Residual risk Is LDL target enough An interpretation



























- Slides: 55

Residual risk: Is LDL target enough?

An interpretation of the continuous relationship between LDL-C and CVD That there is no cut-off cholesterol number below which coronary heart disease cannot develop. Therefore, many men and most women with heart disease have lipid problems other than high total or LDL cholesterol that put them at risk for heart disease. Edward F Gibbons MD Editor of New England Journal Medicine Heart Watch June 2001 Vol 5 #5 p 3

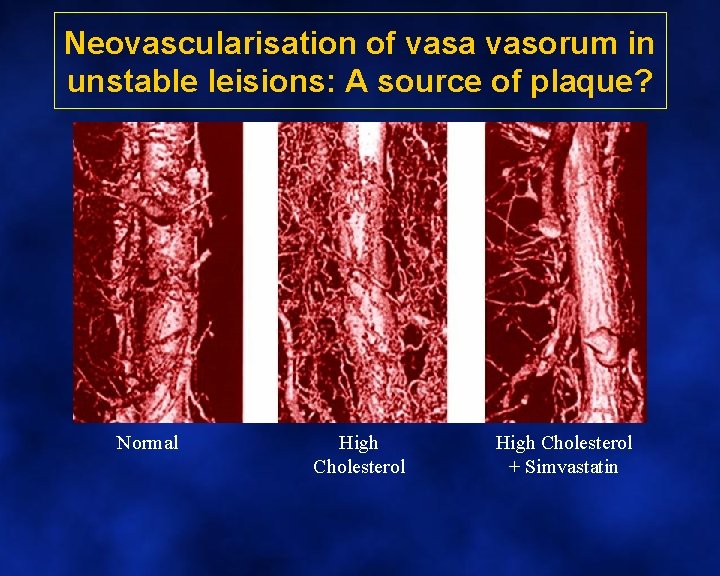
Neovascularisation of vasa vasorum in unstable leisions: A source of plaque? Normal High Cholesterol + Simvastatin

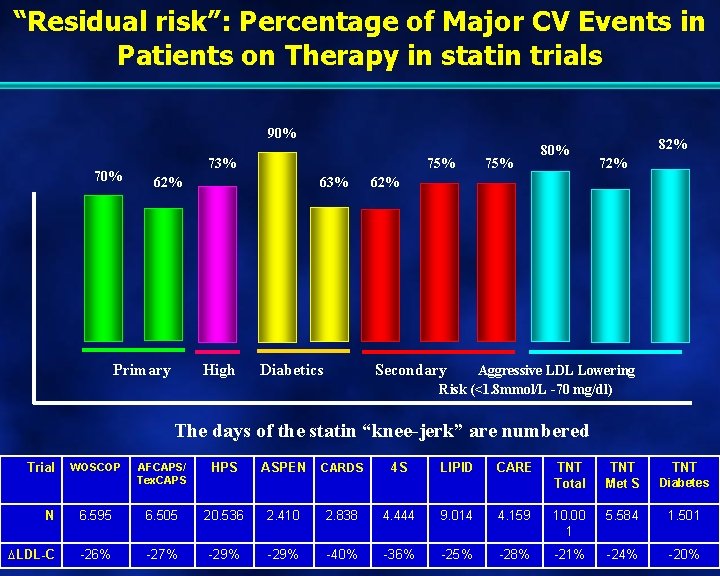
“Residual risk”: Percentage of Major CV Events in Patients on Therapy in statin trials 90% 73% 75% 62% Primary 63% High Diabetics 75% 80% 82% 72% 62% Secondary Aggressive LDL Lowering Risk (<1. 8 mmol/L -70 mg/dl) The days of the statin “knee-jerk” are numbered Trial WOSCOP AFCAPS/ Tex. CAPS HPS ASPEN CARDS 4 S LIPID CARE TNT Total TNT Met S Diabetes TNT N 6. 595 6. 505 20. 536 2. 410 2. 838 4. 444 9. 014 4. 159 10. 00 1 5. 584 1. 501 LDL-C -26% -27% -29% -40% -36% -25% -28% -21% -24% -20%

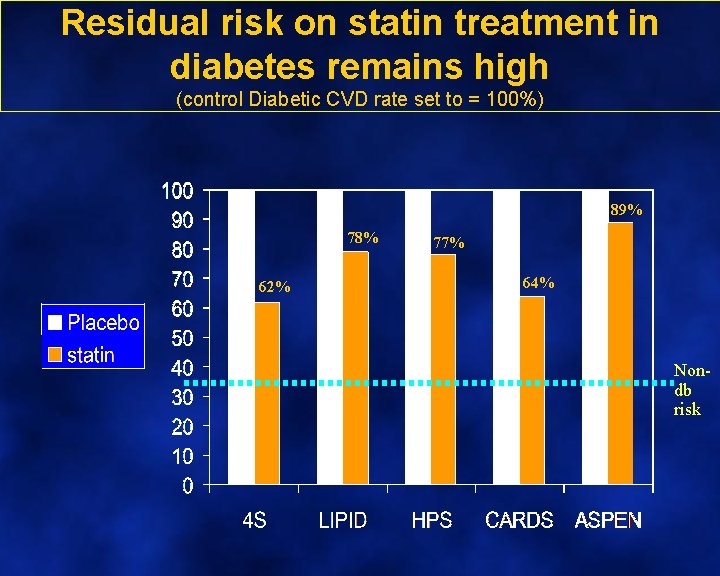
Residual risk on statin treatment in diabetes remains high (control Diabetic CVD rate set to = 100%) 89% 78% 62% 77% 64% Nondb risk

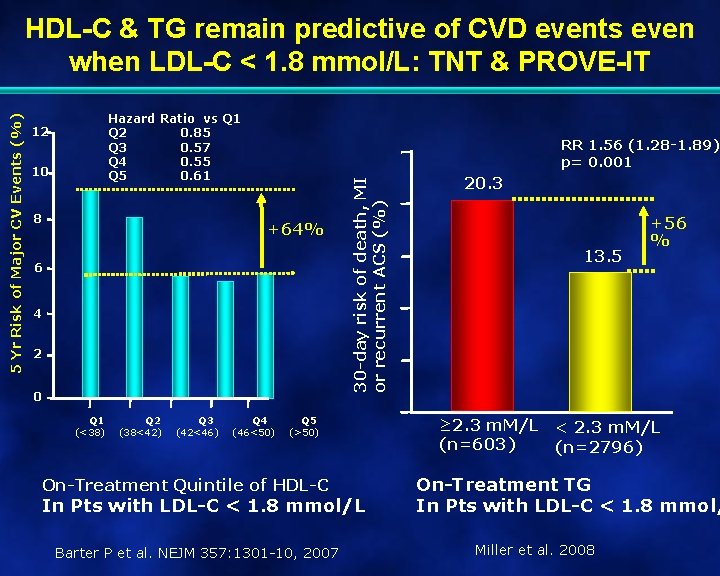
Hazard Ratio vs Q 1 Q 2 0. 85 Q 3 0. 57 Q 4 0. 55 Q 5 0. 61 12 10 8 RR 1. 56 (1. 28 -1. 89) p= 0. 001 +64% 6 4 2 0 Q 1 (<38) Q 2 (38<42) Q 3 (42<46) Q 4 (46<50) 30 -day risk of death, MI or recurrent ACS (%) 5 Yr Risk of Major CV Events (%) HDL-C & TG remain predictive of CVD events even when LDL-C < 1. 8 mmol/L: TNT & PROVE-IT Q 5 (>50) On-Treatment Quintile of HDL-C In Pts with LDL-C < 1. 8 mmol/L Barter P et al. NEJM 357: 1301 -10, 2007 20. 3 13. 5 +56 % ≥ 2. 3 m. M/L < 2. 3 m. M/L (n=603) (n=2796) On-Treatment TG In Pts with LDL-C < 1. 8 mmol/ Miller et al. 2008

