscattered 440 nm ± 30 nm lincident 440 nm Background: H 2 O, sodium benzoate, t-butanol, KOH (p. H = 13. 2), and degassed with N 2. Signal + Background: H 2 O, sodium benzoate, t-butanol, KOH (p. H = 13. 2), degassed with N 2, and benzoate dianion 7

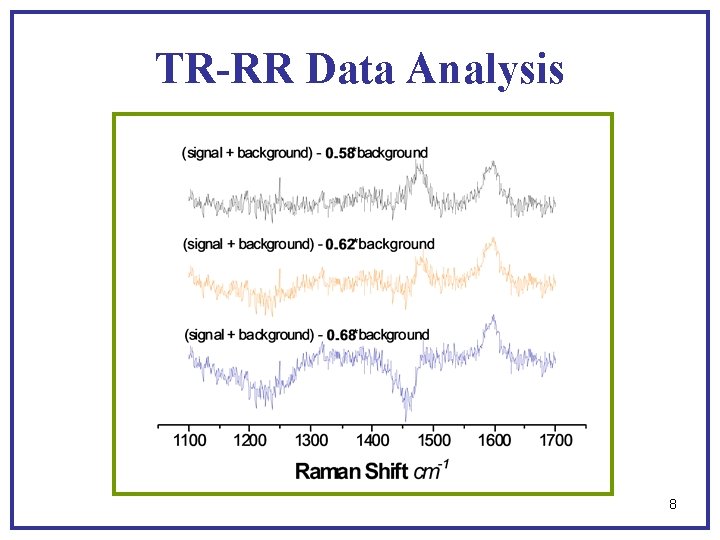
TR-RR Data Analysis 8

Radiolysis of Benzoate H 2 O e-aq + OH + H Benzoate anion Dianion Monoanion 9

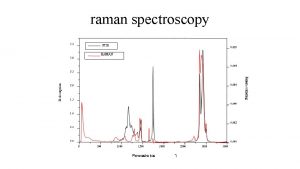
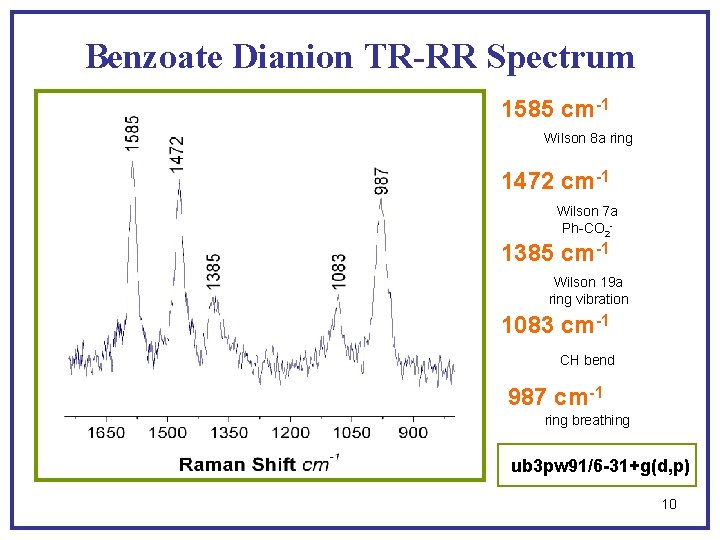
Benzoate Dianion TR-RR Spectrum 1585 cm-1 Wilson 8 a ring 1472 cm-1 Wilson 7 a Ph-CO 2 - 1385 cm-1 Wilson 19 a ring vibration 1083 cm-1 CH bend 987 cm-1 ring breathing ub 3 pw 91/6 -31+g(d, p) 10

