STRUCTURAL CHARACTERIZATION OF HYDROXYL RADICAL ADDUCTS IN AQUEOUS
![Hemibonding of OH and halide ion in aqueous solution Lambda max [nm] Epsilon/103 [M-1 Hemibonding of OH and halide ion in aqueous solution Lambda max [nm] Epsilon/103 [M-1](https://slidetodoc.com/presentation_image_h2/cfd01aceee2688a8e51f7e33c1be5d7d/image-11.jpg)

- Slides: 17

STRUCTURAL CHARACTERIZATION OF HYDROXYL RADICAL ADDUCTS IN AQUEOUS MEDIA Irek Janik, GNR Tripathi Notre Dame Radiation Laboratory

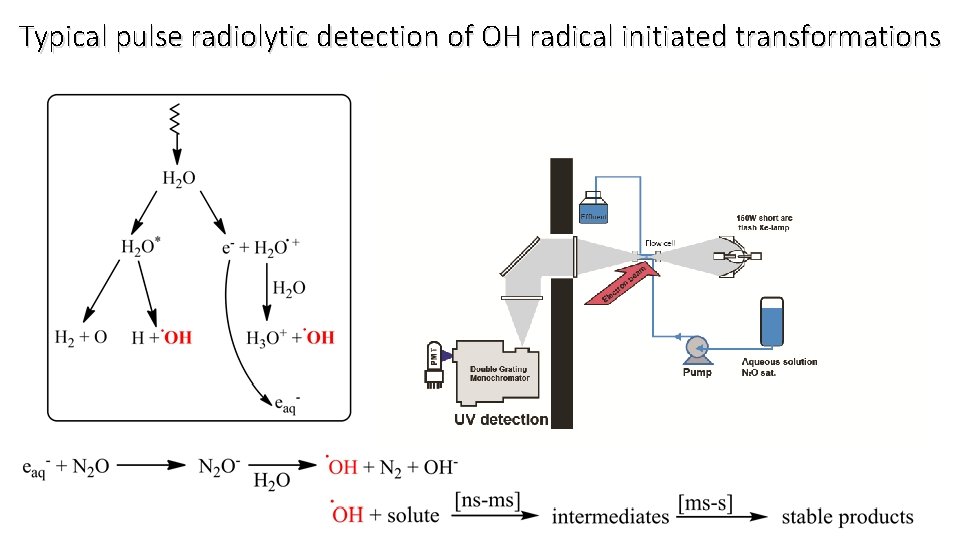
Typical pulse radiolytic detection of OH radical initiated transformations

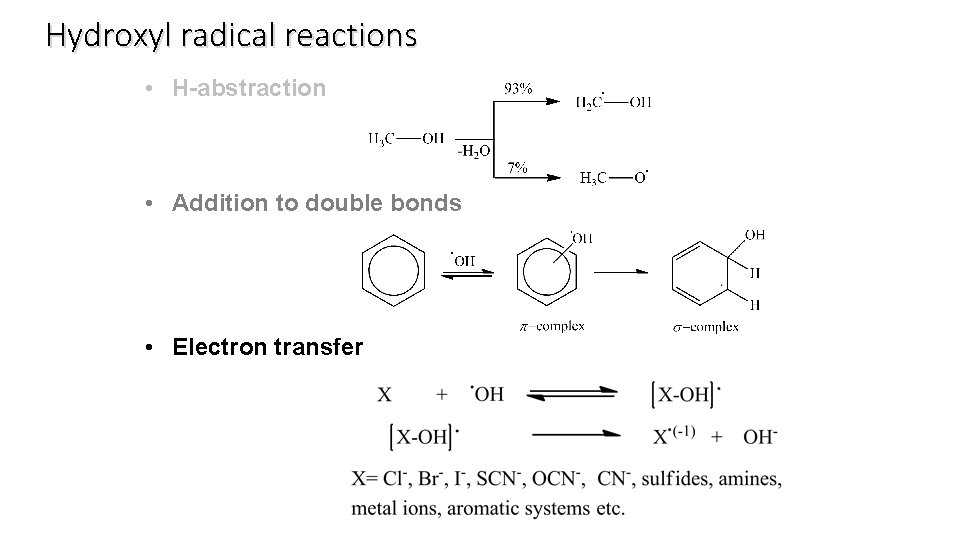
Hydroxyl radical reactions • H-abstraction • Addition to double bonds • Electron transfer