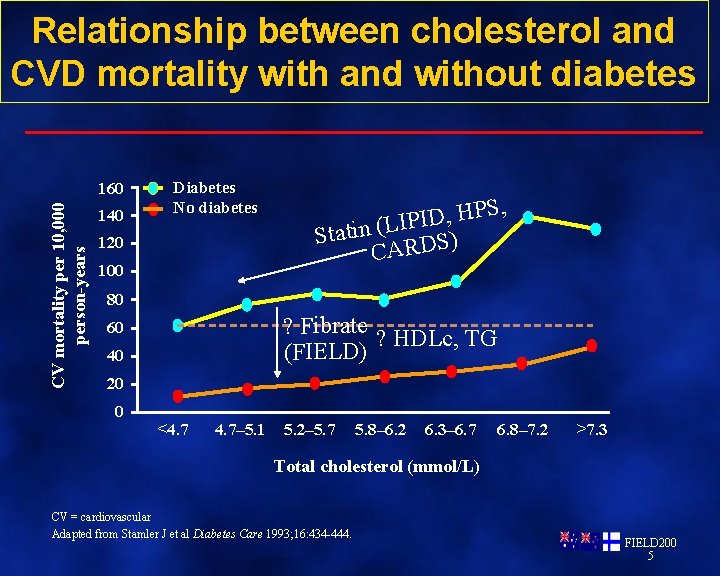
Relationship between cholesterol and CVD mortality with and without diabetes CV mortality per 10, 000 person-years 160 140 Diabetes No diabetes 120 100 S, P H , D I IP L ( n i t a t S ) CARDS 80 ? Fibrate ? HDLc, TG (FIELD) 60 40 20 0 <4. 7– 5. 1 5. 2– 5. 7 5. 8– 6. 2 6. 3– 6. 7 6. 8– 7. 2 >7. 3 Total cholesterol (mmol/L) CV = cardiovascular Adapted from Stamler J et al Diabetes Care 1993; 16: 434 -444. FIELD 200 5

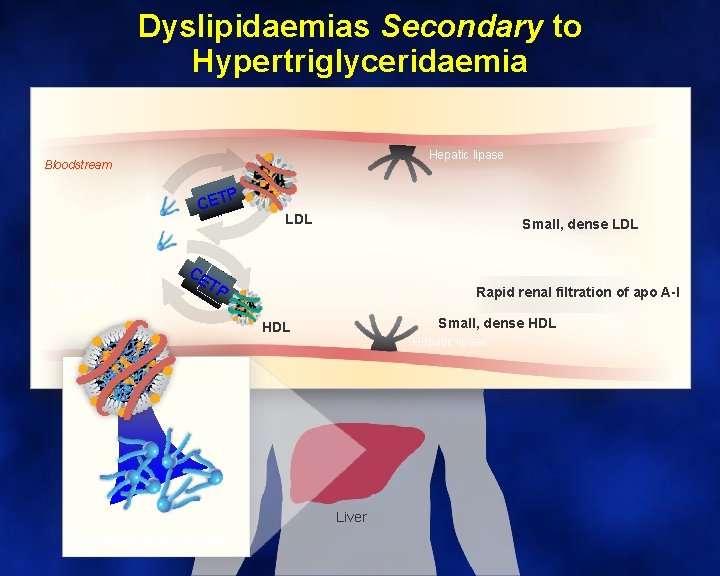
Dyslipidaemias Secondary to Hypertriglyceridaemia Hepatic lipase Bloodstream P CET Increased VLDL Small, dense LDL CE TP Rapid renal filtration of apo A-I Small, dense HDL Hepatic lipase Liver Increased triglycerides

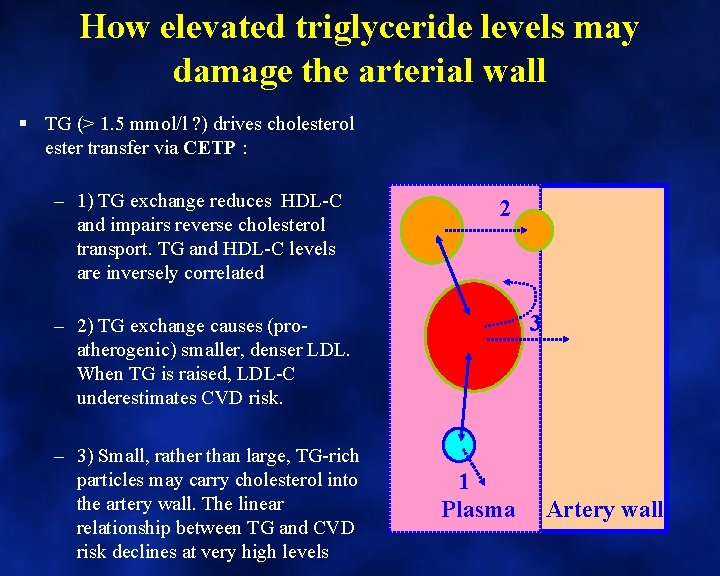
How elevated triglyceride levels may damage the arterial wall § TG (> 1. 5 mmol/l ? ) drives cholesterol ester transfer via CETP : – 1) TG exchange reduces HDL-C and impairs reverse cholesterol transport. TG and HDL-C levels are inversely correlated 2 3 – 2) TG exchange causes (proatherogenic) smaller, denser LDL. When TG is raised, LDL-C underestimates CVD risk. – 3) Small, rather than large, TG-rich particles may carry cholesterol into the artery wall. The linear relationship between TG and CVD risk declines at very high levels 1 Plasma Artery wall

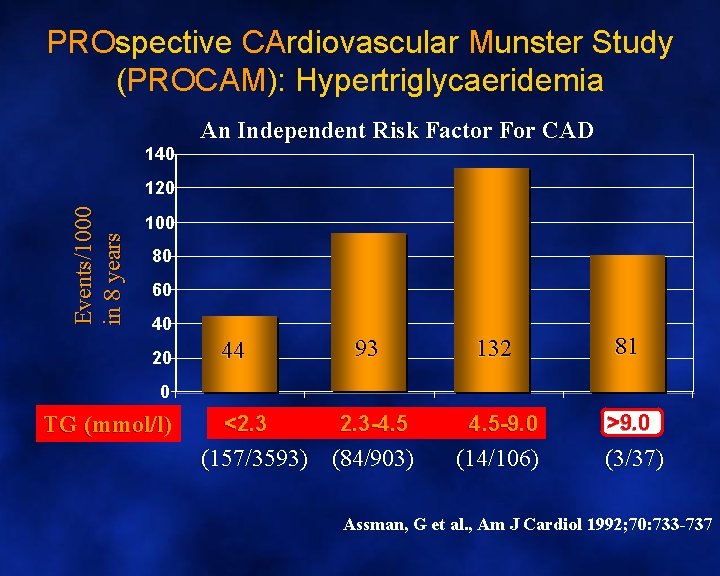
PROspective CArdiovascular Munster Study (PROCAM): Hypertriglycaeridemia 140 An Independent Risk Factor For CAD Events/1000 in 8 years 120 100 80 60 40 20 44 93 132 81 0 TG (mmol/l) <2. 3 -4. 5 -9. 0 (157/3593) (84/903) (14/106) >9. 0 (3/37) Assman, G et al. , Am J Cardiol 1992; 70: 733 -737