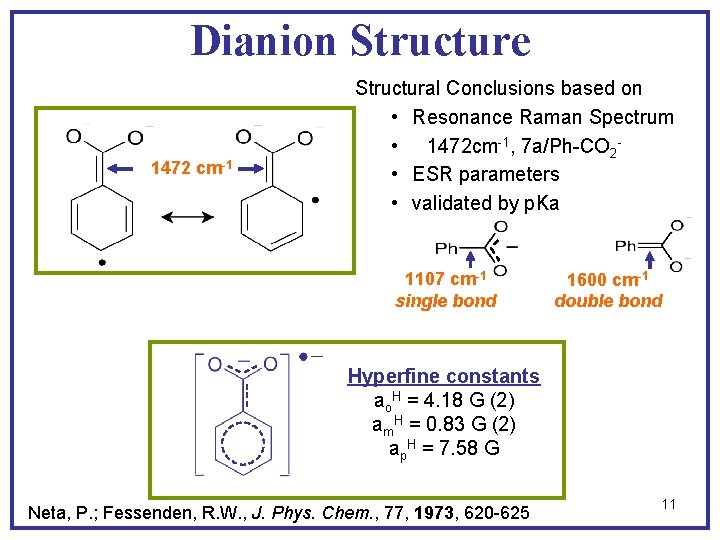
Dianion Structure 1472 cm-1 Structural Conclusions based on • Resonance Raman Spectrum • 1472 cm-1, 7 a/Ph-CO 2 • ESR parameters • validated by p. Ka 1107 cm-1 single bond 1600 cm-1 double bond Hyperfine constants ao. H = 4. 18 G (2) am. H = 0. 83 G (2) ap. H = 7. 58 G Neta, P. ; Fessenden, R. W. , J. Phys. Chem. , 77, 1973, 620 -625 11

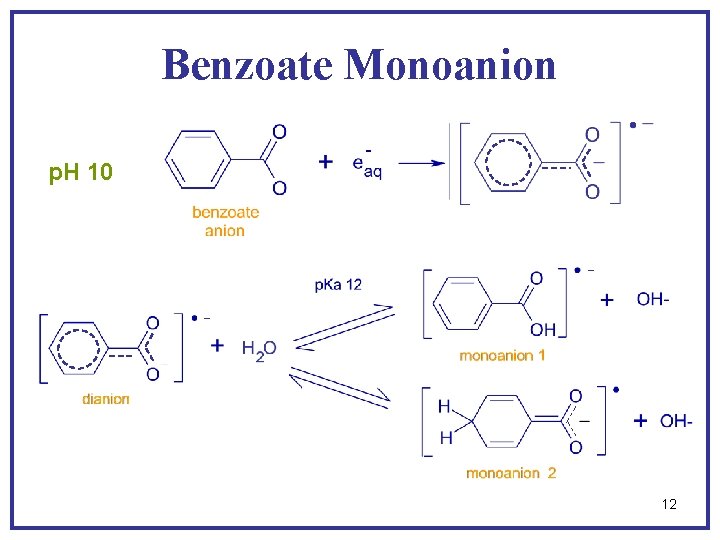
Benzoate Monoanion p. H 10 12

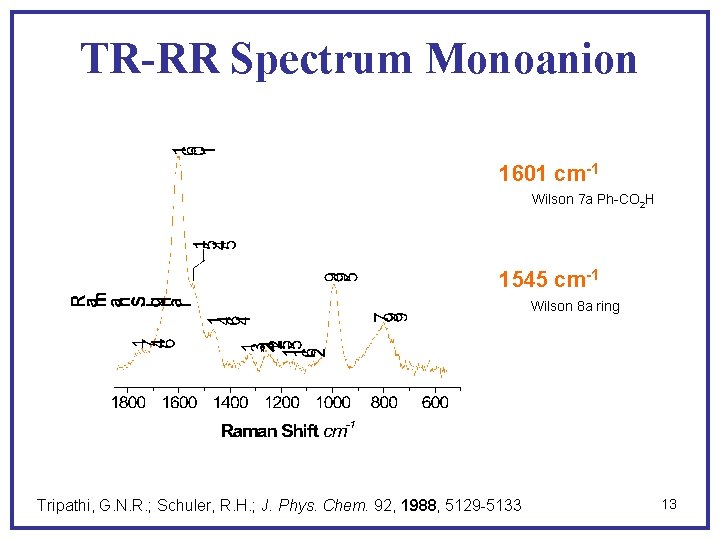
TR-RR Spectrum Monoanion 1601 cm-1 Wilson 7 a Ph-CO 2 H 1545 cm-1 Wilson 8 a ring Tripathi, G. N. R. ; Schuler, R. H. ; J. Phys. Chem. 92, 1988, 5129 -5133 13