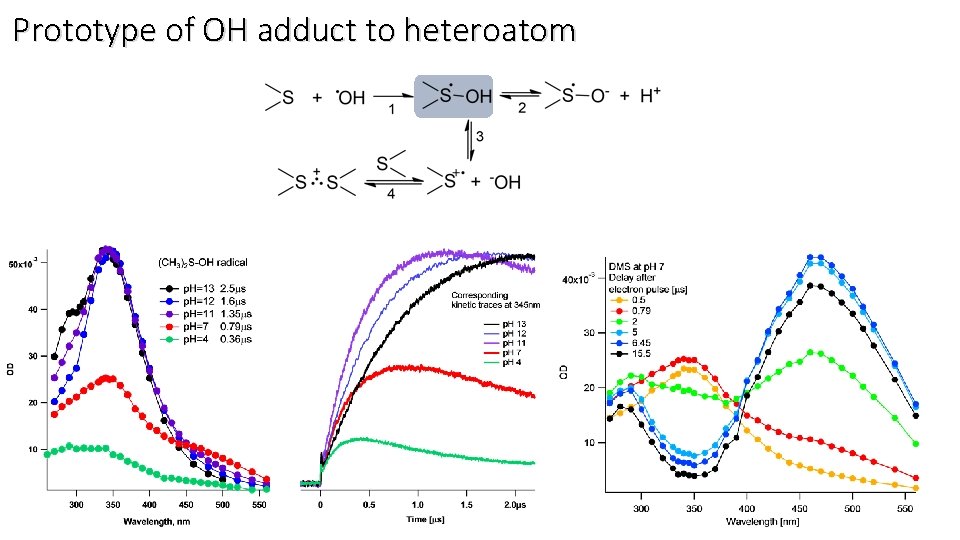
Prototype of OH adduct to heteroatom

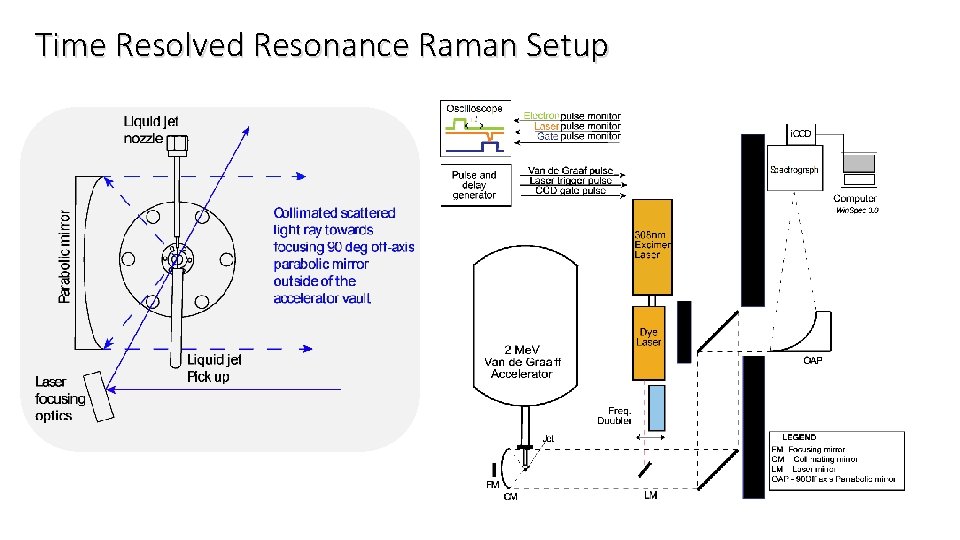
Time Resolved Resonance Raman Setup

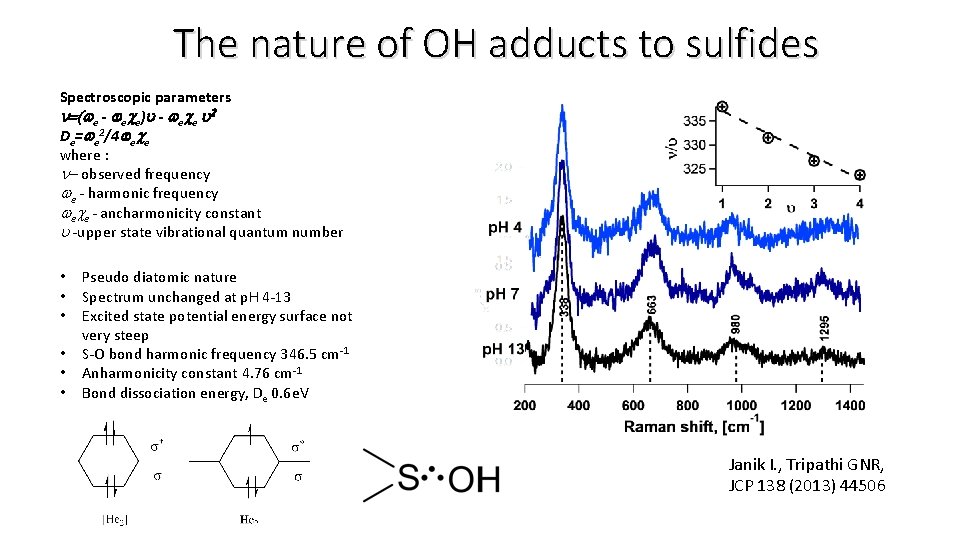
The nature of OH adducts to sulfides Spectroscopic parameters n=(we - wece)u - wece u 2 De=we 2/4 wece where : n- observed frequency we - harmonic frequency wece - ancharmonicity constant u -upper state vibrational quantum number • • • Pseudo diatomic nature Spectrum unchanged