Randomized Controlled Trials Dr Poonam R Naik Professor


















- Slides: 23

Randomized Controlled Trials Dr Poonam R Naik Professor Community Medicine

Why Intervention studies have such an important place in epidemiological research ? • Provides a very high degree of assurance about the validity of a result • Randomized trial will yield the strongest and most direct epidemiological evidence on which to base a judgement whether an observed association is one of cause and effect

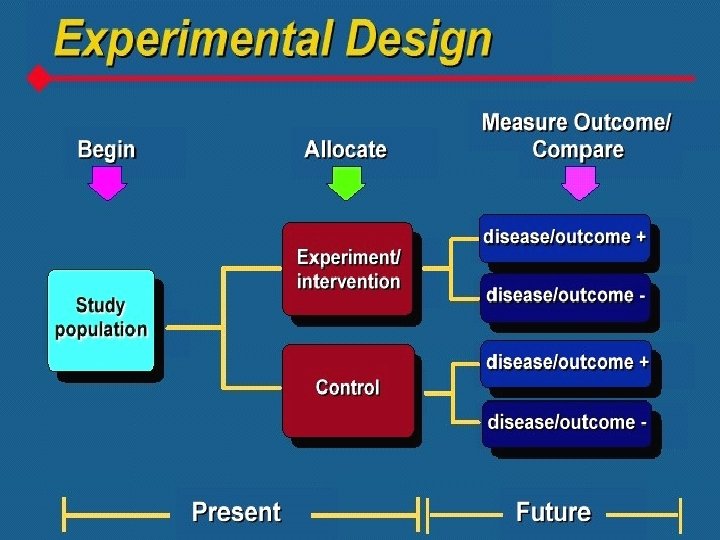
What is a Clinical Trial? • A clinical trial is defined as a prospective study comparing the effect and value of intervention(s) against a control in human subjects

What are the features of a Clinical Trial • Prospective: looking forward in time • Intervention must be employed which may be drugs, devices or procedures that may be diagnostic, prophylactic or therapeutic • Control group is a must • Human beings: safety and ethical considerations

What is the purpose of a clinical trial? • Patient management • To improve diagnostic, therapeutic and prophylactic procedures • To understand the etiology and pathogenesis of the disease • Marketing

What are the types of Clinical trials ? • Randomized vs Non-Randomized • Blind vs Open • Multicentre vs Single centre • Parallel vs Cross over


Randomized Controlled Trial • A controlled trial is a study in which participants are assigned to a study group. • Study groups are also called study arms or treatment conditions. • In a randomized controlled trial, participants are assigned to treatment conditions at random (i. e. , they have an equal probability of being assigned to any group). • Procedures are controlled to ensure that all participants in all study groups are treated the same except for the factor that is unique to their group. The unique factor is the type of intervention they receive.

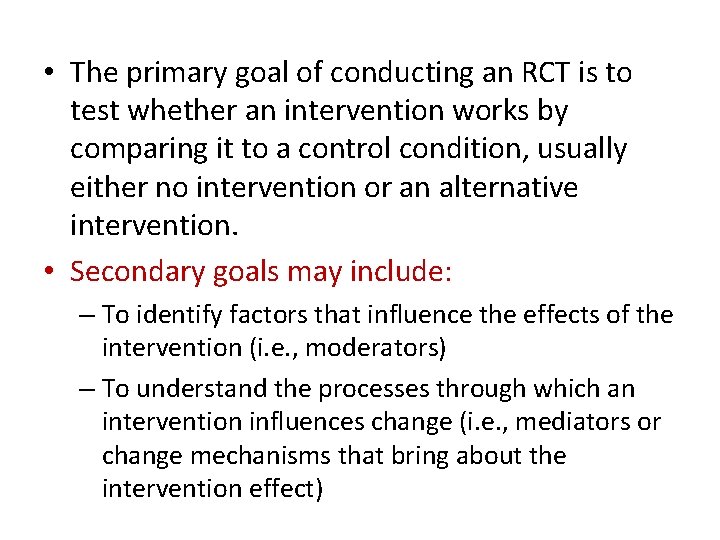
• The primary goal of conducting an RCT is to test whether an intervention works by comparing it to a control condition, usually either no intervention or an alternative intervention. • Secondary goals may include: – To identify factors that influence the effects of the intervention (i. e. , moderators) – To understand the processes through which an intervention influences change (i. e. , mediators or change mechanisms that bring about the intervention effect)