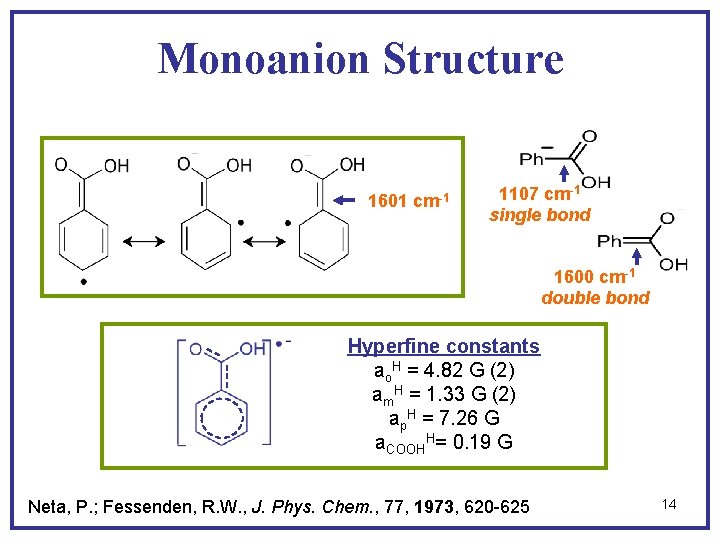
Monoanion Structure 1601 cm-1 1107 cm-1 single bond 1600 cm-1 double bond Hyperfine constants ao. H = 4. 82 G (2) am. H = 1. 33 G (2) ap. H = 7. 26 G a. COOHH= 0. 19 G Neta, P. ; Fessenden, R. W. , J. Phys. Chem. , 77, 1973, 620 -625 14

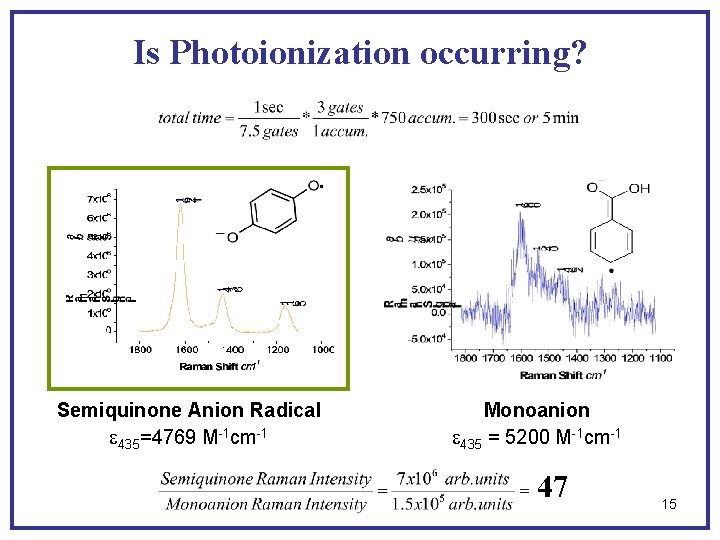
Is Photoionization occurring? Semiquinone Anion Radical e 435=4769 M-1 cm-1 Monoanion e 435 = 5200 M-1 cm-1 47 15

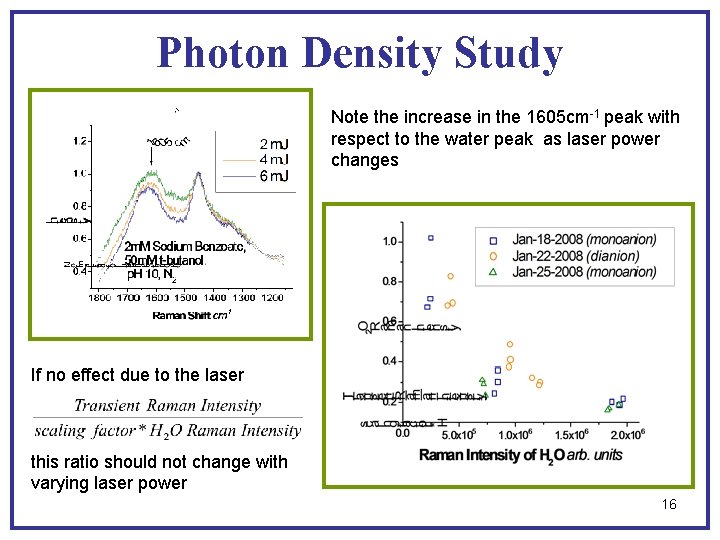
Photon Density Study Note the increase in the 1605 cm-1 peak with respect to the water peak as laser power changes If no effect due to the laser this ratio should not change with varying laser power 16

