Rare due to low packing density only Po



![چگﺎﻟی ﺧﻄی • Linear Density of Atoms LD = [110] a Number of چگﺎﻟی ﺧﻄی • Linear Density of Atoms LD = [110] a Number of](https://slidetodoc.com/presentation_image_h/0cbb663e23b480dbe0d066bcd6434f34/image-22.jpg)



- Slides: 31







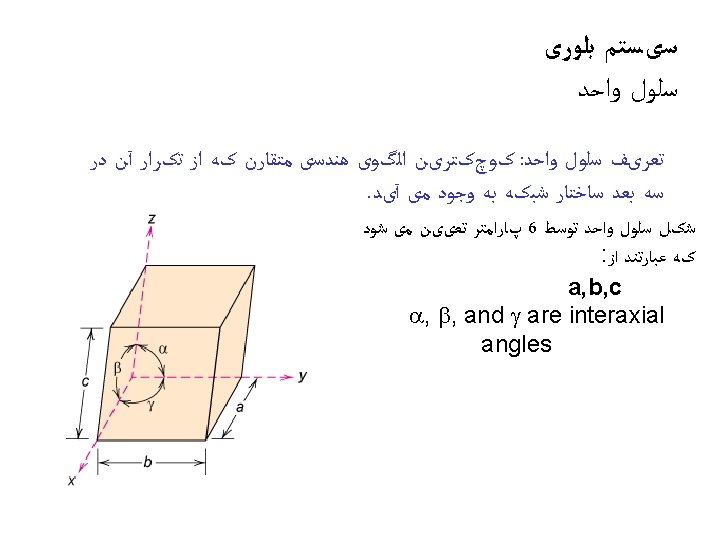
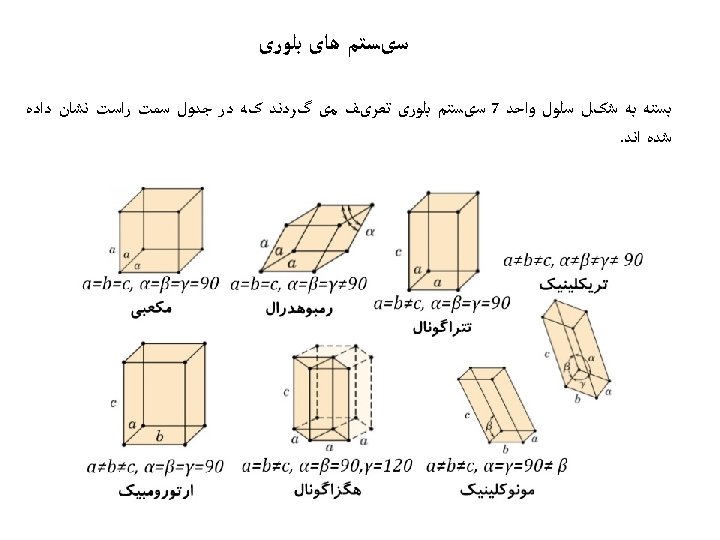
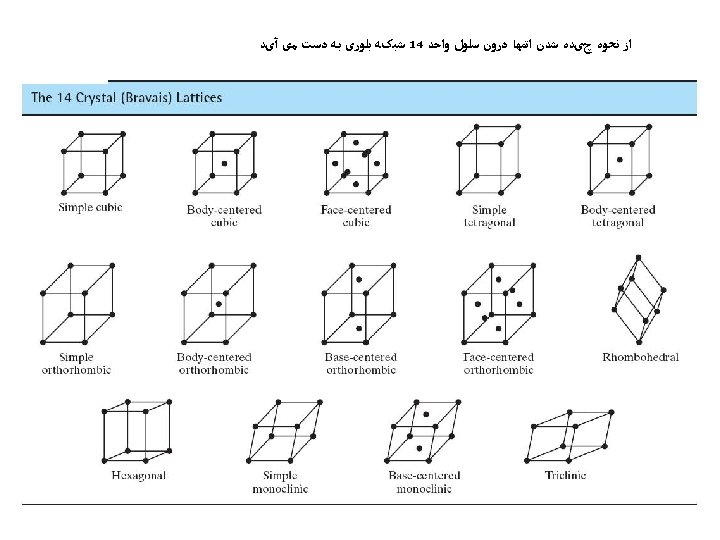
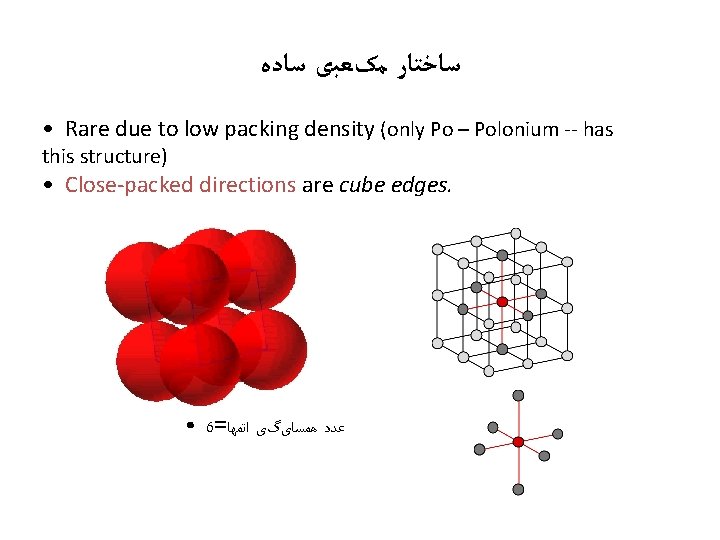
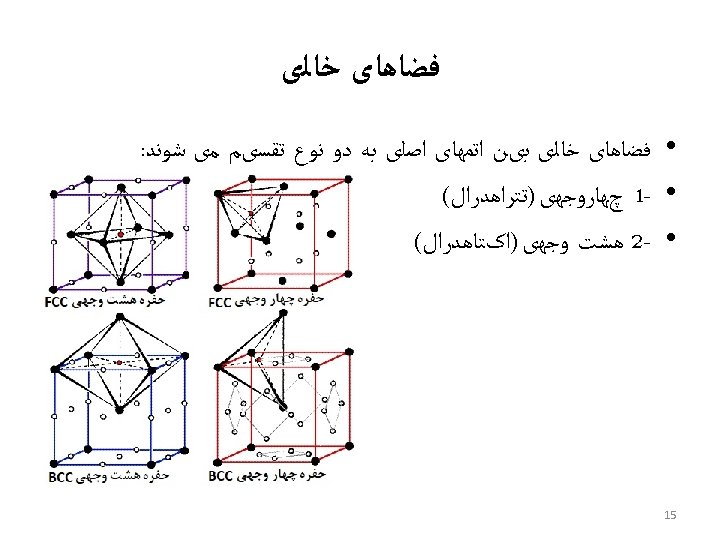
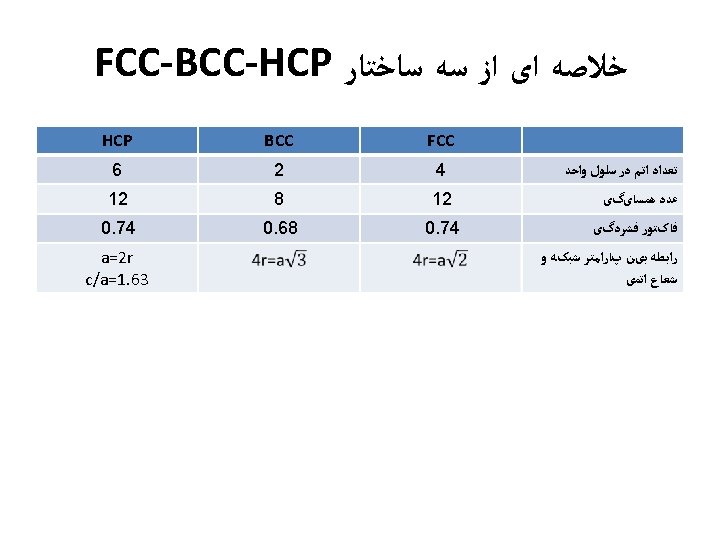
ﺳﺎﺧﺘﺎﺭ ﻣکﻌﺒی ﺳﺎﺩﻩ • Rare due to low packing density (only Po – Polonium -- has this structure) • Close-packed directions are cube edges. • 6= ﺍﺗﻤﻬﺎ ﻫﻤﺴﺎیگی ﻋﺪﺩ

