Quantification of hydroxyl radical during phacoemulsification Department of






- Slides: 12

Quantification of hydroxyl radical during phacoemulsification. Department of Ophthalmology, Dokkyo Medical University (JAPAN) Norihito Gotoh MD Ph. D, Hiroyuki Matsushima MD Ph. D, Koichiro Mukai Ph. D, Makoto Takayama MD, and Tadashi Senoo MD Ph. D The authors have no financial interest in the subject matter of this poster.

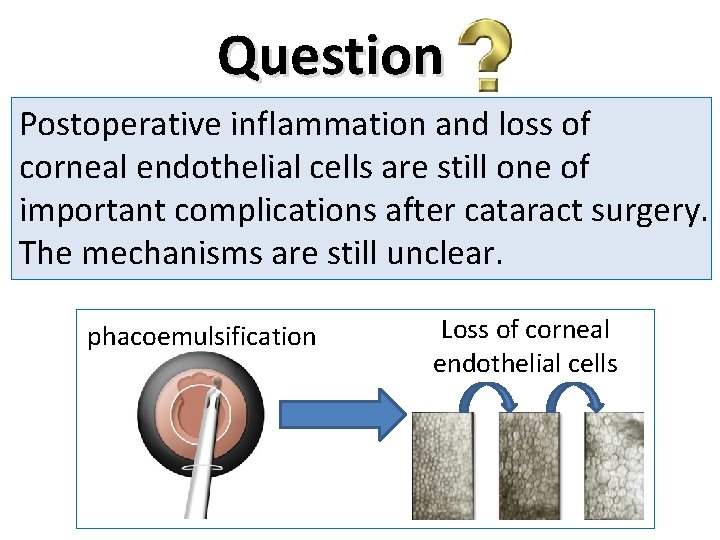
Question Postoperative inflammation and loss of corneal endothelial cells are still one of important complications after cataract surgery. The mechanisms are still unclear. phacoemulsification Loss of corneal endothelial cells

Theories about loss of corneal cells after cataract surgery Oxidation, thermal reaction, water flow, etc… We drew attention to oxidation by phacoemulsification and aspiration (PEA) may be one of causes for the complication. Purpose (1) In this study, we detect the hydroxyl radical during PEA.

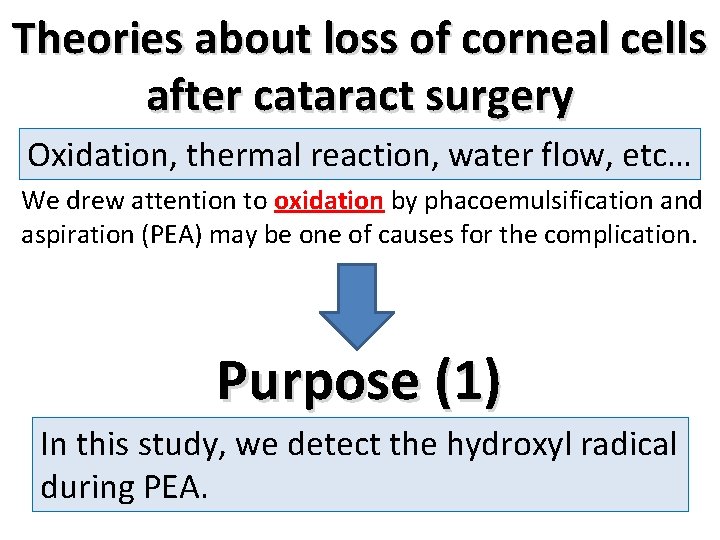
Method to detect hydroxyl radical INFINITITM system (Alcon), a PEA unit was prepared. To trap the hydroxyl radical, 1. 5 ml of distilled water with 1% trapping agent (5, 5 -dimethyl-1 -pyrroline N-oxide, LABOTEC) was added in test chamber. After the oscillation the trapping solution was obtained immediately and electron spin resonance (ESR) trapping methods (JES-RE 1 X, JEOL) were used to detect the hydroxyl radical production.