Summary • Benzoate electron adducts – Benzoate Dianion Radical – Benzoate Monoanion Radical • Photoionization • Future Work includes: – OH adduct – product analysis – para-substituted benzoate 17

Acknowledgments • Dr. G. N. R. Tripathi • Dr. Ian Carmichael • Dr. Irek Janik • This work was sponsored by the Department of Energy 18

19

Extra Slides 20

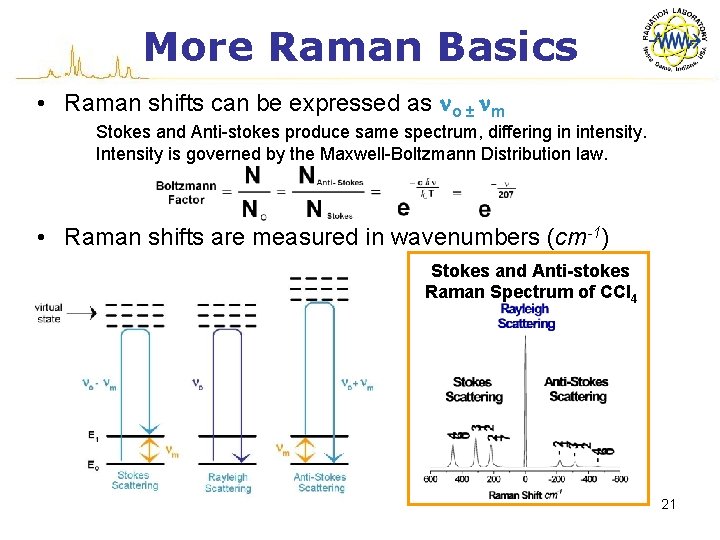
More Raman Basics • Raman shifts can be expressed as no ± nm Stokes and Anti-stokes produce same spectrum, differing in intensity. Intensity is governed by the Maxwell-Boltzmann Distribution law. • Raman shifts are measured in wavenumbers (cm-1) Stokes and Anti-stokes Raman Spectrum of CCl 4 21

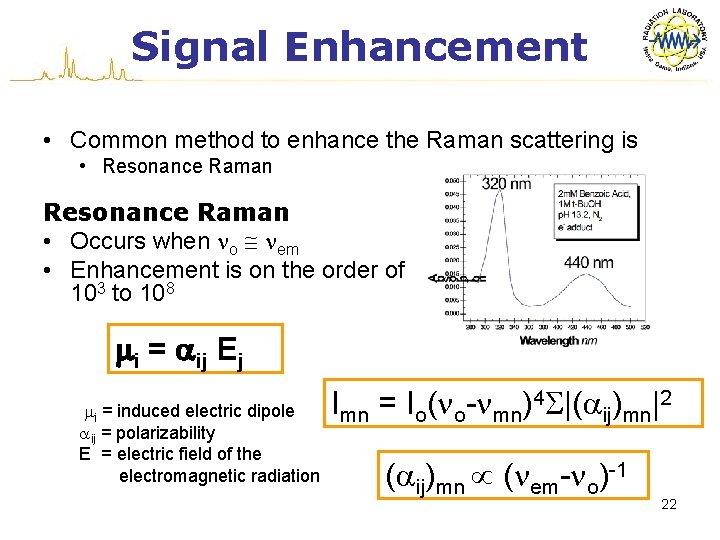
Signal Enhancement • Common method to enhance the Raman scattering is • Resonance Raman • Occurs when no nem • Enhancement is on the order of 103 to 108 mi = aij Ej mi = induced electric dipole aij = polarizability E = electric field of the iiiiielectromagnetic radiation Imn = Io(no-nmn)4 S|(aij)mn|2 (aij)mn (nem-no)-1 22