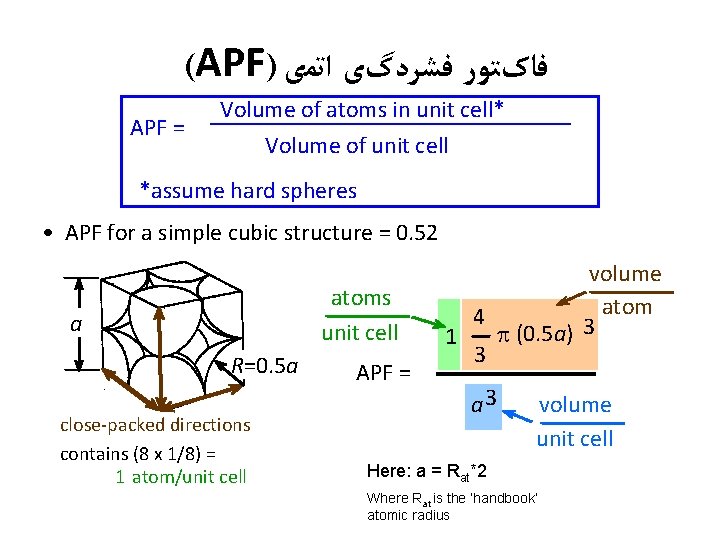
(APF) ﻓﺎکﺘﻮﺭ ﻓﺸﺮﺩگی ﺍﺗﻤی APF = Volume of atoms in unit cell* Volume of unit cell *assume hard spheres • APF for a simple cubic structure = 0. 52 atoms unit cell a R=0. 5 a close-packed directions contains (8 x 1/8) = 1 atom/unit cell APF = volume atom 4 p (0. 5 a) 3 1 3 a 3 volume unit cell Here: a = Rat*2 Where Rat is the ‘handbook’ atomic radius