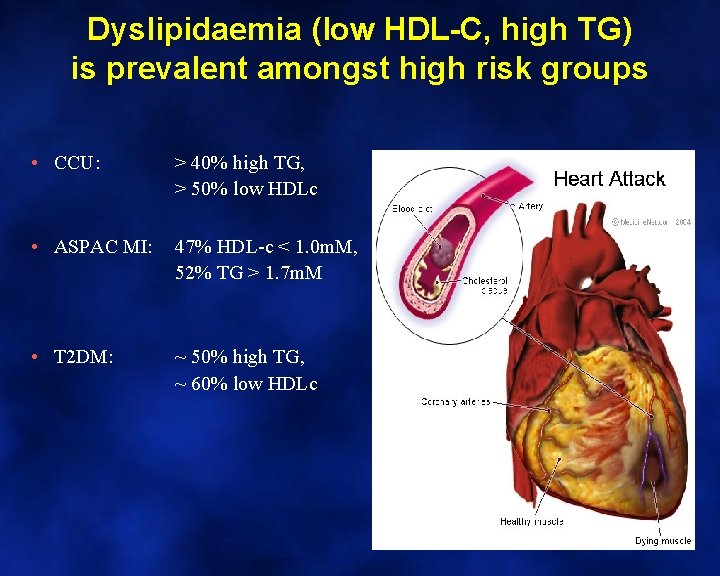
Dyslipidaemia (low HDL-C, high TG) is prevalent amongst high risk groups • CCU: > 40% high TG, > 50% low HDLc • ASPAC MI: 47% HDL-c < 1. 0 m. M, 52% TG > 1. 7 m. M • T 2 DM: ~ 50% high TG, ~ 60% low HDLc

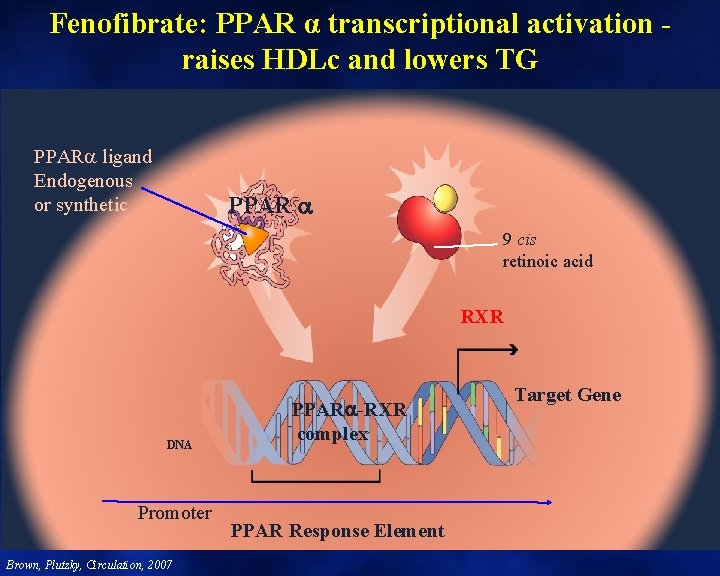
Fenofibrate: PPAR α transcriptional activation raises HDLc and lowers TG PPAR ligand Endogenous or synthetic PPAR 9 cis retinoic acid RXR DNA Promoter Brown, Plutzky, Circulation, 2007 PPAR -RXR complex PPAR Response Element Target Gene

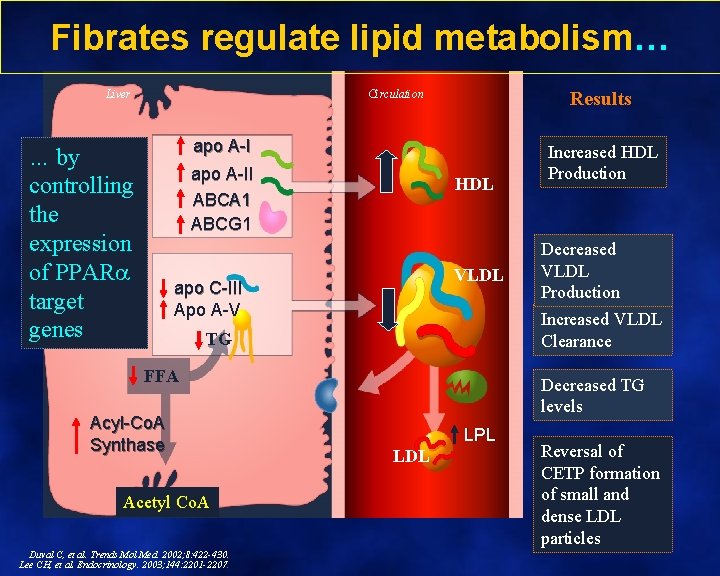
Fibrates regulate lipid metabolism… Liver Circulation Results apo A-I … by controlling the expression of PPAR target genes apo A-II ABCA 1 ABCG 1 HDL VLDL apo C-III Apo A-V FFA Acetyl Co. A Duval C, et al. Trends Mol Med. 2002; 8: 422 -430. Lee CH, et al. Endocrinology. 2003; 144: 2201 -2207. Decreased VLDL Production Increased VLDL Clearance TG Acyl-Co. A Synthase Increased HDL Production Decreased TG levels LPL LDL Reversal of CETP formation of small and dense LDL particles

