Principles of endocrinology Semmelweis University First Department of


- Slides: 25

Principles of endocrinology Semmelweis University First Department of Medicine Dr. Szathmári Miklós 01. February 2010.

Scope of endocrinology 1. • The term endocrinology contrast the actions of hormones secreted internally (endocrine) with those secreted externally (exocrine) or into a lumen, such as the gastrointestinal tract • The hormone, derived from a Greek phrase meaning „to set in motion” aptly describes the dynamic actions of hormones as they elicit cellular responses and regulate physiologic processes through feedback mechanisms.

Scope of endocrinology 2. Unlike many other specialties in medicine, it is not possible to define endocrinology strictly along anatomic lines • The classic endocrine glands – pituitary, thyroid, parathyroid, pancreatic islets, adrenal, and gonadscommunicate with other organs through the nervous system, hormones, cytokins, and growth factors. • The brain – in addition to its traditional synaptic function – produces peptide hormones, such as hypothalamic releasing factors, which exert regulatory influence over pituitary hormonal secretion. • The peripheral nervous system stimulates adrenal medulla • The immune and endocrine systems are also intimately intertwined. Autoimmune thyroiditis and type 1 diabetes mellitus are caused by dysregulation of immune tolerance

Scope of endocrinology 3. • The cytokins and interleukins have profound effects on the functions of the pituitary, adrenal, thyroid, and gonads • The kidney produces erythropoietin that stimulates erythropoiesis in the bone marrow. Moreover the kidney is also integrally involved in the renin-angiotensin axis, and is a target of several hormones. • The gastrointestinal tract produces a large number of peptid hormones (cholecystokinin, ghrelin, gastrin, secretin, VIP, etc. ) • The heart is the source of atrial natriuretic peptide, which acts in classic endocrine fashion to induce natriuresis at a distant target organ in the kidney).

Nature of hormones • Amino acid derivates: dopamine, catecholamine and thyroid homones • Small neuropeptides: Gn. RH, TRH, somatostatin, and vasopressin • Large proteins: insulin, LH, and PTH produced by claasic endocrine glands • Steroid hormones: cortisol, estrogen that are synthesized from cholesterol-based precursors • Vitamin derivates: retinoid (vitamin A) and vitamin D – Amino acid derivates and peptide hormones interact with cell surface membrane receptors – Steroids, thyroid hormones, vitamin D and retinoids are lipid soluble and interact with intracellular nuclear receptors

Hormone and receptor families • The hormones can be grouped into families, reflecting their structural similarities. – Glycoprotein hormones (TSH, FSH, LH, h. CG) have the α-subunit in common, the β subunits are distinct and confer specific biologic action. The related GPCRs have evolved for each of the glycoprotein hormones. These receptors are structurally similar, and each coupled to the Gsα signaling pathway • Minimal overlap of hormone binding with subtle physiological consequences (h. CG stimulates TSH receptor, and increase thyroid hormone levels. – Insulin, IGF-I and IGF II • Structural similarities with moderate cross-talk among the members of the insulin/IGF family – PTH and PTHrp • These hormones share amino acid sequence similarity. Both hormones bind to a single PTH receptor that is expressed in bones and kidney

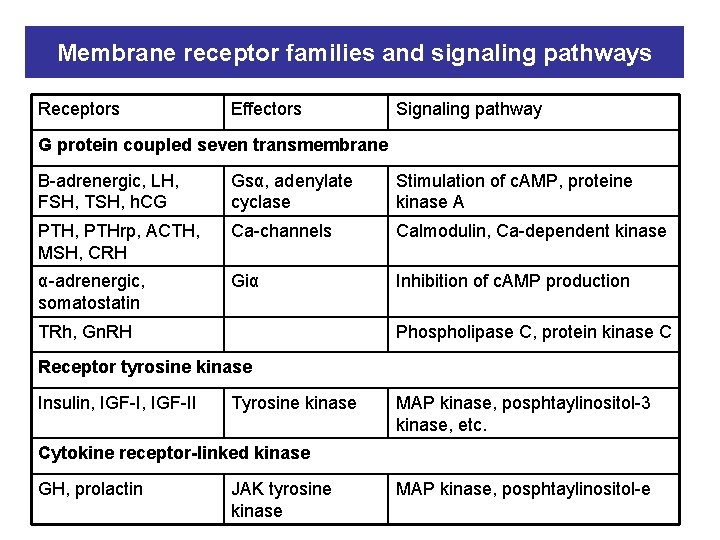
Membrane receptor families and signaling pathways Receptors Effectors Signaling pathway G protein coupled seven transmembrane Β-adrenergic, LH, FSH, TSH, h. CG Gsα, adenylate cyclase Stimulation of c. AMP, proteine kinase A PTH, PTHrp, ACTH, MSH, CRH Ca-channels Calmodulin, Ca-dependent kinase α-adrenergic, somatostatin Giα Inhibition of c. AMP production TRh, Gn. RH Phospholipase C, protein kinase C Receptor tyrosine kinase Insulin, IGF-II Tyrosine kinase MAP kinase, posphtaylinositol-3 kinase, etc. Cytokine receptor-linked kinase GH, prolactin JAK tyrosine kinase MAP kinase, posphtaylinositol-e