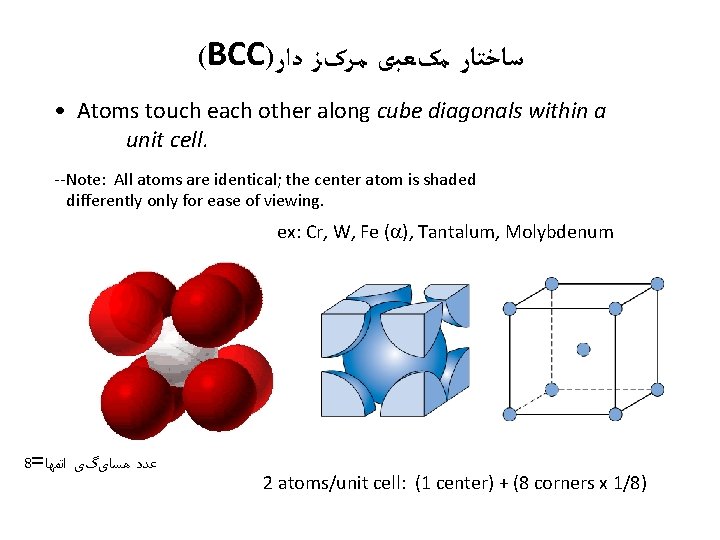
(BCC) ﺳﺎﺧﺘﺎﺭ ﻣکﻌﺒی ﻣﺮکﺰ ﺩﺍﺭ • Atoms touch each other along cube diagonals within a unit cell. --Note: All atoms are identical; the center atom is shaded differently only for ease of viewing. ex: Cr, W, Fe ( ), Tantalum, Molybdenum 8= ﺍﺗﻤﻬﺎ ﻫﺴﺎیگی ﻋﺪﺩ 2 atoms/unit cell: (1 center) + (8 corners x 1/8)

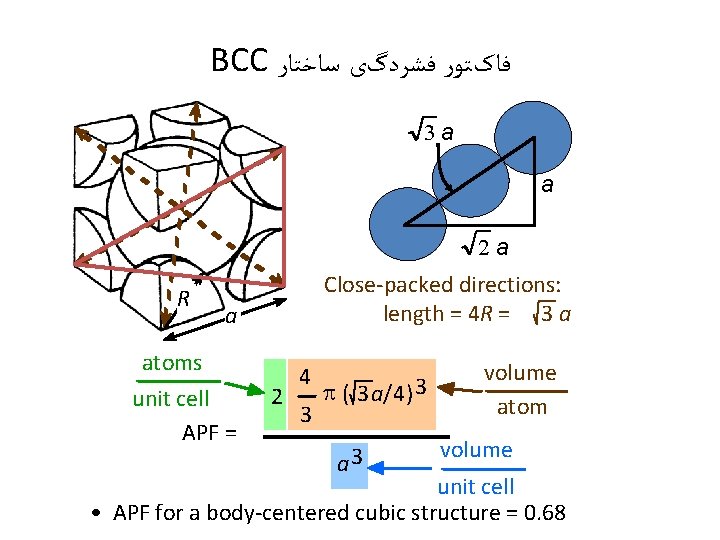
BCC ﻓﺎکﺘﻮﺭ ﻓﺸﺮﺩگی ﺳﺎﺧﺘﺎﺭ 3 a a 2 a R Close-packed directions: length = 4 R = 3 a a atoms unit cell APF = 2 4 3 p ( 3 a/4) 3 a 3 volume atom volume unit cell • APF for a body-centered cubic structure = 0. 68