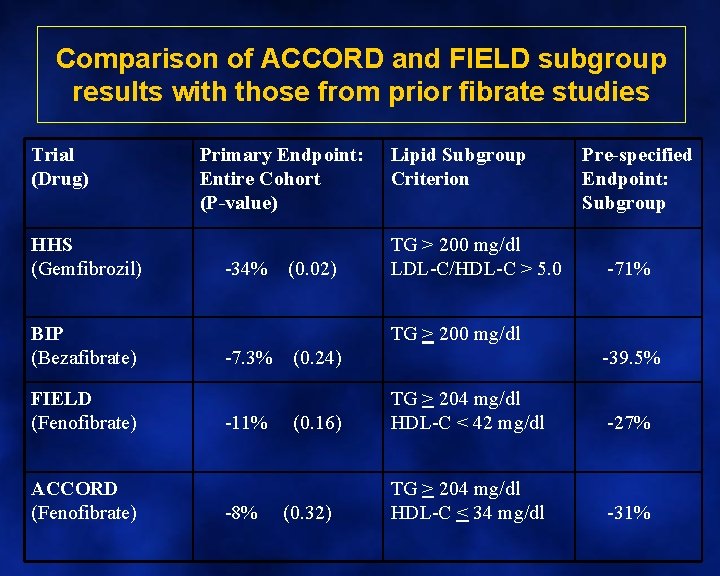
Comparison of ACCORD and FIELD subgroup results with those from prior fibrate studies Trial (Drug) HHS (Gemfibrozil) BIP (Bezafibrate) FIELD (Fenofibrate) ACCORD (Fenofibrate) Primary Endpoint: Entire Cohort (P-value) -34% (0. 02) Lipid Subgroup Criterion TG > 200 mg/dl LDL-C/HDL-C > 5. 0 TG > 200 mg/dl -7. 3% (0. 24) Pre-specified Endpoint: Subgroup -71% -39. 5% -11% (0. 16) TG > 204 mg/dl HDL-C < 42 mg/dl -27% -8% (0. 32) TG > 204 mg/dl HDL-C < 34 mg/dl -31%

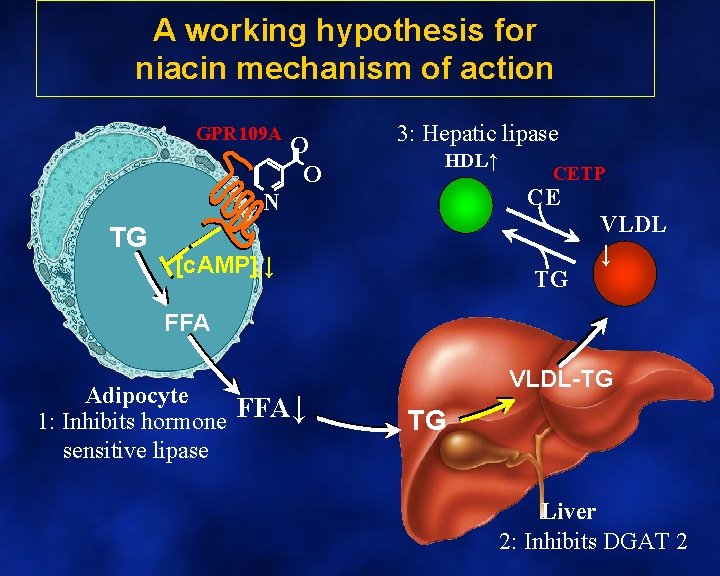
A working hypothesis for niacin mechanism of action GPR 109 A O O 3: Hepatic lipase HDL↑ CE N TG CETP [c. AMP]i↓ TG VLDL ↓ FFA Adipocyte 1: Inhibits hormone FFA↓ sensitive lipase VLDL-TG TG Liver 2: Inhibits DGAT 2

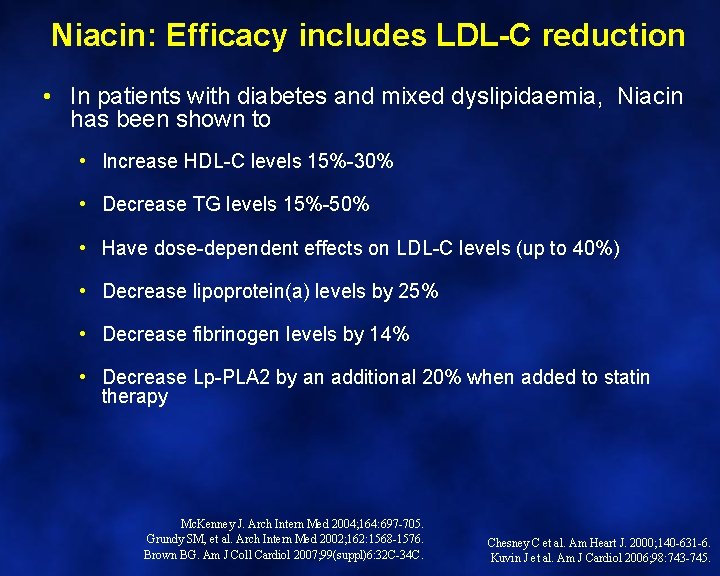
Niacin: Efficacy includes LDL-C reduction • In patients with diabetes and mixed dyslipidaemia, Niacin has been shown to • Increase HDL-C levels 15%-30% • Decrease TG levels 15%-50% • Have dose-dependent effects on LDL-C levels (up to 40%) • Decrease lipoprotein(a) levels by 25% • Decrease fibrinogen levels by 14% • Decrease Lp-PLA 2 by an additional 20% when added to statin therapy Mc. Kenney J. Arch Intern Med 2004; 164: 697 -705. Grundy SM, et al. Arch Intern Med 2002; 162: 1568 -1576. Brown BG. Am J Coll Cardiol 2007; 99(suppl)6: 32 C-34 C. Chesney C et al. Am Heart J. 2000; 140 -631 -6. Kuvin J et al. Am J Cardiol 2006; 98: 743 -745.

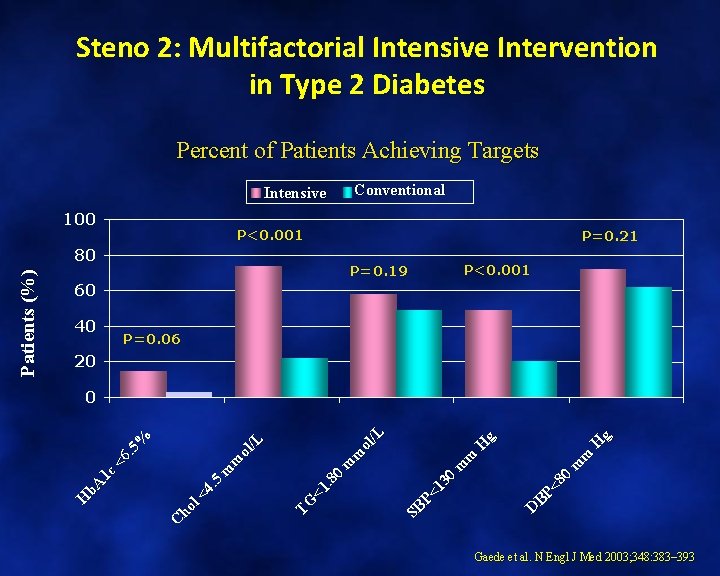
Steno 2: Multifactorial Intensive Intervention in Type 2 Diabetes Percent of Patients Achieving Targets Intensive 100 P<0. 001 80 P=0. 21 P=0. 19 P<0. 001 60 40 P=0. 06 20 H g D BP <8 0 m m H g SB P< 13 0 m m TG <1. 8 0 m m ol /L C ho l< 4. 5 <6. 5 % 0 H b. A 1 c Patients (%) Conventional Gaede et al. N Engl J Med 2003; 348: 383– 393

