Quality Assurance in Clinical Trials Stephanie de Rijke






















- Slides: 32

Quality Assurance in Clinical Trials Stephanie de. Rijke Clinical Trials Audit and Compliance

Objectives • Discuss the importance of quality assurance in clinical research • Describe regulatory impact of monitoring and how to respond to monitoring reports • Outline the steps to take when a problem occurs • Detail the processes of root cause analysis and corrective and preventive actions 2

Quality Assurance: what • QA is the process by which sponsors and researchers monitor and evaluate the protocol implementation. • QA prevents patterns of error • QA leads to reliable research data 3

Quality Assurance: how • • • Sponsor monitoring Self-checks Standard tools Written procedures 4

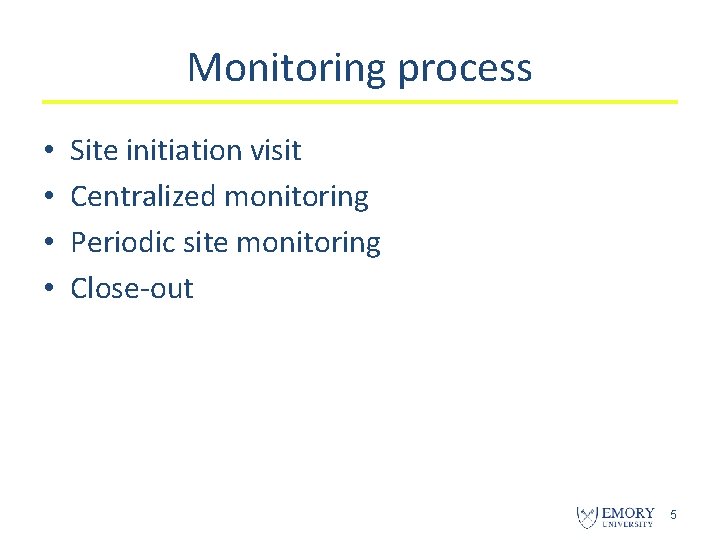
Monitoring process • • Site initiation visit Centralized monitoring Periodic site monitoring Close-out 5

Reports • • • Ask monitor to send you a written report Verify findings Use report as a to-do list Write on report as you resolve things Consider the IRB’s reporting requirements Review with PI; PI should sign if possible 6

Tips on reviewing reports • First look for anything awful • Look for ongoing or pending items that weren’t resolved after the previous visit • Look for things they ask you to report to IRB – “Report to IRB” vs “Report per IRB policies” • Look for hidden attachments or appendices 7

8

9

Responding to monitoring findings • If findings are wrong, provide documentation to monitor and ask for a revised letter or at least keep documentation of correspondence • Report items to IRB if monitor asks • If something does not need to be reported to IRB, respond to the monitor that you don’t plan to report and provide them with IRB P&P • If there becomes a pattern of problems, respond with a CAPA 10

Self-monitoring • Emory University Self-Monitoring Tool – www. ctac. emory. edu • Review a minimum of twice per year – More for high risk or high enrolling studies • Consider reporting requirements of sponsor and IRB 11

Problems in Research • Steps to resolve problems – Identify problems – Make immediate corrections – Evaluate risk – Identify root cause of each problem – Identify corrective and preventive action for each root cause 12

Problem Statement • A problem statement should include – Description of the problem – Where and when it happened – Weight of the problem – Requirements that were not met – Evidence to show that requirements weren’t met 13

Corrections “Immediate corrections” include – Evaluating rights, welfare, and safety of subject – Reporting, if applicable: subject, subject’s family, sponsor, and/or IRB 14

Evaluate Risk 15

Risk • Severity – Rights, welfare, and safety of current and future subjects • Frequency – Systematic problem – Protocol design – Departmental, local problem • If Risk is there, move to RCA and CAPA 16

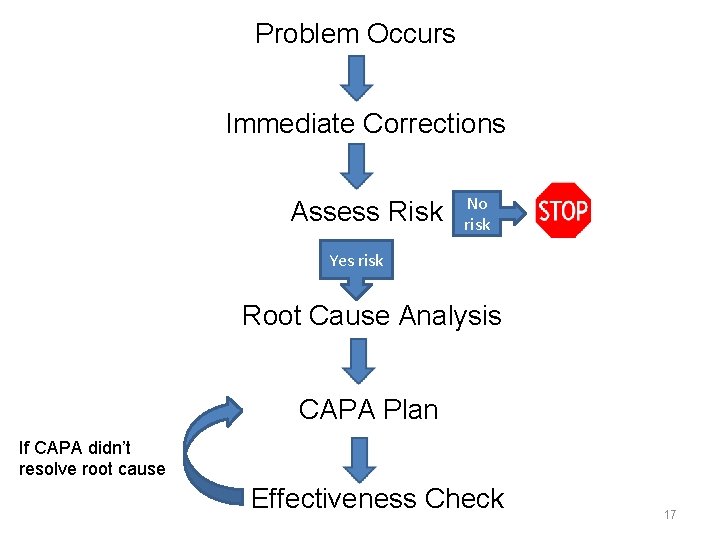
Problem Occurs Immediate Corrections Assess Risk No risk Yes risk Root Cause Analysis CAPA Plan If CAPA didn’t resolve root cause Effectiveness Check 17