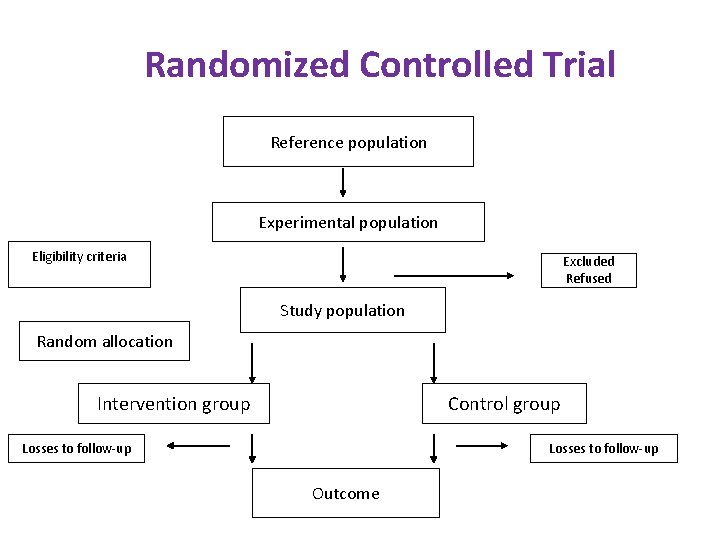
Randomized Controlled Trials Steps i. iii. iv. v. vi. Drawing up a protocol Selecting a reference population Randomization Manipulation or Intervention Follow up Assessment of outcome

Randomized Controlled Trial Reference population Experimental population Eligibility criteria Excluded Refused Study population Random allocation Intervention group Control group Losses to follow-up Outcome

1. Protocol: Follow the protocol drawn Ø Aims, objectives, research questions, Ø selection criteria: study and control group, allocation procedures, Ø sample size, Ø treatment procedures: when, where, how Ø standardization of working procedures and schedules, Multi centric study: Prevent bias and reduce errors Pilot study:

2. Selecting reference and experimental populations: 1. Reference/ Target population: Population to which the trial findings if successful will be applicable. May be the whole community or a defined geographical population or specific groups 2. Experimental/ Study population: Derived from the reference population. Actual population which participates in the experimental study Randomly chosen from the reference population so as to generalize the findings to the reference population.

Experimental population: i. Invite the members to participate officially ii. Select a stable population iii. Avoid losses to follow up Criteria to be fulfilled by the participants: i. Informed consent ii. Representative of the reference population iii. Eligible for the trial Example: anemia, Vaccine efficacy

Randomization: A statistical procedure by which the participants are allocated into groups called study and control groups: To receive or not to receive Randomization is an attempt to eliminate bias and allow for comparability Randomization: Heart of a controlled trial Ensures groups are comparable Investigator has no control over allocation of participants to study and control group, thus eliminating the selection bias

Randomization ensures that every participant gets an equal chance of being allocated into study and control group The whole group could be stratified into sub groups according to the variable and individuals within each subgroup can then be randomly allocated into study and control group Randomization is done only after the participant has entered the study and qualified for the trial

Manipulation: Intervene or manipulate the study and control group Independent variable and outcome Follow up: Examination of the study and control groups Defined intervals of time, in a standard manner, in the given time frame Minimize the losses to follow up

Assessment: Positive results: Negative results: Bias: 3 sources: Participants: Subject variation Observer bias: Measurement of outcome Evaluation: Reporting of results

Blinding: Single blind: Participant Double blind: Doctor and participant Triple blind: Doctor, participant and investigator

Study designs of controlled trials 1. Concurrent parallel study design: 2. Cross over study design:

Types of RCT 1. Clinical Trials 2. Preventive trials: Outcome: i. The community will derive from the measure ii. Risks involved iii. Cost to the health service (MMM) 3. Risk factor trials: A type of preventive trial Modification of the risk factor 4. Cessation of experiments: Evaluate the termination of a habit 5. Evaluation of health services: Community DOT Provider

Types of randomization 1. Simple (Complete) 2. Block

CONSORT Guidelines Consolidated Standards of Reporting Trials: 25 point checklist to report Clinical trials