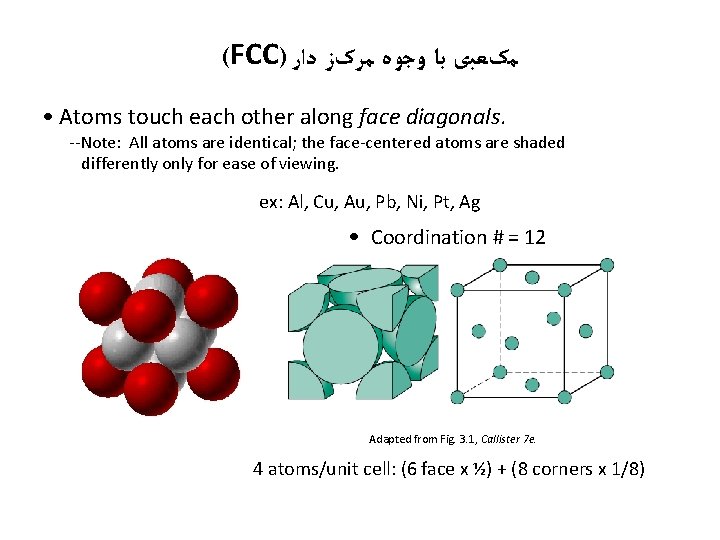
(FCC) ﻣکﻌﺒی ﺑﺎ ﻭﺟﻮﻩ ﻣﺮکﺰ ﺩﺍﺭ • Atoms touch each other along face diagonals. --Note: All atoms are identical; the face-centered atoms are shaded differently only for ease of viewing. ex: Al, Cu, Au, Pb, Ni, Pt, Ag • Coordination # = 12 Adapted from Fig. 3. 1, Callister 7 e. 4 atoms/unit cell: (6 face x ½) + (8 corners x 1/8)

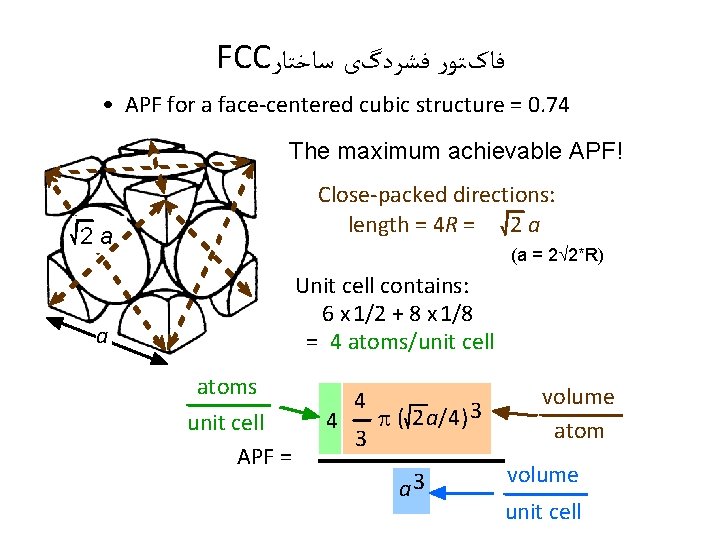
FCC ﻓﺎکﺘﻮﺭ ﻓﺸﺮﺩگی ﺳﺎﺧﺘﺎﺭ • APF for a face-centered cubic structure = 0. 74 The maximum achievable APF! Close-packed directions: length = 4 R = 2 a a (a = 2 2*R) Unit cell contains: 6 x 1/2 + 8 x 1/8 = 4 atoms/unit cell atoms unit cell APF = 4 4 3 p ( 2 a/4) 3 a 3 volume atom volume unit cell