Root Cause • The root cause is the initiating, most basic cause of a problem that may or may not lead to a chain of causes or other problems (domino effect) • Eliminating the root cause should prevent recurrence of the problem 18

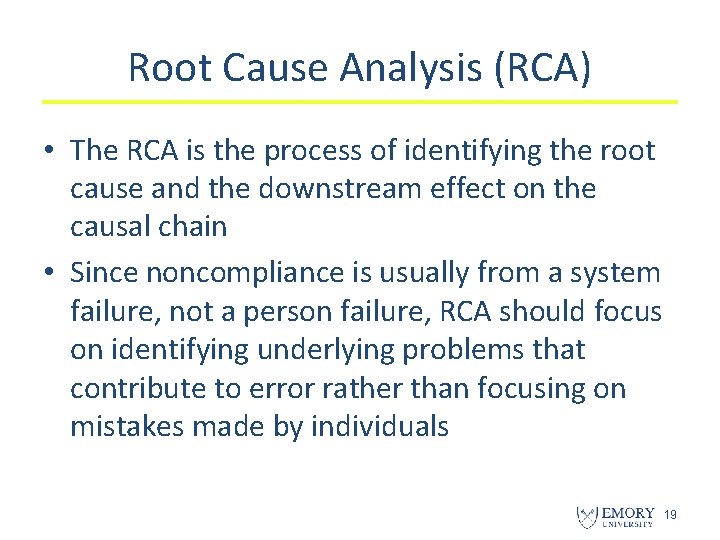
Root Cause Analysis (RCA) • The RCA is the process of identifying the root cause and the downstream effect on the causal chain • Since noncompliance is usually from a system failure, not a person failure, RCA should focus on identifying underlying problems that contribute to error rather than focusing on mistakes made by individuals 19

RCA Steps • Questions to ask – Why and how did the problem occur? – What were the steps? – Who was impacted by the problem? – Keep asking “why” and “how” until you reach the root cause 20

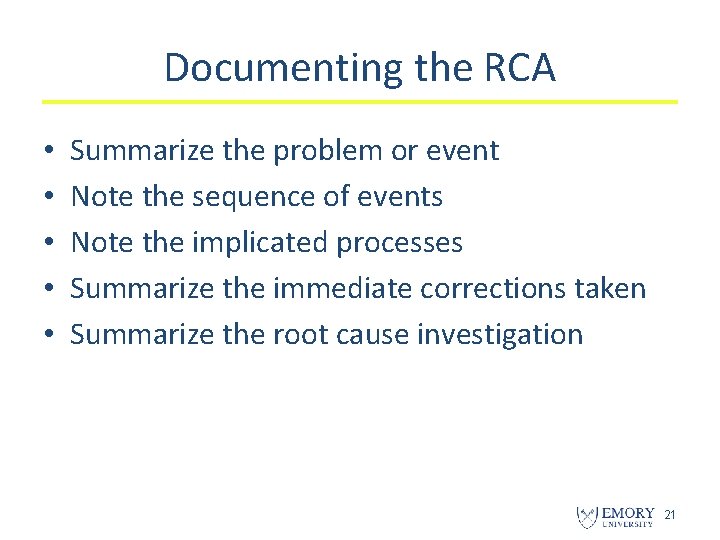
Documenting the RCA • • • Summarize the problem or event Note the sequence of events Note the implicated processes Summarize the immediate corrections taken Summarize the root cause investigation 21

Corrections vs Corrective Actions • Corrections – Immediate actions taken to resolve a problem before the root cause is known – Putting out fires – Could resolve minor deviations/noncompliance • Corrective Actions – If risk of problem (severity and frequency) is greater, do RCA and move to CAPA – Process 22

What is CAPA? • Corrective and Preventive Actions – Corrective Actions • The process of reacting to an existing problem and fixing it – Preventive Actions • The process of detecting potential problems and eliminating them 23