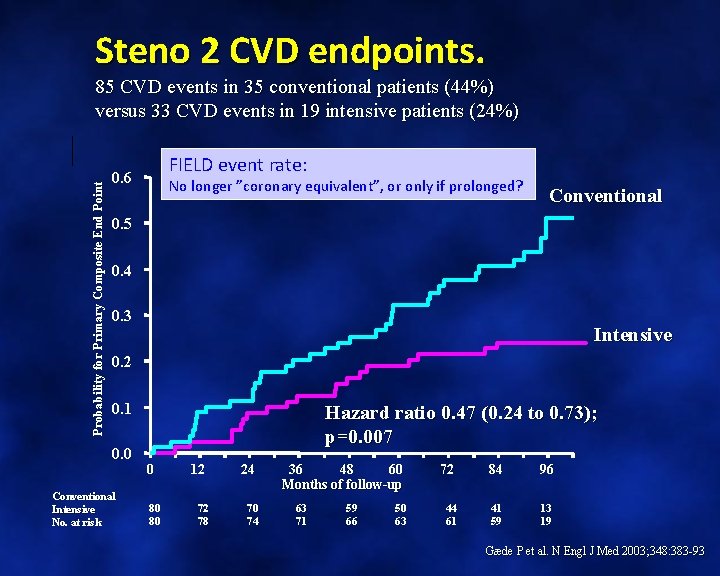
Steno 2 CVD endpoints. Probability for Primary Composite End Point 85 CVD events in 35 conventional patients (44%) versus 33 CVD events in 19 intensive patients (24%) FIELD event rate: 0. 6 No longer ”coronary equivalent”, or only if prolonged? Conventional 0. 5 0. 4 0. 3 Intensive 0. 2 0. 1 0. 0 Conventional Intensive No. at risk Hazard ratio 0. 47 (0. 24 to 0. 73); p=0. 007 0 80 80 12 24 72 78 70 74 36 48 60 Months of follow-up 63 71 59 66 50 63 72 84 96 44 61 41 59 13 19 Gæde P et al. N Engl J Med 2003; 348: 383 -93

Prevention of High-Risk and Recurrent Vascular Disease in 2012? • • Fish/fish oils Fenofibrate Statin Aspirin ACE-I/ARB Beta-blocker Clopidogrel * in hypertension Primary Secondary ? 1 B 1 A 1 A** 1 A 1 A small 1 A 1 A* 1 A small 1 B ** In dyslipidaemic subjects

Darapladib (opposite genetic paradigm)

Summary and link to cases

Mrs M. L. • This 56 year-old lady, who recently suffered a TIA, has a history of SVT, type 2 diabetes diagnosed 4 years ago, and hypertension treated from that point in time. • Drug treatment includes Metformin 500 mg x 2 BD, Aspirin 100 mg, enalapril 10 mg BD, indapamide 2. 5 mg, atenolol 50 mg and maximal tolerated statin dose (simvastatin 40 mg) • Her weight is 68 kg, BMI 28 kg/m 2, • BP 155/98 mm. Hg • Total cholesterol 5. 4 mmol/l, fasting TG 3. 9 mmol/l, HDL 1. 1 mmol/l, calculated LDL 2. 5 mmol/l. • Urinary ACR is 4. 2 • Hb. A 1 C is 7. 3%

Questions concerning Mrs M. L. • Which aspect of her risk factor profile is of the greatest concern? A) BP and ACR B) Weight and BMI C) Total and LDL-C D) TG and HDL-C E) Plasma glucose and Hb. A 1 C • Which aspect of her risk factor profile is most amenable to intervention? A) BP and ACR B) Weight and BMI C) Total and LDL-C D) TG and HDL-C E) Plasma glucose and Hb. A 1 C • If refusal to take more than 1 extra tablet was a limitation, what would you do? A) Add an AII Receptor Blocker B) Add Ezetimibe C) Add a Calcium Channel Blocker D) Add a sulphonylurea E) Add fenofibrate

• This 56 year-old lady, who recently suffered a TIA, has history of SVT, type 2 diabetes diagnosed 4 years ago, and hypertension treated from then. • Drugs: Metformin 500 mg x 2 BD, Aspirin 100 mg, enalapril 10 mg BD, indapamide 2. 5 mg, atenolol 50 mg and maximal tolerated statin dose (simvastatin 40 mg) • Weight is 68 kg, BMI 28 kg/m 2, BP 155/98 mm. Hg, • Total cholesterol 5. 4 mmol/l, fasting TG 3. 9 mmol/l, HDL 1. 1 mmol/l, calculated LDL-C 2. 5 mmol/l. • Urinary ACR is 4. 2 • Hb. A 1 C is 7. 3% Which aspect of her risk factor profile is of the greatest concern? A) BP and ACR B) Weight and BMI C) Total and LDL-C D) TG and HDL-C E) Plasma glucose and Hb A 1 C • Which aspect of her risk factor profile is most amenable to intervention? A) BP and ACR B) Weight and BMI C) Total and LDL-C D) TG and HDL-C E) Plasma glucose and Hb A 1 C • If refusal to take more than 1 extra tablet was a limitation, what would you do? A) Add an AII Receptor Blocker B) Add Ezetimibe C) Add a Calcium Channel Blocker D) Add a sulphonylurea E) Add fenofibrate

Which aspect of her risk factor profile is of the greatest concern? A) BP and ACR B) Weight and BMI C) Total and LDL-C D) TG and HDL-C E) Plasma glucose and Hb. A 1 C

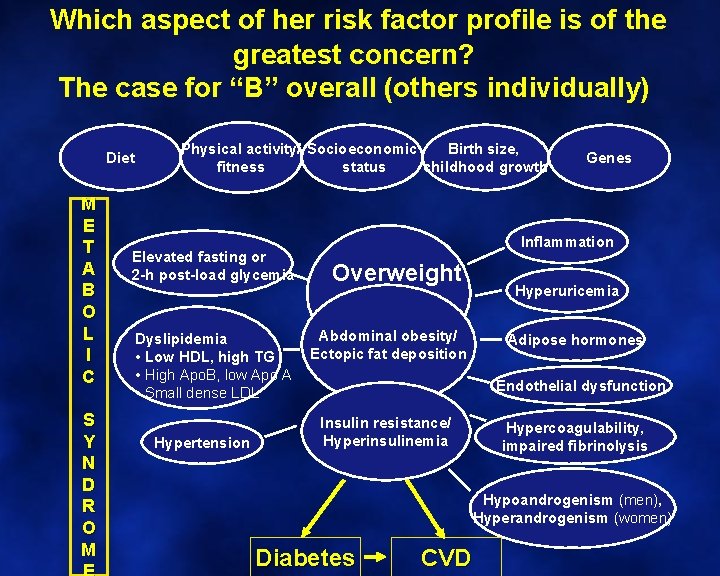
Which aspect of her risk factor profile is of the greatest concern? The case for “B” overall (others individually) Diet M E T A B O L I C S Y N D R O M Birth size, Physical activity/ Socioeconomic status childhood growth fitness Elevated fasting or 2 -h post-load glycemia Dyslipidemia • Low HDL, high TG • High Apo. B, low Apo A • Small dense LDL Hypertension Genes Inflammation Overweight Abdominal obesity/ Ectopic fat deposition Hyperuricemia Adipose hormones Endothelial dysfunction Insulin resistance/ Hyperinsulinemia Hypercoagulability, impaired fibrinolysis Hypoandrogenism (men), Hyperandrogenism (women) Diabetes CVD