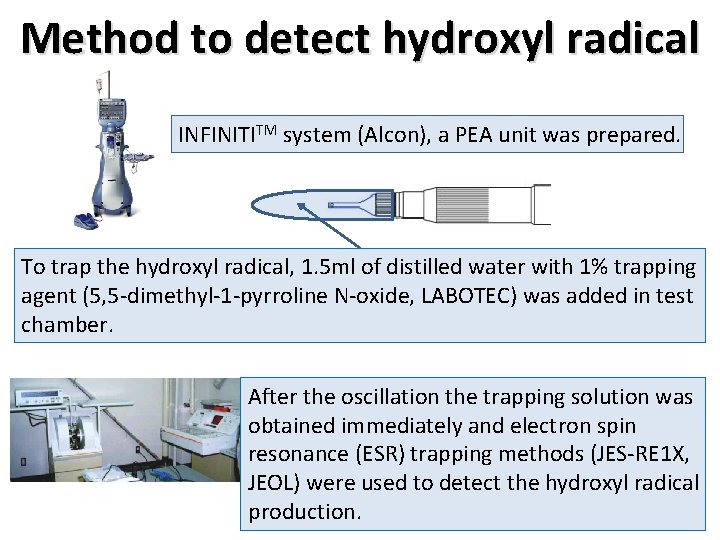
Typical hydroxyl radical by ESR (positive control: Fenton reaction) Fe 2+ Control: manganese . + H 2 O 2 → Fe 3+ + HO- + HO Control: manganese Hydroxyl radical is a strongest oxidation in some free radicals. Hydroxyl radical bound trapping agent is detected 4 peaks by electron spin resonance (ESR). Fenton reaction is a typical reaction of hydroxyl radical production (positive control).

Result to detect hydroxyl radical by ESR Condition: 100% traditional-PEA for 30 seconds. Mn Mn Answer (1) Hydroxyl radical was produced during PEA.

The relationship between different systems of PEA (traditional & torsional) and loss of corneal endothelial cells have been reported in the following. Comparison of a torsional handpiece through microincision versus standard clear corneal cataract wounds. Berdahl JP, Jun B, De. Stafeno JJ, Kim T. J Cataract Refract Surg. 2008; 34(12): 2091 -5. Comparison of endothelial changes and power settings between torsional and longitudinal phacoemulsification. Reuschel A, Bogatsch H, Barth T, Wiedemann R. J Cataract Refract Surg. 2010; 36(11): 1855 -61 Purpose (2) Next, we quantitate the hydroxyl radical during oscillations using traditional- and torsional-PEAs.

Method to quantitate hydroxyl radical <Different settings of PEA> 100% traditional-PEA 50% traditional-PEA 100% torsional-PEA 50% torsional-PEA Traditional- and torsional-PEA was oscillated for 30 seconds with fixing test chamber and cumulative dissipated energy (CDE) was measured. ESR was also used to detect the radical production. Method to calculate hydroxyl radical level The level of hydroxyl radical production were calculated by percentage of manganese peak. B Level of hydroxyl radical (%) = x 100 A Control: manganese A B