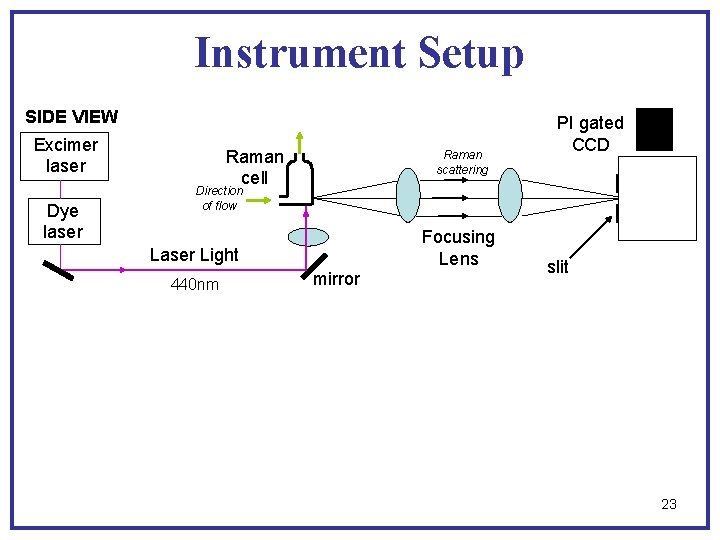
Instrument Setup SIDE VIEW Excimer laser Dye laser Raman cell Raman scattering PI gated CCD Direction of flow Focusing Lens Laser Light 440 nm mirror slit 23

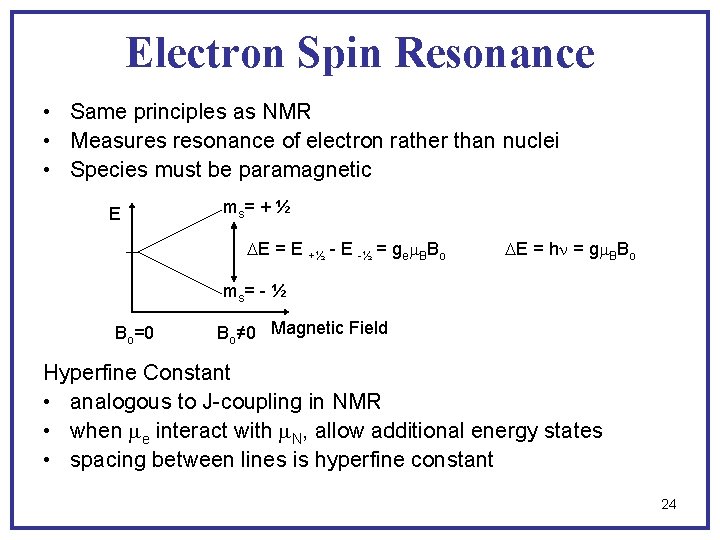
Electron Spin Resonance • Same principles as NMR • Measures resonance of electron rather than nuclei • Species must be paramagnetic E ms = + ½ E = E +½ - E -½ = gem. BBo E = hn = gm. BBo ms = - ½ Bo=0 Bo≠ 0 Magnetic Field Hyperfine Constant • analogous to J-coupling in NMR • when me interact with m. N, allow additional energy states • spacing between lines is hyperfine constant 24

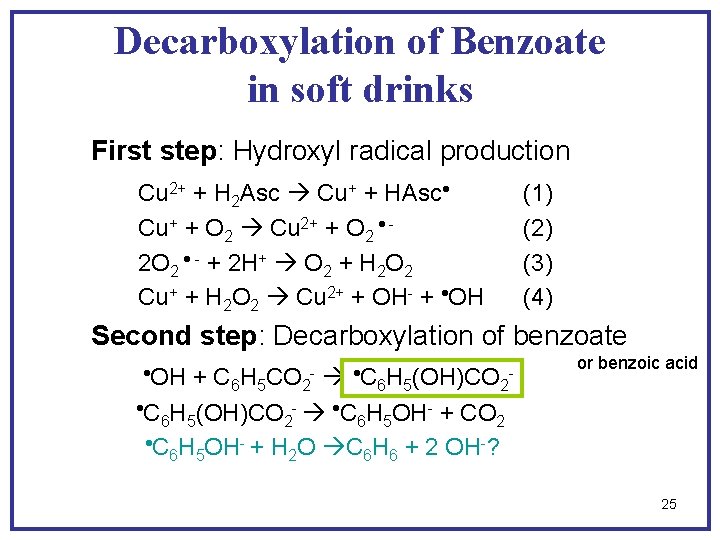
Decarboxylation of Benzoate in soft drinks First step: Hydroxyl radical production Cu 2+ + H 2 Asc Cu+ + HAsc Cu+ + O 2 Cu 2+ + O 2 2 O 2 - + 2 H+ O 2 + H 2 O 2 Cu+ + H 2 O 2 Cu 2+ + OH- + OH (1) (2) (3) (4) Second step: Decarboxylation of benzoate OH C + C 6 H 5 CO 2 6 H 5(OH)CO 2 C H (OH)CO - C H OH- + CO 6 5 2 C H OH- + H O C H + 2 OH-? 6 5 2 6 6 - - or benzoic acid 25