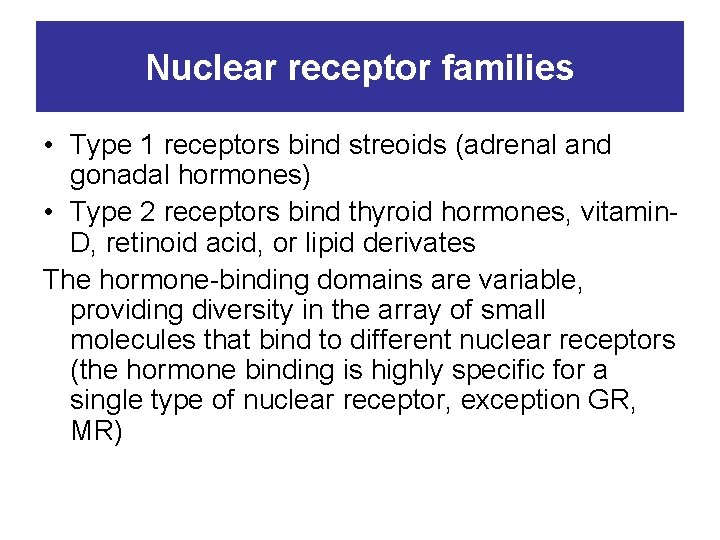
Nuclear receptor families • Type 1 receptors bind streoids (adrenal and gonadal hormones) • Type 2 receptors bind thyroid hormones, vitamin. D, retinoid acid, or lipid derivates The hormone-binding domains are variable, providing diversity in the array of small molecules that bind to different nuclear receptors (the hormone binding is highly specific for a single type of nuclear receptor, exception GR, MR)