Which aspect of her risk factor profile is most amenable to intervention? • A) BP and ACR • B) Weight and BMI • C) Total and LDL-C • D) TG and HDL-C • E) Plasma glucose and Hb A 1 C

Which aspect of her risk factor profile is most amenable to intervention? The case for “D” • The three specific primary ACCORD hypotheses were as follow. In middleaged or older people with type 2 diabetes who are at high risk for having a cardiovascular disease (CVD) event because of existing clinical or subclinical CVD or CVD risk factors: • does a therapeutic strategy that targets a Hb. A 1 c of < 6. 0% reduce the rate of CVD events more than a strategy that targets a Hb. A 1 c of 7. 0% to 7. 9% (with the expectation of achieving a median level of 7. 5%) ? • in the context of good glycaemic control, does a therapeutic strategy that uses a fibrate to raise HDL-C/lower triglyceride levels and uses a statin for treatment of LDL-C reduce the rate of CVD events compared to a strategy that only uses a statin for treatment of LDL-C? • In the context of good glycaemic control, does a therapeutic strategy that targets a systolic blood pressure (SBP) < 120 mm Hg reduce the rate of CVD events compared to a strategy that targets a SBP of < 140 mm Hg?

If refusal to take more than 1 extra tablet was • a limitation, what would you do? A) Add an AII Receptor Blocker B) Add Ezetimibe C) Add a Calcium Channel Blocker D) Add a sulphonylurea E) Add fenofibrate

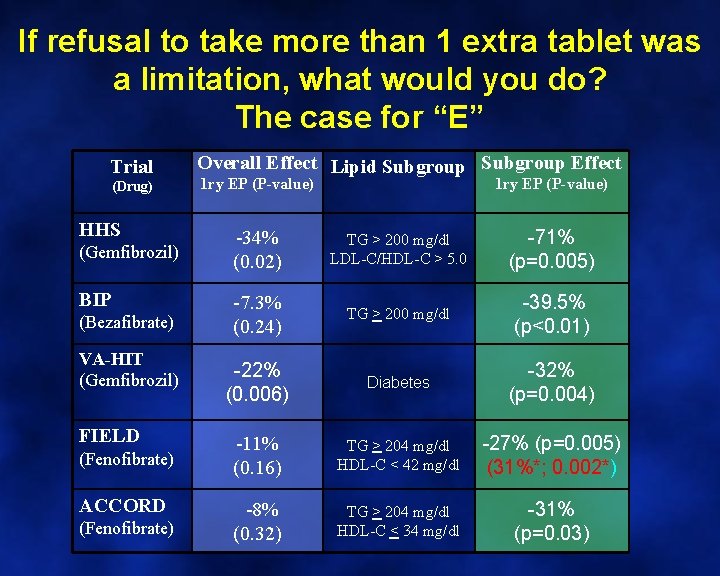
If refusal to take more than 1 extra tablet was a limitation, what would you do? The case for “E” Trial (Drug) HHS (Gemfibrozil) BIP (Bezafibrate) VA-HIT (Gemfibrozil) FIELD (Fenofibrate) ACCORD (Fenofibrate) Overall Effect Lipid Subgroup Effect 1 ry EP (P-value) -34% (0. 02) TG > 200 mg/dl LDL-C/HDL-C > 5. 0 -71% (p=0. 005) -7. 3% (0. 24) TG > 200 mg/dl -39. 5% (p<0. 01) -22% (0. 006) Diabetes -32% (p=0. 004) -11% (0. 16) TG > 204 mg/dl HDL-C < 42 mg/dl -27% (p=0. 005) (31%*; 0. 002*) -8% (0. 32) TG > 204 mg/dl HDL-C < 34 mg/dl -31% (p=0. 03)

Mrs M. L. • • • You cheat. You replace her Enalapril and Indapamide with a higher dose combination agent and replace her simvastatin with a tolerable dose of the atorvastatin felodipine combination. (or change her metformin 500 mg ii bd to I g bd or Metformin XR daily) Having done so, this leaves room to add Fenofibrate 145 mg/day. Repeat results reveal : weight is 65 kg (3 kg decrease), BP 125/85, Total cholesterol 4. 4 mmol/l, fasting TG 1. 9 mmol/l, HDL 1. 4 mmol/l, calculated LDL 2. 1 mmol/l. Urinary ACR is 3. 2 and Hb. A 1 C is 7. 1%.

More questions concerning Mrs M. L. • Fenofibrate may have contributed towards: A) Weight loss B) Lower BP C) Lower Hb. A 1 C • Fenofibrate lowers which 1 of the following A) Homocysteine B) Serum Creatinine C) Fibrinogen • Fenofibrate also increases which 1 of the following LDL particle size B) Urinary ACR C) Urate • A) The lipid changes associated with fenofibrate use predict its ability to protect against A) Retinopathy B )Neuropathy C) Nephropathy D) Amputation

• • You cheat. You replace her Enalapril and Indapamide with a higher dose combination agent and repalce her simvastatin with a tolerable dose of the atorvastatin felodipine combination. Having done so, this leaves room to add Fenofibrate 145 mg/day. Repeat results reveal : weight is 65 kg (3 kg decrease), BP 125/85, Total cholesterol 4. 4 mmol/l, fasting TG 1. 9 mmol/l, HDL 1. 4 mmol/l, calculated LDL 2. 1 mmol/l. Urinary ACR is 3. 2 and Hb. A 1 C is 7. 1%. • Fenofibrate may have contributed towards: A) Weight loss B) Lower BP C) Lower Hb. A 1 C • It lowers which 1 of the following A) Homocysteine B) Creatinine • It also increases which 1 of the following A) LDL particle size B) Urinary ACR C) Urate • The lipid changes associated with fenofibrate use predict its ability to protect against A) Retinopathy B )Neuropathy C) Nephropathy D) Amputation C) Fibrinogen

Fenofibrate may have contributed towards: • A) Weight loss • B) Lower BP • C) Lower Hb. A 1 C

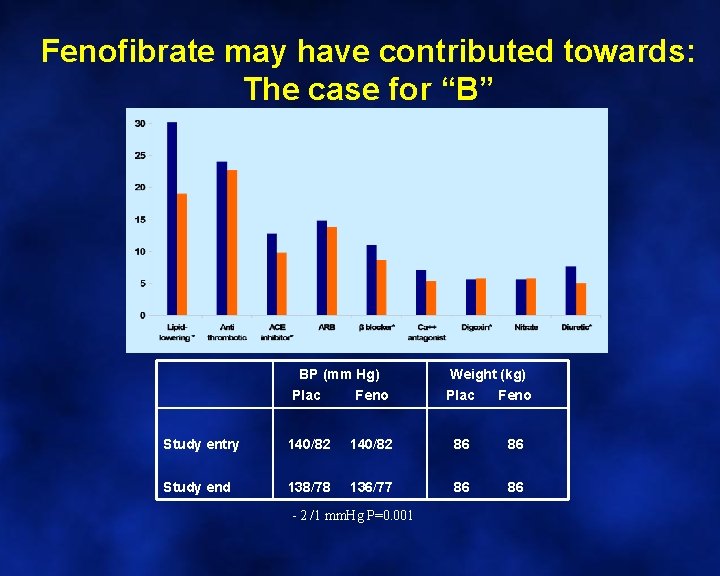
Fenofibrate may have contributed towards: The case for “B” BP (mm Hg) Weight (kg) Plac Feno Study entry 140/82 86 86 Study end 138/78 136/77 86 86 - 2 /1 mm. Hg P=0. 001 Plac Feno