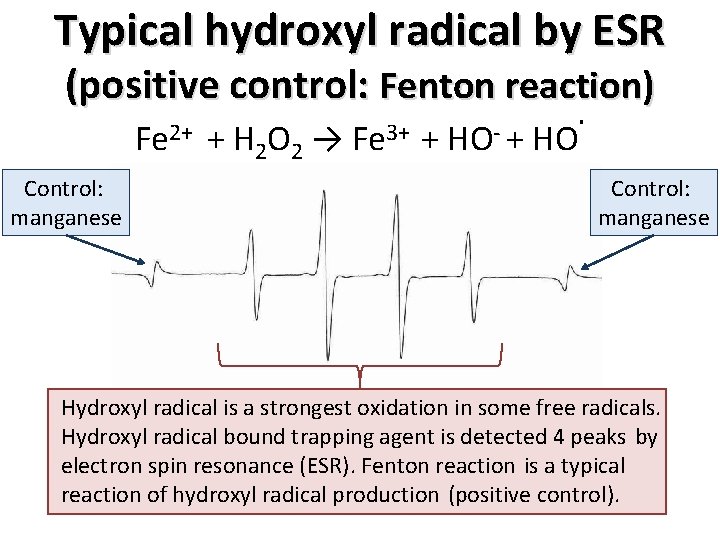
Results: quantity of hydroxyl radical Mn 100% traditional-PEA Mn Mn 50% traditional-PEA Level of hydroxyl radical = 11. 1± 2. 3% CDE = 14. 8± 0. 2 Mn Level of hydroxyl radical = 6. 0± 3. 1% CDE = 12. 1± 0. 2 Level of hydroxyl radical = 18. 9± 8. 9% CDE = 30. 4± 0. 2 Mn 100% torsional-PEA Mn Mn 50% torsional-PEA Mn Level of hydroxyl radical = 2. 5± 0. 3% CDE = 5. 9± 0. 1 The quantity of hydroxyl radical was smaller using torsional-PEA than traditional-PEA, and was increased by an increase in US power. . • torsional-PEA <<traditional PEA • low US power << high US power

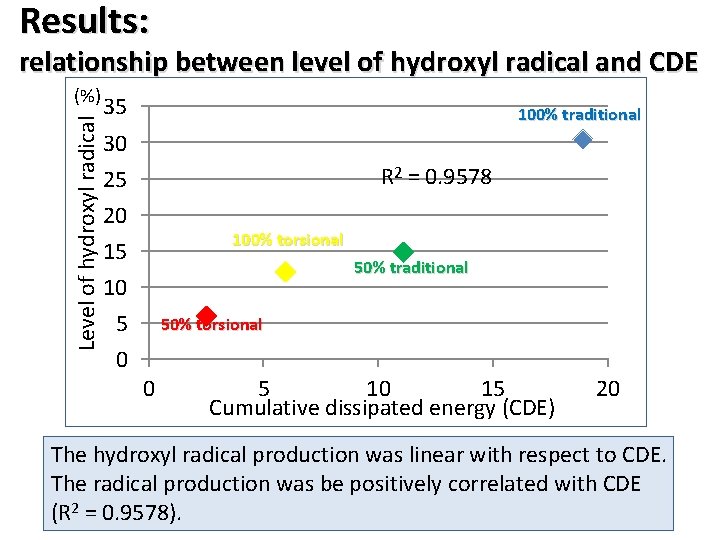
Results: relationship between level of hydroxyl radical and CDE Level of hydroxyl radical (%) 35 100% traditional 30 R 2 = 0. 9578 25 20 100% torsional 15 50% traditional 10 5 50% torsional 0 0 5 10 15 Cumulative dissipated energy (CDE) 20 The hydroxyl radical production was linear with respect to CDE. The radical production was be positively correlated with CDE (R 2 = 0. 9578).

Answer (2) • The quantity of hydroxyl radical was affected by PEA system (traditional or torsional) and US power. torsional-PEA << traditional-PEA low US power << high US power • The radical production was be positively correlated with CDE (R 2 = 0. 9578).

Conclusions • Hydroxyl radical was produced during PEA and its quantity of production was dependent on CDE during PEA. • The quantity of hydroxyl radical was smaller using torsional-PEA than traditional-PEA. • Oxidative stress in the eye may be minimized by using torsional-PEA.
 để cm glucozo có nhiều nhóm hydroxyl
để cm glucozo có nhiều nhóm hydroxyl Vacuous quantification
Vacuous quantification Highly multiplexed protein quantification
Highly multiplexed protein quantification Universal quantification examples
Universal quantification examples Poloacetalový hydroxyl
Poloacetalový hydroxyl Polysacharidy
Polysacharidy Alcohol and hbr
Alcohol and hbr Entire radical
Entire radical Entire radical
Entire radical Radical functions and rational exponents unit test
Radical functions and rational exponents unit test Verbos con cambio radical
Verbos con cambio radical Free radical halogenation
Free radical halogenation 5-8 applying special right triangles
5-8 applying special right triangles