at p. H 4 -13 Excited state potential energy surface not very steep S-O bond harmonic frequency 346. 5 cm-1 Anharmonicity constant 4. 76 cm-1 Bond dissociation energy, De 0. 6 e. V Janik I. , Tripathi GNR, JCP 138 (2013) 44506

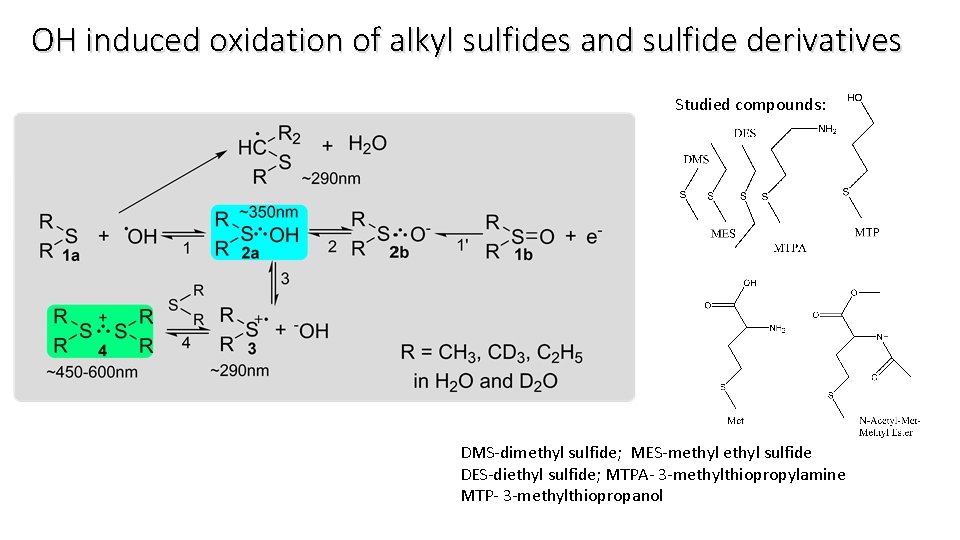
OH induced oxidation of alkyl sulfides and sulfide derivatives Studied compounds: DMS-dimethyl sulfide; MES-methyl sulfide DES-diethyl sulfide; MTPA- 3 -methylthiopropylamine MTP- 3 -methylthiopropanol