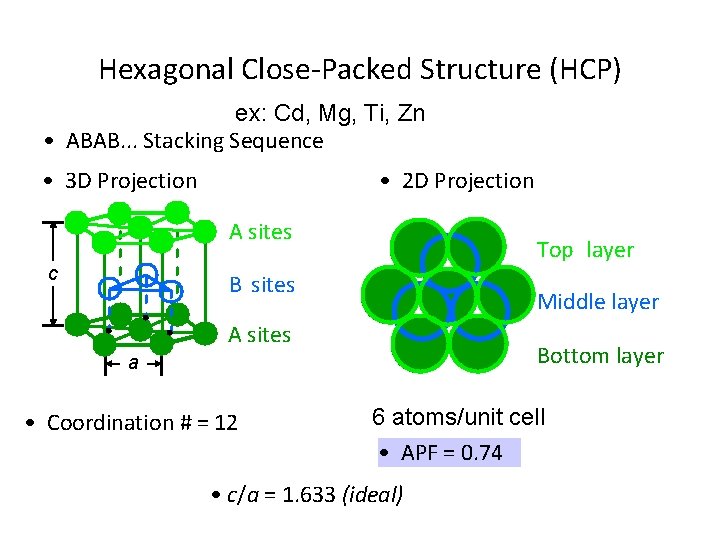
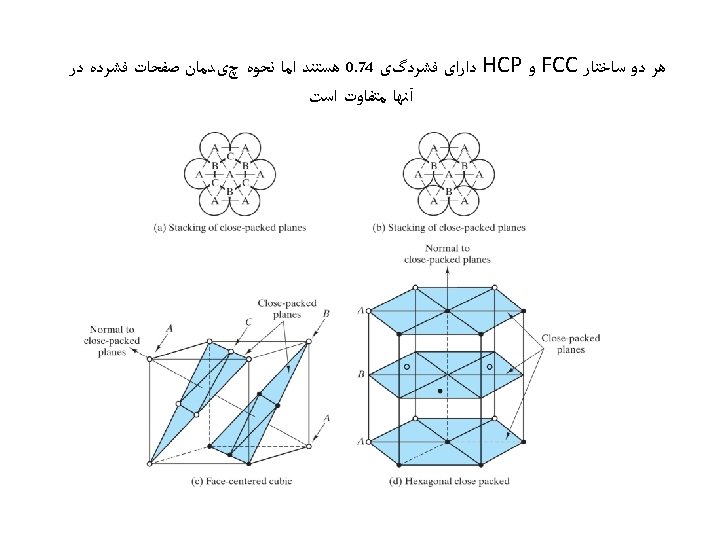
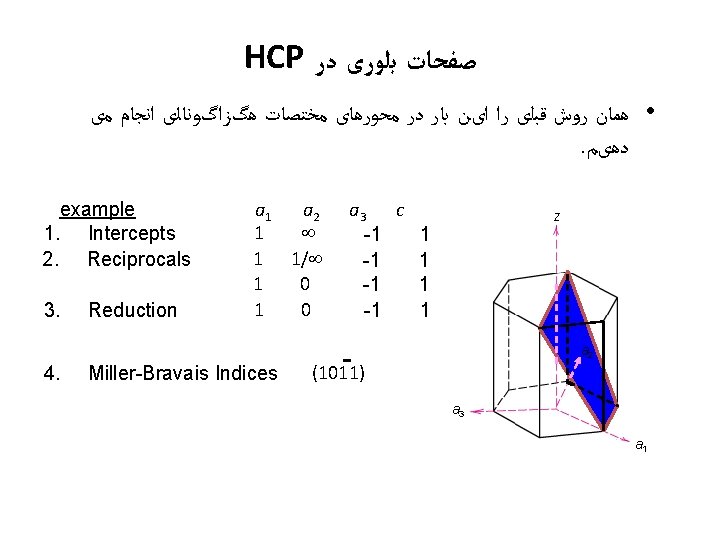
Hexagonal Close-Packed Structure (HCP) ex: Cd, Mg, Ti, Zn • ABAB. . . Stacking Sequence • 3 D Projection • 2 D Projection A sites c Top layer B sites Middle layer A sites Bottom layer a • Coordination # = 12 6 atoms/unit cell • APF = 0. 74 • c/a = 1. 633 (ideal)



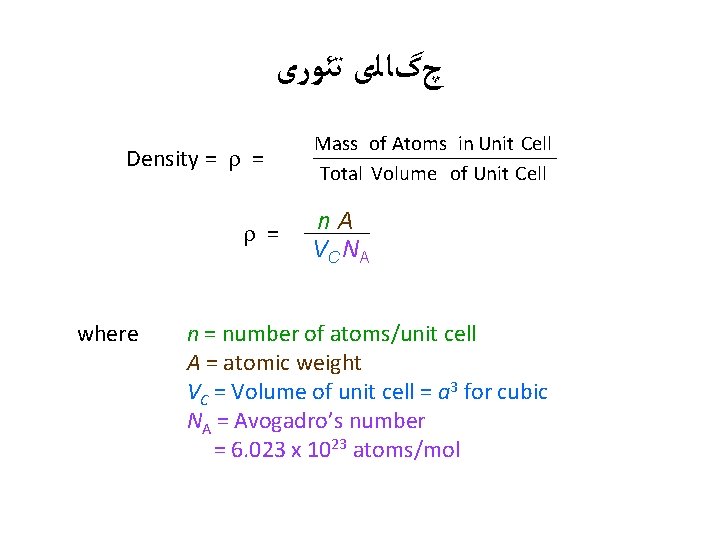
چگﺎﻟی ﺗﺌﻮﺭی Density = = = Mass of Atoms in Unit Cell Total Volume of Unit Cell n A V C NA where n = number of atoms/unit cell A = atomic weight VC = Volume of unit cell = a 3 for cubic NA = Avogadro’s number = 6. 023 x 1023 atoms/mol