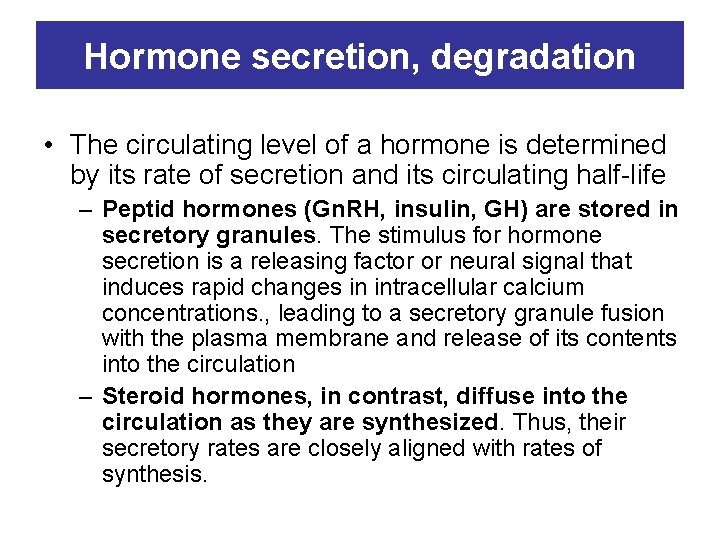
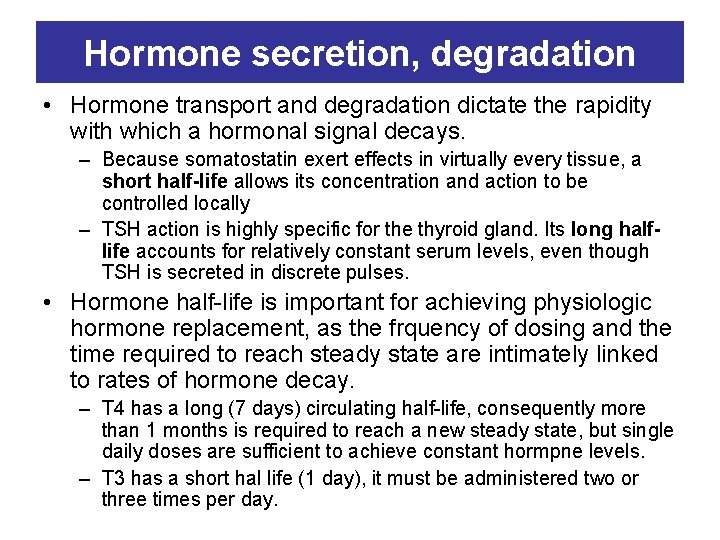
Hormone secretion, degradation • The circulating level of a hormone is determined by its rate of secretion and its circulating half-life – Peptid hormones (Gn. RH, insulin, GH) are stored in secretory granules. The stimulus for hormone secretion is a releasing factor or neural signal that induces rapid changes in intracellular calcium concentrations. , leading to a secretory granule fusion with the plasma membrane and release of its contents into the circulation – Steroid hormones, in contrast, diffuse into the circulation as they are synthesized. Thus, their secretory rates are closely aligned with rates of synthesis.

Hormone secretion, degradation • Hormone transport and degradation dictate the rapidity with which a hormonal signal decays. – Because somatostatin exert effects in virtually every tissue, a short half-life allows its concentration and action to be controlled locally – TSH action is highly specific for the thyroid gland. Its long halflife accounts for relatively constant serum levels, even though TSH is secreted in discrete pulses. • Hormone half-life is important for achieving physiologic hormone replacement, as the frquency of dosing and the time required to reach steady state are intimately linked to rates of hormone decay. – T 4 has a long (7 days) circulating half-life, consequently more than 1 months is required to reach a new steady state, but single daily doses are sufficient to achieve constant hormpne levels. – T 3 has a short hal life (1 day), it must be administered two or three times per day.

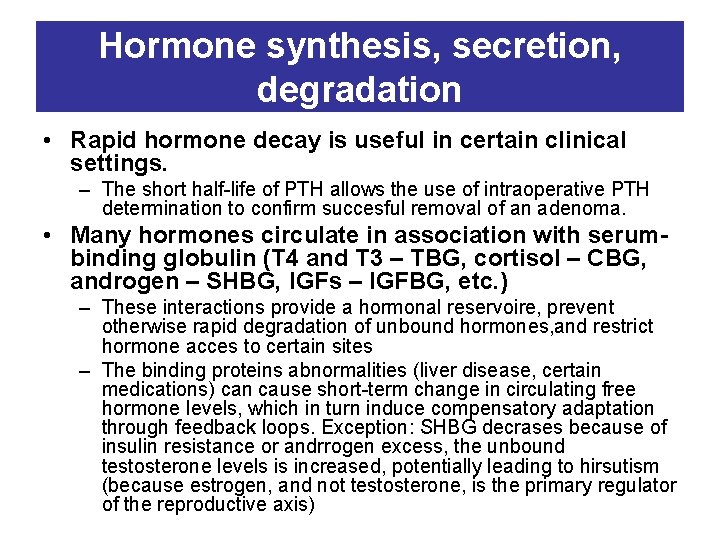
Hormone synthesis, secretion, degradation • Rapid hormone decay is useful in certain clinical settings. – The short half-life of PTH allows the use of intraoperative PTH determination to confirm succesful removal of an adenoma. • Many hormones circulate in association with serumbinding globulin (T 4 and T 3 – TBG, cortisol – CBG, androgen – SHBG, IGFs – IGFBG, etc. ) – These interactions provide a hormonal reservoire, prevent otherwise rapid degradation of unbound hormones, and restrict hormone acces to certain sites – The binding proteins abnormalities (liver disease, certain medications) can cause short-term change in circulating free hormone levels, which in turn induce compensatory adaptation through feedback loops. Exception: SHBG decrases because of insulin resistance or andrrogen excess, the unbound testosterone levels is increased, potentially leading to hirsutism (because estrogen, and not testosterone, is the primary regulator of the reproductive axis)