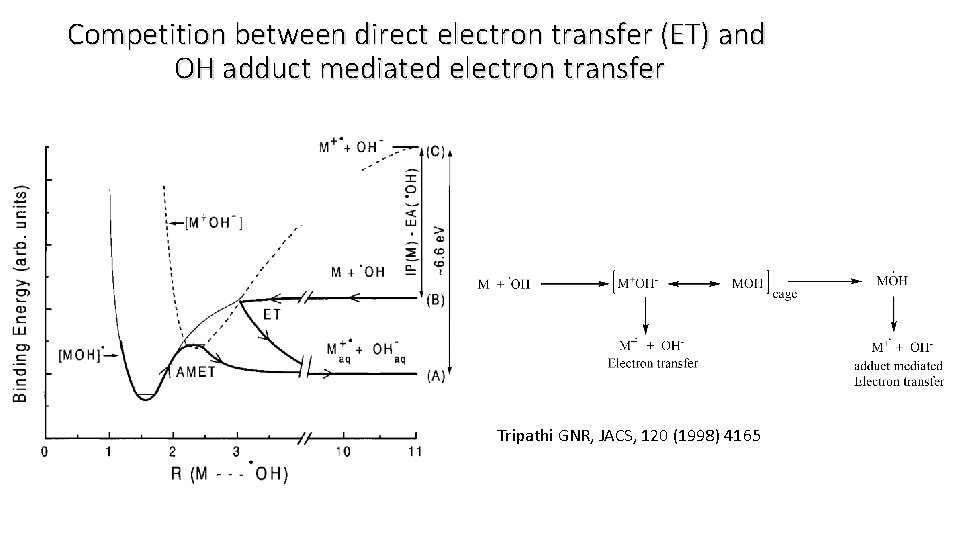
Competition between direct electron transfer (ET) and OH adduct mediated electron transfer Tripathi GNR, JACS, 120 (1998) 4165

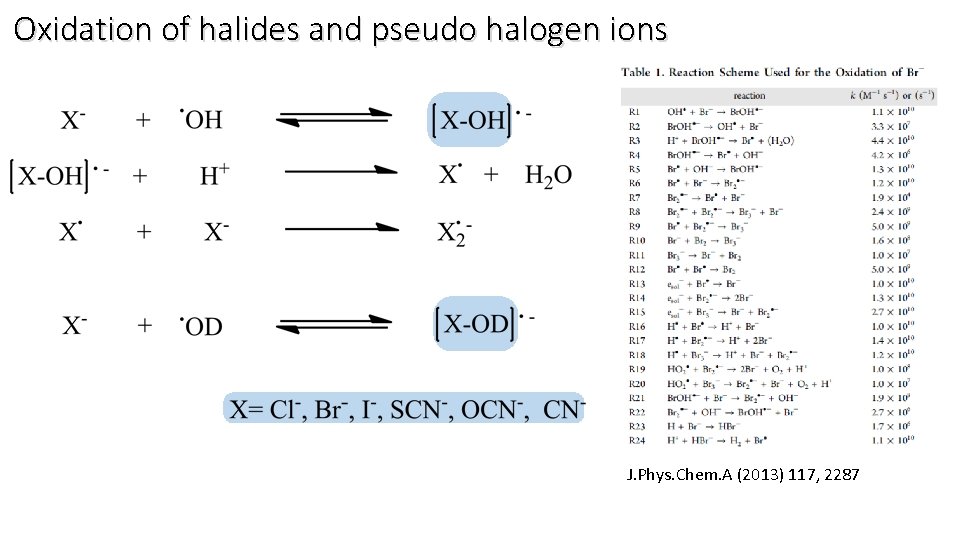
Oxidation of halides and pseudo halogen ions J. Phys. Chem. A (2013) 117, 2287