FDA Warning Letter Excerpts • “You failed to secure the investigator’s compliance and allowed subjects to be put at risk for potential adverse effects, such as…Your response is inadequate in that it does not describe your corrective and preventive actions in sufficient detail. ” • “You stated that study subjects will be re-consented with a revised consent form after it is approved by the IRB. Your response is inadequate as it lacks a corrective and preventive action plan to ensure that adequate consent forms are provided to study subjects prior to any study related procedures in the future. ” 24

CAPA Don’ts! • We will retrain the study team… • Study team members have been terminated or will be reassigned… • We will have 5 people check…. – Don’t overpromise with detailed CAPA plans that we can’t maintain 25

CAPA Musts! • Corrective: Assess and correct safety/rights/welfare of subjects. Report to subjects, IRB, sponsor, and/or internal department. Document corrections. • Preventive: Develop and document a process, train on the process, implement the process, evaluate the process, amend process as necessary. 26

“SMART” CAPAs 27

Effectiveness Check • Build in an effectiveness check as the final step of CAPA • Effectiveness checks verify that the CAPAs resolved the root cause 28

Documenting the CAPA • • • Action type (corrective or preventive) Action description Owner Due date Plan for effectiveness check Effectiveness check outcome 29

In Summary • Problem occurs • Immediate corrections taken • Determine impact of problem and risk of recurrence (risk = severity + frequency) • Root cause analysis • CAPA plan • Effectiveness check 30

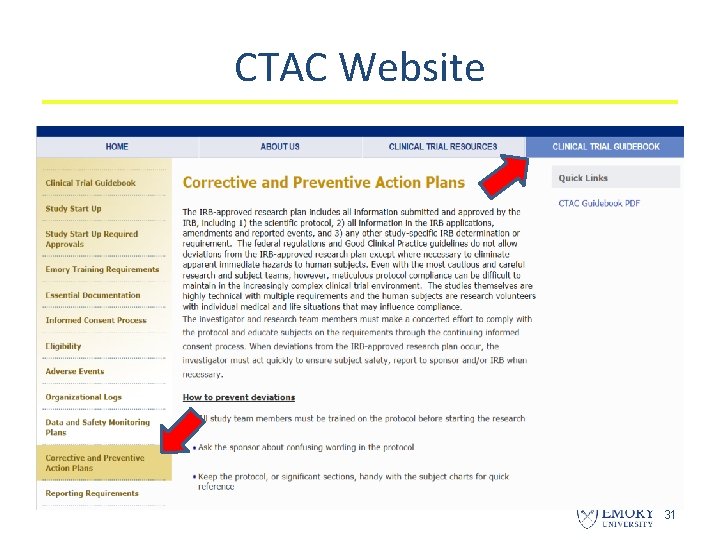
CTAC Website 31

Contacts • Stephanie de. Rijke – Emory University Clinical Trials Audit and Compliance – www. ctac. emory. edu – smickle@emory. edu – (404) 712 -5159 32
 Clinical trials quality by design
Clinical trials quality by design Rijke rekenomgeving
Rijke rekenomgeving External quality assurance in clinical laboratory
External quality assurance in clinical laboratory Pmp quality management
Pmp quality management Basic concepts of quality control
Basic concepts of quality control Pmp gold plating
Pmp gold plating Ana quality assurance model
Ana quality assurance model Perform quality assurance
Perform quality assurance Compliance vs quality
Compliance vs quality Clinicaltrials gov api
Clinicaltrials gov api Readyset ohsu
Readyset ohsu Mpn clinical trials
Mpn clinical trials York trials unit
York trials unit Mpn clinical trials
Mpn clinical trials Role of statistician in clinical trials
Role of statistician in clinical trials Clinical trials prs
Clinical trials prs Hawk irb
Hawk irb Nida clinical trials network
Nida clinical trials network Dhl bishkek
Dhl bishkek Andrew nunn
Andrew nunn Clinical trials.gov login
Clinical trials.gov login Clinical trials
Clinical trials Site initiation visit agenda
Site initiation visit agenda Clinical hysteria salem witch trials
Clinical hysteria salem witch trials Phs human subjects and clinical trials information
Phs human subjects and clinical trials information Randomization
Randomization Ivr iwr for clinical trial
Ivr iwr for clinical trial Audits and inspections of clinical trials
Audits and inspections of clinical trials Clinical assurance
Clinical assurance Introduction to software quality assurance
Introduction to software quality assurance Define quality control
Define quality control Statistical software quality assurance
Statistical software quality assurance Plan de software quality assurance
Plan de software quality assurance