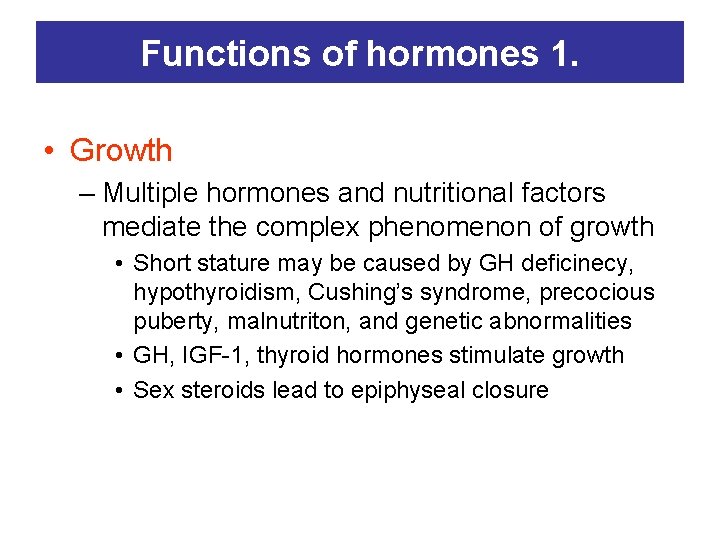
Functions of hormones 1. • Growth – Multiple hormones and nutritional factors mediate the complex phenomenon of growth • Short stature may be caused by GH deficinecy, hypothyroidism, Cushing’s syndrome, precocious puberty, malnutriton, and genetic abnormalities • GH, IGF-1, thyroid hormones stimulate growth • Sex steroids lead to epiphyseal closure

Functions of hormones 2. • Maintenance of homeostasis – Thyroid hormones control about 25% of basal metabolism in different tissues – Cortisol exerts a permissive action for many hormones in addition to its own direct effects – Parathormone regulates calcium and phosphorus levels – Vasopressin regulates serum osmolality by controlling renal free water clearance – Mineralocorticoids control vascular volume and serum electrolyte concentrations – Insulin maintains euglycemia in the fed and fasted states

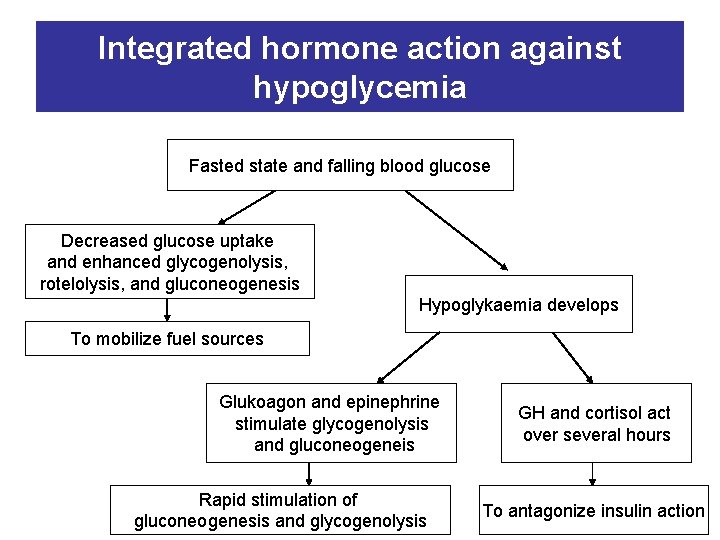
Integrated hormone action against hypoglycemia Fasted state and falling blood glucose Decreased glucose uptake and enhanced glycogenolysis, rotelolysis, and gluconeogenesis Hypoglykaemia develops To mobilize fuel sources Glukoagon and epinephrine stimulate glycogenolysis and gluconeogeneis Rapid stimulation of gluconeogenesis and glycogenolysis GH and cortisol act over several hours To antagonize insulin action