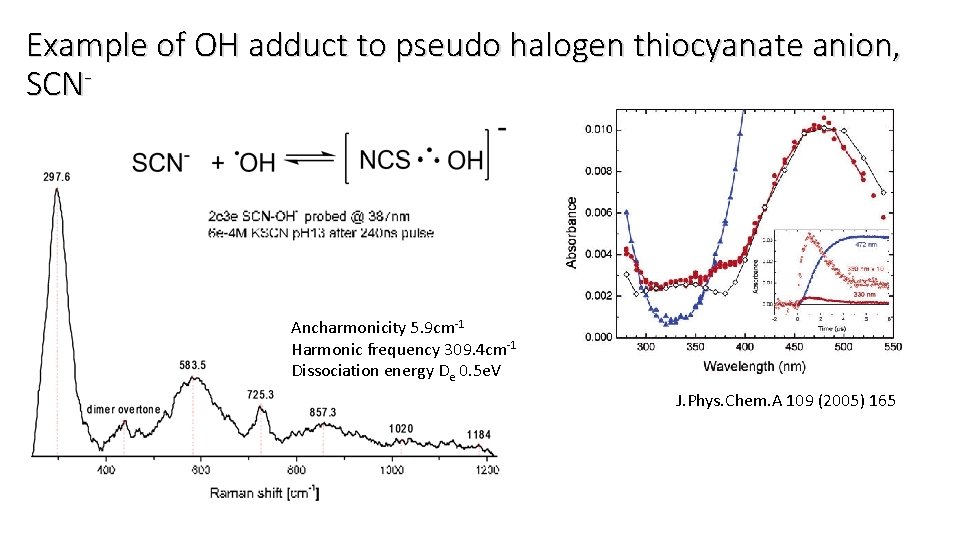
Example of OH adduct to pseudo halogen thiocyanate anion, SCN- Ancharmonicity 5. 9 cm-1 Harmonic frequency 309. 4 cm-1 Dissociation energy De 0. 5 e. V J. Phys. Chem. A 109 (2005) 165
![Hemibonding of OH and halide ion in aqueous solution Lambda max nm Epsilon103 M1 Hemibonding of OH and halide ion in aqueous solution Lambda max [nm] Epsilon/103 [M-1](https://slidetodoc.com/presentation_image_h2/cfd01aceee2688a8e51f7e33c1be5d7d/image-11.jpg)
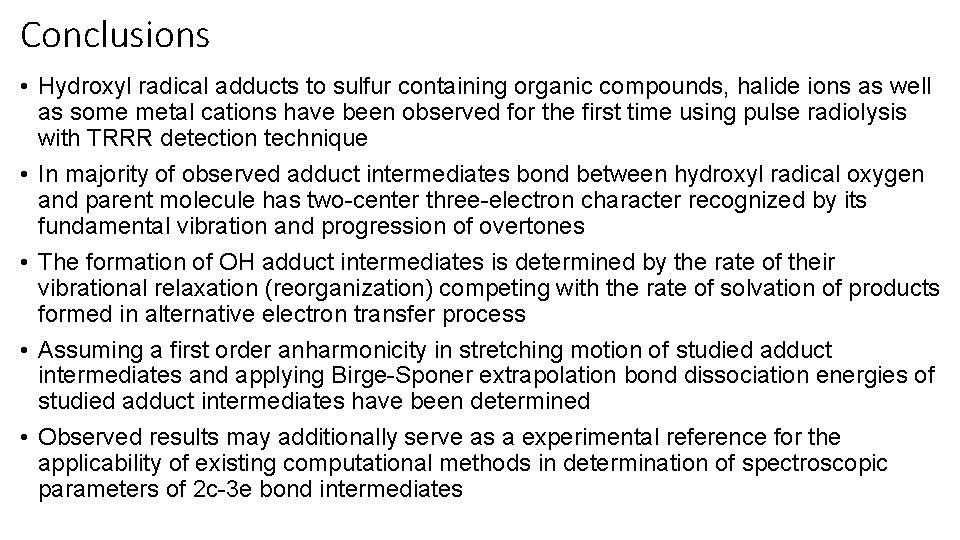
Hemibonding of OH and halide ion in aqueous solution Lambda max [nm] Epsilon/103 [M-1 cm-1] X-O stretching [cm-1] R (X-O) [A] Cl-OH 350 (348) 3. 7 (5. 7) 162, 180 2. 417 Br-OH 360 (343) 8 (7. 1) 285 2. 402 I-OH 335 (324) 4. 5 (6. 9) 277 2. 485 DFT with range-separated hybrid (RSH) exchange-correlation functional M. Yamaguchi, J. Phys. Chem. A 2011, 115, 14620