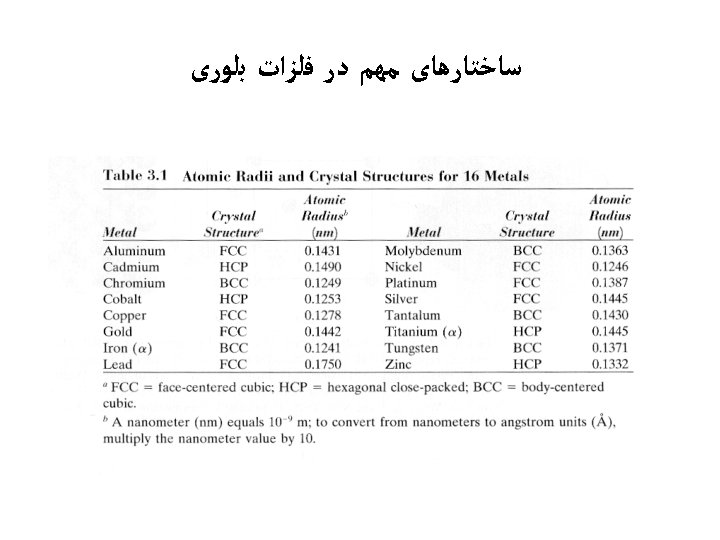
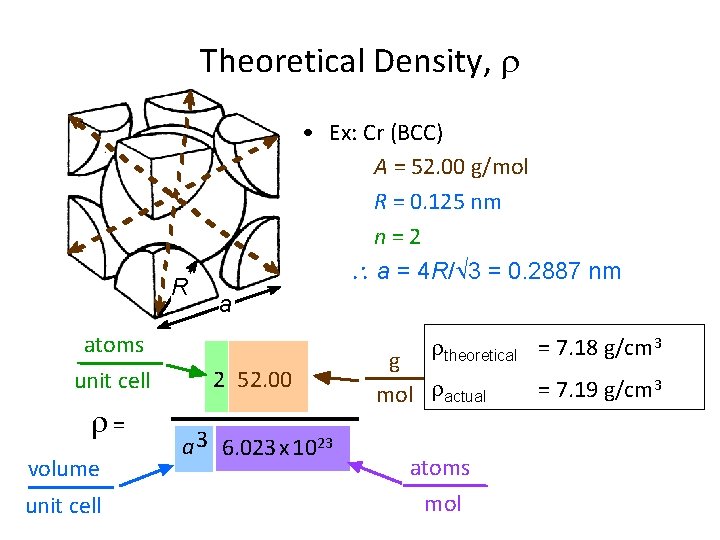
Theoretical Density, R atoms unit cell = volume unit cell • Ex: Cr (BCC) A = 52. 00 g/mol R = 0. 125 nm n = 2 a = 4 R/ 3 = 0. 2887 nm a 2 52. 00 a 3 6. 023 x 1023 theoretical = 7. 18 g/cm 3 g mol actual atoms mol = 7. 19 g/cm 3



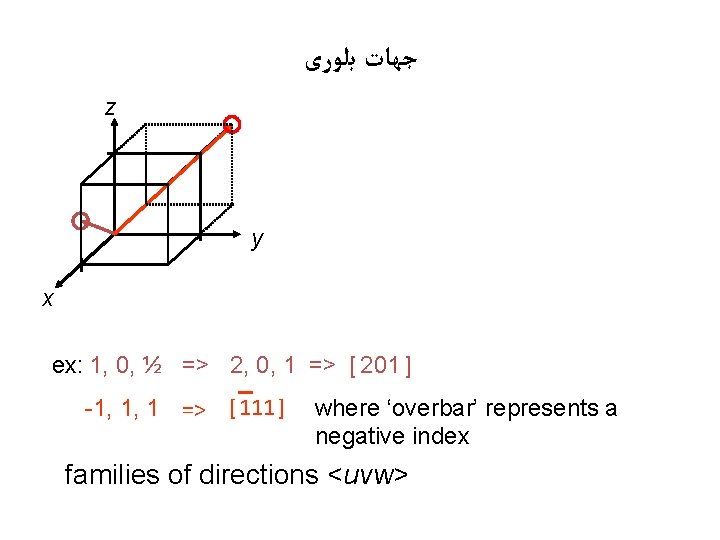
ﺟﻬﺎﺕ ﺑﻠﻮﺭی z y x ex: 1, 0, ½ => 2, 0, 1 => [ 201 ] -1, 1, 1 => [ 111 ] where ‘overbar’ represents a negative index families of directions <uvw>