Fenofibrate lowers which 1 of the following • A) Homocysteine • B) Serum Creatinine • C) Fibrinogen

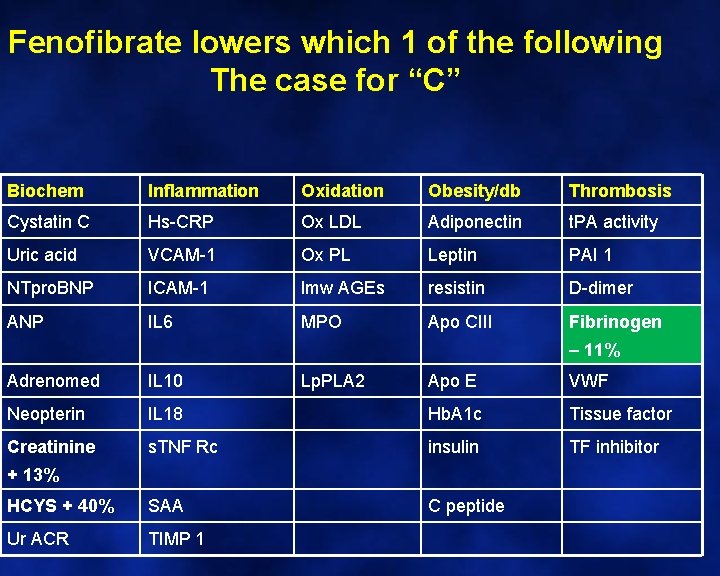
Fenofibrate lowers which 1 of the following The case for “C” Biochem Inflammation Oxidation Obesity/db Thrombosis Cystatin C Hs-CRP Ox LDL Adiponectin t. PA activity Uric acid VCAM-1 Ox PL Leptin PAI 1 NTpro. BNP ICAM-1 lmw AGEs resistin D-dimer ANP IL 6 MPO Apo CIII Fibrinogen – 11% Adrenomed IL 10 Neopterin Creatinine Lp. PLA 2 Apo E VWF IL 18 Hb. A 1 c Tissue factor s. TNF Rc insulin TF inhibitor HCYS + 40% SAA C peptide Ur ACR TIMP 1 + 13%

Fenofibrate also increases which 1 of the following • A) LDL particle size • B) Urinary ACR • C) Urate

Fenofibrate also increases which 1 of the following? The case for “A” Lemieux I, Laperrière L, Dzavik V, Tremblay G, Bourgeois J, Després JP, Atherosclerosis 2002, 162: 363 -371 RESULTS: Whereas significant improvements in the plasma lipoprotein-lipid variables were observed with both fenofibrate and pravastatin treatments, LDL peak particle size was only significantly increased withfenofibrate therapy (+2. 11+/5. 18 A, P<0. 05). Among patients under fenofibrate therapy, changes in TG levels were negatively associated with changes in LDL peak particle size (r=-0. 54, P<0. 0007)

The lipid changes associated with fenofibrate use predict its ability to protect against • • A) Retinopathy B) Neuropathy C) Nephropathy D) Amputation

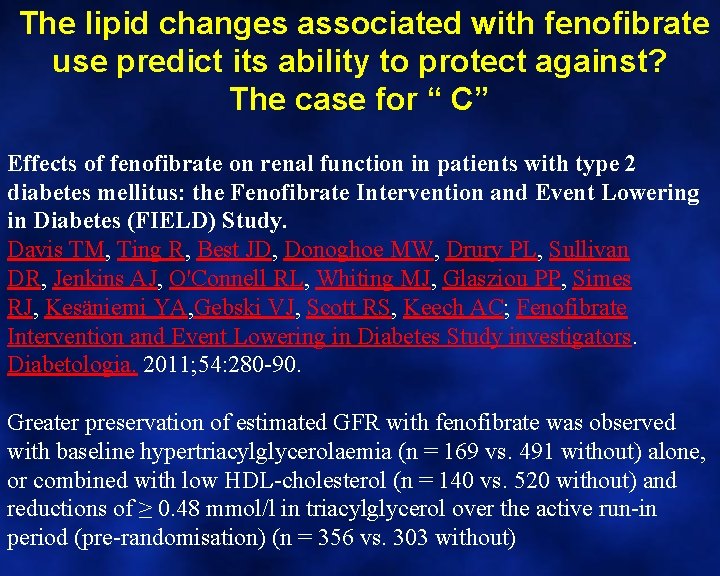
The lipid changes associated with fenofibrate use predict its ability to protect against? The case for “ C” Effects of fenofibrate on renal function in patients with type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Study. Davis TM, Ting R, Best JD, Donoghoe MW, Drury PL, Sullivan DR, Jenkins AJ, O'Connell RL, Whiting MJ, Glasziou PP, Simes RJ, Kesäniemi YA, Gebski VJ, Scott RS, Keech AC; Fenofibrate Intervention and Event Lowering in Diabetes Study investigators. Diabetologia. 2011; 54: 280 -90. Greater preservation of estimated GFR with fenofibrate was observed with baseline hypertriacylglycerolaemia (n = 169 vs. 491 without) alone, or combined with low HDL-cholesterol (n = 140 vs. 520 without) and reductions of ≥ 0. 48 mmol/l in triacylglycerol over the active run-in period (pre-randomisation) (n = 356 vs. 303 without)

Mr G. E. • This non-diabetic 49 year - old male was a smoker until he required CABG at age 43. • Despite treatment with aspirin 100 mg and Vytorin 40/10 mg he required stenting recently. Hypercholesterolaemia persists at a level of 5. 2 mmol/l (fasting TG 1. 1 mmol/l, HDL 0. 9 mmol/l, calculated LDL 3. 8 mmol/l). • He had resumed occasional use of cannabis.

Questions concerning Mr G. E. • What test would be the most helpful to investigate “residual risk” A) hs-CRP B) Total homocysteine C) Lipoprotein (a) D) Lipoprotein-associated Phospholipase A 2 E) Urinary ACR • Which of the following interventions might help to improve HDL-C? A) Increased activity B) Weight loss C) Cessation of cannabis D) All of the above E) Change from beer to wine consumption

• This non-diabetic 49 year - old male was a smoker until he required CABG at age 43. • Despite treatment with aspirin 100 mg and Vytorin 40/10 mg he required stenting recently. • Hypercholesterolaemia persists at a level of 5. 2 mmol/l (fasting TG 1. 1 mmol/l, HDL 0. 9 mmol/l, calculated LDL 3. 8 mmol/l). • He had resumed occasional use of cannabis. • What test would be the most helpful to investigate “residual risk” A) hs-CRP B) Total homocysteine C) Lipoprotein (a) D) Lipoprotein-associated Phospholipase A 2 E) Urinary ACR • Which of the following interventions might help to improve HDL-C? A) Increased activity B) Weight loss C) Cessation of cannabis D) All of the above E) Change from beer to wine consumption