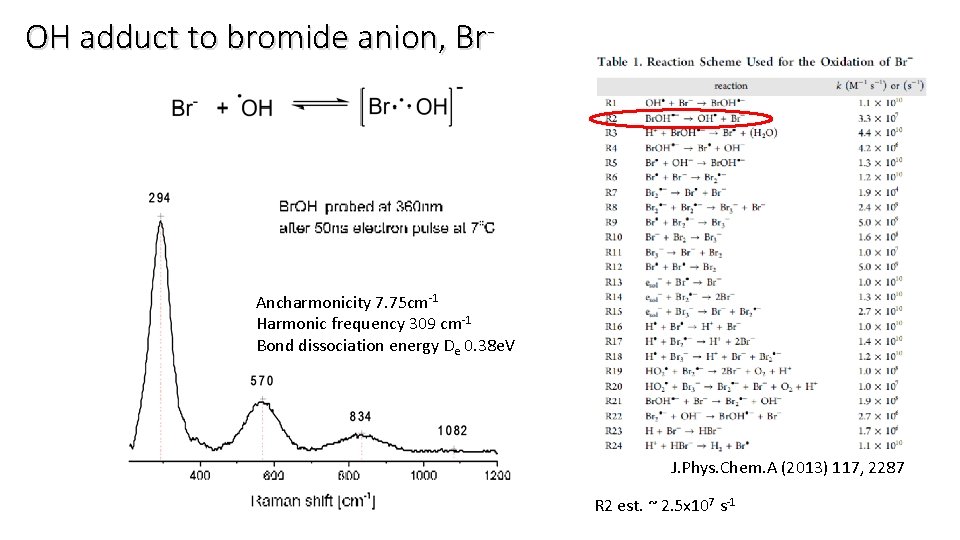
OH adduct to bromide anion, Br- Ancharmonicity 7. 75 cm-1 Harmonic frequency 309 cm-1 Bond dissociation energy De 0. 38 e. V J. Phys. Chem. A (2013) 117, 2287 R 2 est. ~ 2. 5 x 107 s-1

Oxidation of transition metal cations

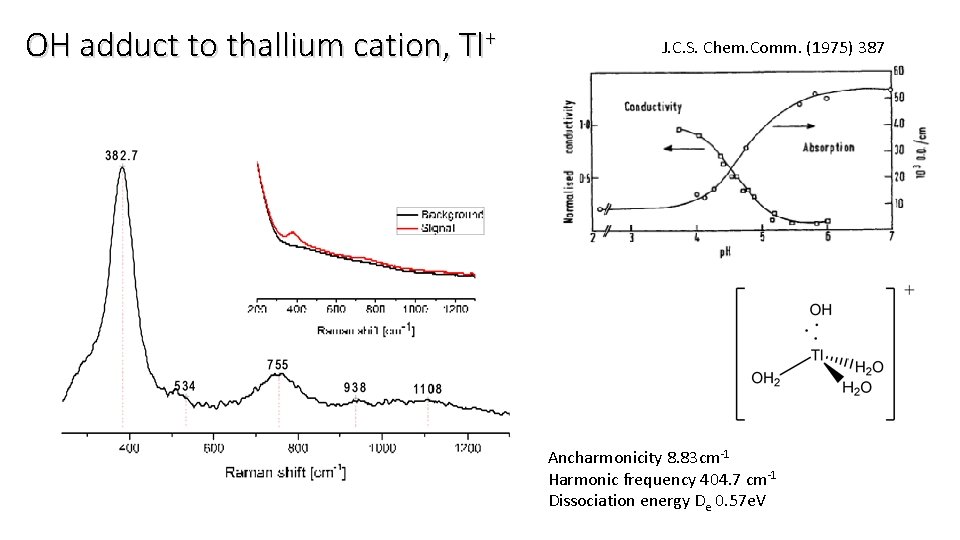
OH adduct to thallium cation, Tl+ J. C. S. Chem. Comm. (1975) 387 Ancharmonicity 8. 83 cm-1 Harmonic frequency 404. 7 cm-1 Dissociation energy De 0. 57 e. V