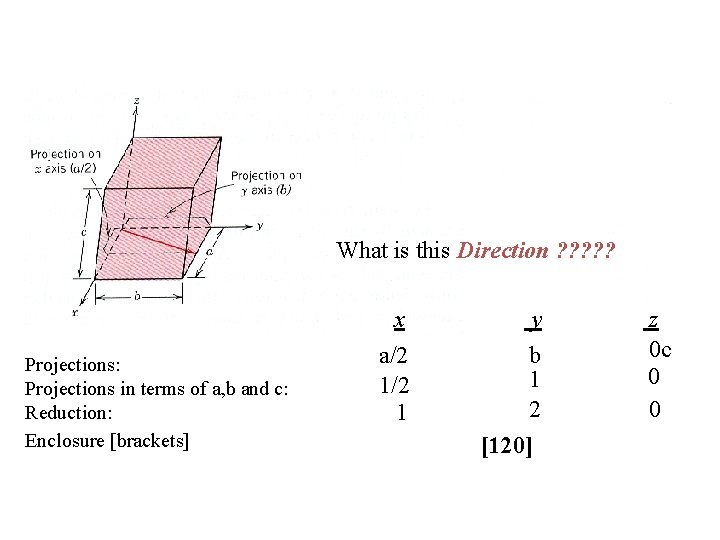
What is this Direction ? ? ? Projections: Projections in terms of a, b and c: Reduction: Enclosure [brackets] x a/2 1 y b 1 2 [120] z 0 c 0 0
![چگﺎﻟی ﺧﻄی Linear Density of Atoms LD 110 a Number of چگﺎﻟی ﺧﻄی • Linear Density of Atoms LD = [110] a Number of](https://slidetodoc.com/presentation_image_h/0cbb663e23b480dbe0d066bcd6434f34/image-22.jpg)
چگﺎﻟی ﺧﻄی • Linear Density of Atoms LD = [110] a Number of atoms Unit length of direction vector ﺭﺍ [110] ﺟﻬﺖ ﺩﺭ آﻠﻮﻣیﻨیﻮﻡ ﺧﻄی چگﺎﻟی : ﻣﺜﺎﻝ . ﺑیﺎﺑیﺪ a = 0. 405 nm # atoms LD = length 2 2 a = 3. 5 nm # atoms CENTERED on the direction of interest! Length is of the direction of interest within the Unit Cell -1


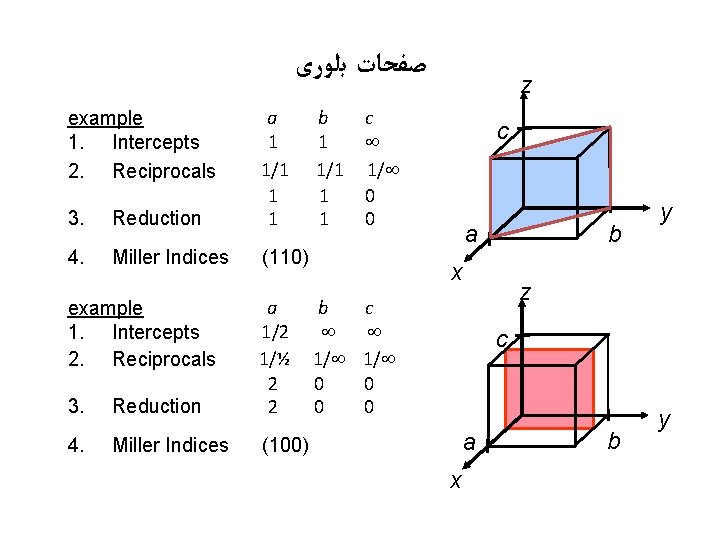
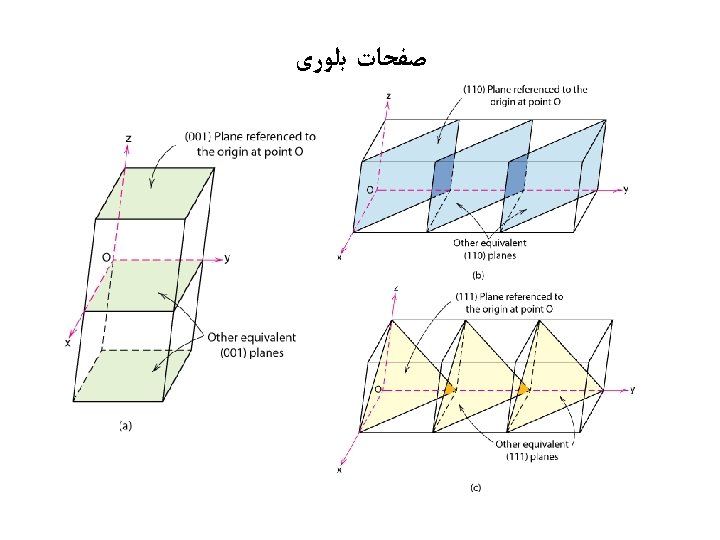
ﺻﻔﺤﺎﺕ ﺑﻠﻮﺭی example 1. Intercepts 2. Reciprocals 3. Reduction a b c 1 1/1 1/ 1 1 0 4. Miller Indices (110) example 1. Intercepts 2. Reciprocals 3. Reduction z c b a x a b c 1/2 1/½ 1/ 1/ 2 0 0 y z c a 4. Miller Indices (100) x b y