What test would be the most helpful to investigate “residual risk” • A) hs-CRP • B) Total homocysteine • C) Lipoprotein (a) • D) Lipoprotein-associated Phospholipase A 2 • E) Urinary ACR

What test would be the most helpful to investigate “residual risk” The case for “C” Emerging Risk Factor Collaboration. JAMA 2009; 302: 412 -23

Which of the following interventions might help to improve HDL-C? • A) Increased activity • B) Weight loss • C) Cessation of cannabis • D) All of the above • E) Change from beer to wine consumption

Which of the following interventions might help to improve HDL-C? The case for “C”, but “D” rarely considered The mean triglyceride level in the 18 marijuana users was 1. 5 mmol/l, compared with 1. 0 mmol/l in the 24 controls. This is consistent with a previous paper that reported significant increases in HDL-triglyceride concentrations in marijuana users compared with controls. Jayanthi S, Buie S, Moore S, et al. Heavy marijuana users show increased serum apolipoprotein C 3 levels: evidence from proteomic analyses. Mol Psychiatry 2008.

More questions concerning Mr G. E. • Mr G. E’s Lipoprotein (a) level was very high (1, 783 mg/l). With this in mind, how would you intensify lipid management? A) Add a fibrate B) Add a bile acid resin C) Increase Vytorin (to 80/10 mg) D) Replace Vytorin with Rosuvastatin plus Ezetimibe E) Add Niacin 1 to 2 gm / day, as tolerated. • How much improvement do you anticipate? A) 50% decrease in TG B) 30% increase in HDL-C C) 25% decrease in Lp(a) D) 30% decrease in LDL-C E) All of the above • Niacin affects plasma glucose because A) It affects pancreatic beta cell function B) It increases plasma free fatty acid levels C) It reduces plasma free fatty acid levels which then re-bound D) It increases the speed of intestinal absorption. E) The mechanism is unknown

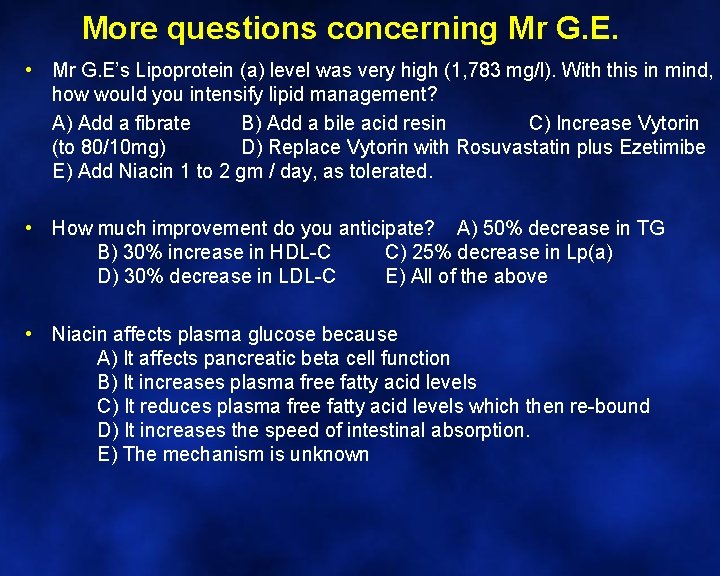
How would you intensify lipid management? A) Add a fibrate B) Add a bile acid resin C) Increase Vytorin (to 80/10 mg) D) Replace Vytorin with Rosuvastatin plus Ezetimibe E) Add Niacin 1 to 2 gm / day, as tolerated.

How would you intensify lipid management? The case for “E” Hepatocyte Nascent HDL apo. A-I No effect on increasing Apo A 1 synthesis Inhibits hepatic lipase activity which reduces lipolysis of large HDL 3 CE A-I HDL 2

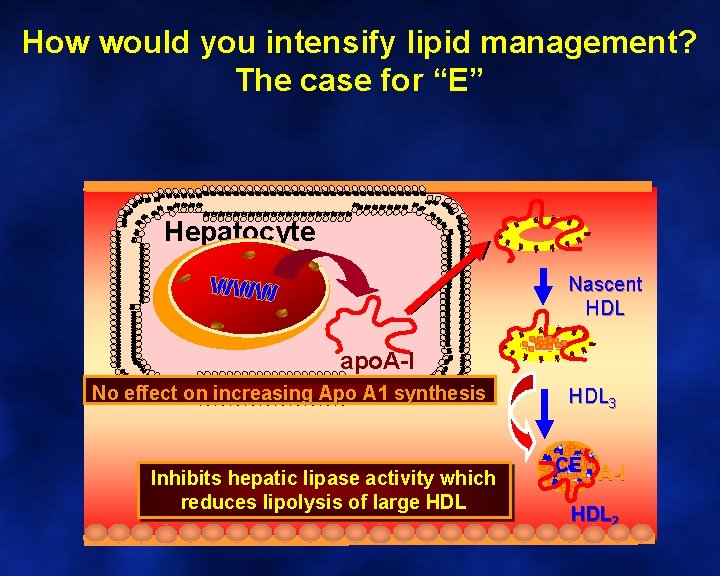
How much improvement do you anticipate? • A) 50% decrease in TG • B) 30% increase in HDL-C • C) 25% decrease in Lp(a) • D) 30% decrease in LDL-C • E) All of the above

How much improvement do you anticipate? The case for “E” Niacin used at pharmacologic doses (2 g/d) has been shown to reduce serum levels of Lp(a) by 20% to 25%.

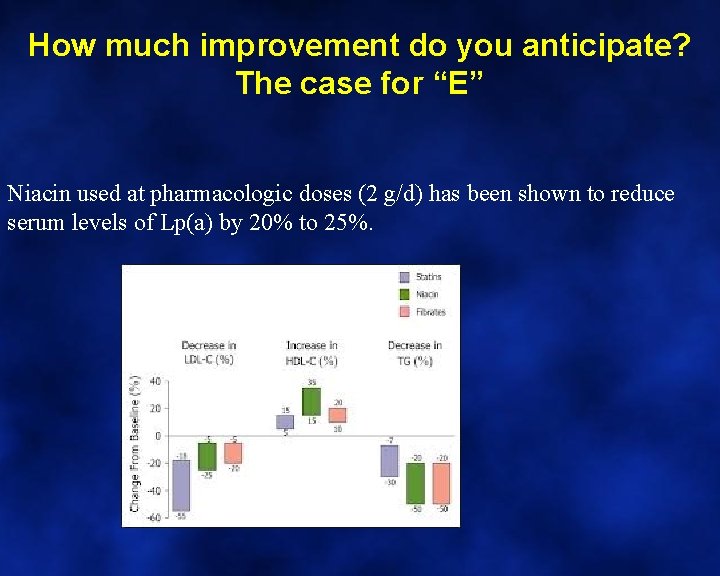
Niacin affects plasma glucose because. . . • A) It affects pancreatic beta cell function • B) It increases plasma free fatty acid levels • C) It reduces plasma free fatty acid levels which then re-bound • D) It increases the speed of intestinal absorption. • E) The mechanism is unknown

Niacin affects plasma glucose because. . . The case for “C”