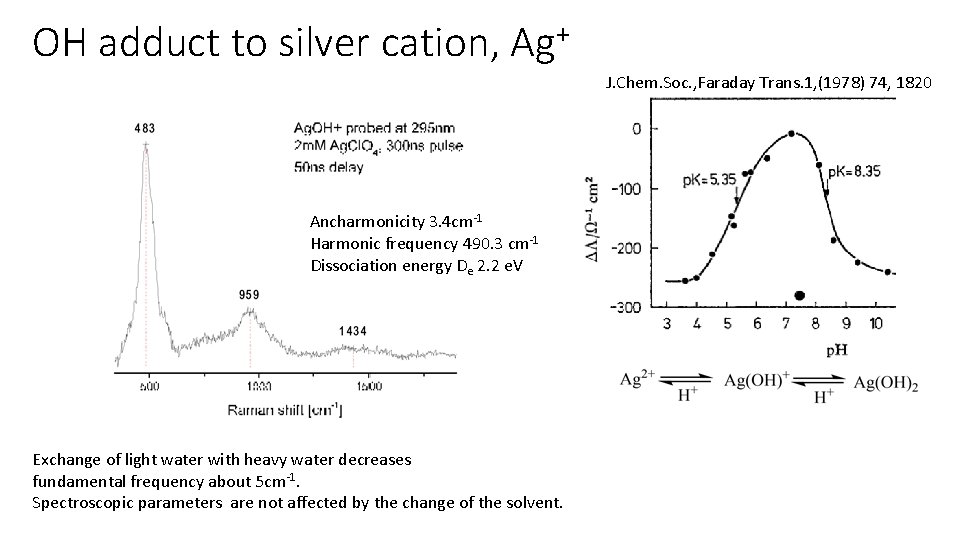
OH adduct to silver cation, + Ag J. Chem. Soc. , Faraday Trans. 1, (1978) 74, 1820 Ancharmonicity 3. 4 cm-1 Harmonic frequency 490. 3 cm-1 Dissociation energy De 2. 2 e. V Exchange of light water with heavy water decreases fundamental frequency about 5 cm-1. Spectroscopic parameters are not affected by the change of the solvent.

Conclusions • Hydroxyl radical adducts to sulfur containing organic compounds, halide ions as well as some metal cations have been observed for the first time using pulse radiolysis with TRRR detection technique • In majority of observed adduct intermediates bond between hydroxyl radical oxygen and parent molecule has two-center three-electron character recognized by its fundamental vibration and progression of overtones • The formation of OH adduct intermediates is determined by the rate of their vibrational relaxation (reorganization) competing with the rate of solvation of products formed in alternative electron transfer process • Assuming a first order anharmonicity in stretching motion of studied adduct intermediates and applying Birge-Sponer extrapolation bond dissociation energies of studied adduct intermediates have been determined • Observed results may additionally serve as a experimental reference for the applicability of existing computational methods in determination of spectroscopic parameters of 2 c-3 e bond intermediates

Acknowledgements Students Daniel Stuart Rohit Chikkaraddy Faculty Gordon Hug Ian Carmichael
 Temporalis
Temporalis Aldotrióza
Aldotrióza để cm glucozo có nhiều nhóm hydroxyl
để cm glucozo có nhiều nhóm hydroxyl Aldopentóza
Aldopentóza Pcc with aldehyde
Pcc with aldehyde Unit 6 test radical functions
Unit 6 test radical functions Radical section
Radical section Entire radical examples
Entire radical examples Indirect characterization
Indirect characterization Indirect characterization
Indirect characterization An artificial cell consisting of an aqueous solution
An artificial cell consisting of an aqueous solution Solid liquid, gas aqueous chart
Solid liquid, gas aqueous chart Vestibulocochlear nerve
Vestibulocochlear nerve Aqueous ammonia
Aqueous ammonia Aqueous solution
Aqueous solution Manipulation of impression compound
Manipulation of impression compound Chemical reactions section 3 reactions in aqueous solutions
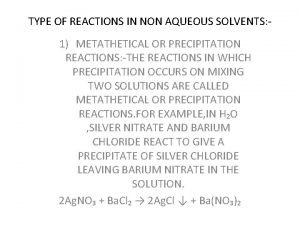
Chemical reactions section 3 reactions in aqueous solutions Types of reaction in non aqueous solvents
Types of reaction in non aqueous solvents