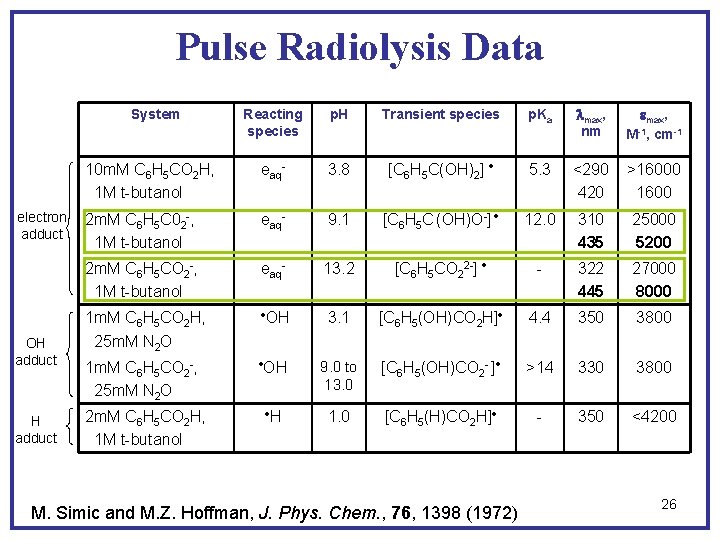
Pulse Radiolysis Data Reacting species p. H Transient species p. Ka lmax, nm emax, M-1, cm-1 10 m. M C 6 H 5 CO 2 H, 1 M t-butanol eaq- 3. 8 [C 6 H 5 C(OH)2] 5. 3 <290 420 >16000 1600 2 m. M C 6 H 5 C 02 -, 1 M t-butanol eaq- 9. 1 [C 6 H 5 C (OH)O-] 12. 0 310 435 25000 5200 2 m. M C 6 H 5 CO 2 -, 1 M t-butanol eaq- 13. 2 [C 6 H 5 CO 22 -] - 322 445 27000 8000 1 m. M C 6 H 5 CO 2 H, 25 m. M N 2 O OH 3. 1 [C 6 H 5(OH)CO 2 H] 4. 4 350 3800 1 m. M C 6 H 5 CO 2 -, 25 m. M N 2 O OH 9. 0 to 13. 0 [C 6 H 5(OH)CO 2 - ] >14 330 3800 H 1. 0 [C 6 H 5(H)CO 2 H] - 350 <4200 System electron adduct OH adduct 2 m. M C 6 H 5 CO 2 H, 1 M t-butanol M. Simic and M. Z. Hoffman, J. Phys. Chem. , 76, 1398 (1972) 26
 Adducts arm
Adducts arm Paradigm shift from women studies to gender studies
Paradigm shift from women studies to gender studies Water an elixir of life essay
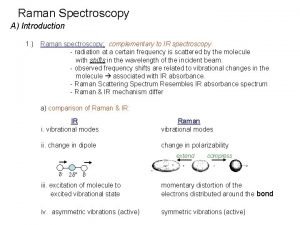
Water an elixir of life essay Why are raman and ir complementary
Why are raman and ir complementary Difference between internet and www
Difference between internet and www Edfa gain spectrum
Edfa gain spectrum فونت رامان
فونت رامان Applications of raman effect in daily life
Applications of raman effect in daily life Laser coherence
Laser coherence Portable raman analyzer for hazmat and narcotics
Portable raman analyzer for hazmat and narcotics Narayanan raman
Narayanan raman Raman saçılması
Raman saçılması Raman letchumanan
Raman letchumanan Raman 1
Raman 1 Raman spectroscopy selection rules
Raman spectroscopy selection rules Rayleigh vs raman scattering
Rayleigh vs raman scattering Vijayshankar raman
Vijayshankar raman Acharyamarch
Acharyamarch Fizik raman
Fizik raman Sanjay raman mit
Sanjay raman mit Raman spectroscopy basics
Raman spectroscopy basics What is the imaginary elixir of life
What is the imaginary elixir of life Kameswari chebrolu
Kameswari chebrolu Aina kuu mbili za ramani
Aina kuu mbili za ramani Sers wiki
Sers wiki Amplificador raman
Amplificador raman Dispersion raman
Dispersion raman