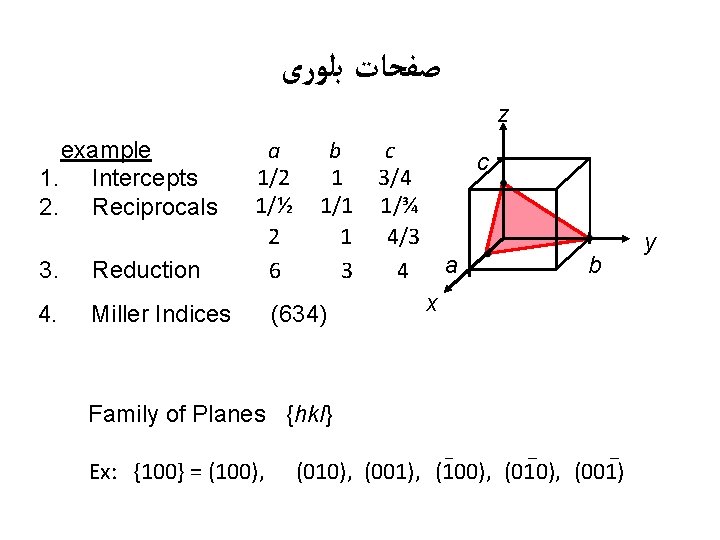
ﺻﻔﺤﺎﺕ ﺑﻠﻮﺭی z example 1. Intercepts 2. Reciprocals a b c 1/2 1 3/4 1/½ 1/1 1/¾ 2 1 4/3 6 3 4 c 3. Reduction 4. Miller Indices (634) a b x Family of Planes {hkl} Ex: {100} = (100), (010), (001), (100), (010), (001) y


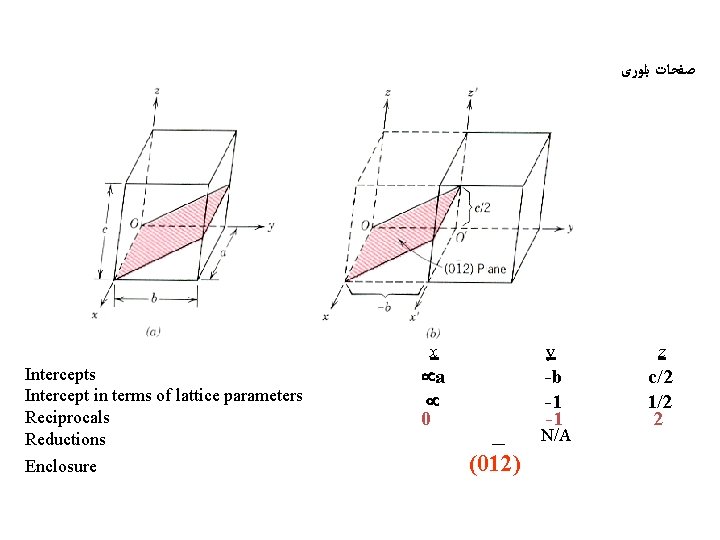
ﺻﻔﺤﺎﺕ ﺑﻠﻮﺭی Intercepts Intercept in terms of lattice parameters Reciprocals Reductions Enclosure x y z a 0 -b -1 -1 c/2 1/2 2 N/A (012)