Functions of hormones 3. • Reproduction – Sex determination during fetal development – Sexual maturation during puberty – Conception, pregnancy, lactation – Cessation of reproductive capability at menopause Each of these stages involves an orchestrated interplay of multiple hormones

Regulatory systems of hormone production 1. • Feedback control: both negative and positive, is fundamental feature of endocrine system. – Each of the major hypothalamic-pitutary-hormone axes is governed by negative feedback: • • Thyroid hormones on the TRH-TSH axis Cortisol on the CRH-ACTH axis Gonadal steroids on the Gn. RH-LH/FSH axis IGF-1 on the GHRH-GH axis – Feedback regulation also occurs for endocrine systems that do not involve the pituitary gland: • Calcium inhibits PTH secretion • Glucose inhibition of insulin secretion – Positive feedback control: • Estrogen mediated stimulation of mid-cycle LH-surge

Regulatory systems of hormone production 2. • Local regulatory systems, often involving growth factors: – Paracrine regulation (factors released by one cell that act on an adjacent cell in the same tissue: somatostatin secretion of pancreatic δ-cells inhibits insulin secretion from nearby β-cells – Autocrine regulation (the action of a factor on the same cell from which it is produced): IGF-1 acts on many cells that produce it (gonadal cells etc. )

Regulatory systems of hormone production 3. • Hormonal rhythms. The feedback regulatory systems are superimposed on hormonal rhythms that are used for adaptation to the environment (seasonal changes, the daily occurence of lightdark cycle, sleep, meals, and stress) – Menstrual cycle is repeated on every 28 days – All pituitary hormone rhythms are entrained to sleep and to the circadian cycle, generating reproducible patterns that are repeated appr. every 24 h. – Other endocrine rhythms occur on a more rapid time scale. LH and FSH secretion are exquisitely sensitive to Gn. RH pulse frequency. Intermittant pulses of Gn. RH are required to maintain pituitary sensitivity, whereas continuous exposure to Gn. RH causes pituitary gonadotrop desensitization

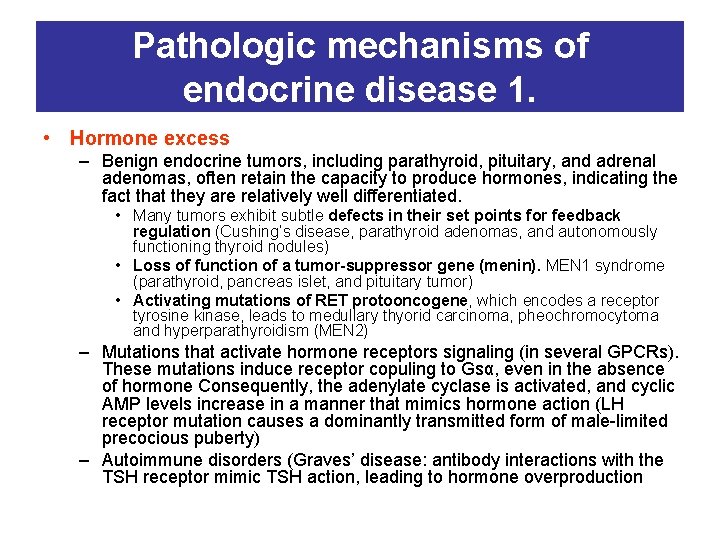
Pathologic mechanisms of endocrine disease 1. • Hormone excess – Benign endocrine tumors, including parathyroid, pituitary, and adrenal adenomas, often retain the capacity to produce hormones, indicating the fact that they are relatively well differentiated. • Many tumors exhibit subtle defects in their set points for feedback regulation (Cushing’s disease, parathyroid adenomas, and autonomously functioning thyroid nodules) • Loss of function of a tumor-suppressor gene (menin). MEN 1 syndrome (parathyroid, pancreas islet, and pituitary tumor) • Activating mutations of RET protooncogene, which encodes a receptor tyrosine kinase, leads to medullary thyorid carcinoma, pheochromocytoma and hyperparathyroidism (MEN 2) – Mutations that activate hormone receptors signaling (in several GPCRs). These mutations induce receptor copuling to Gsα, even in the absence of hormone Consequently, the adenylate cyclase is activated, and cyclic AMP levels increase in a manner that mimics hormone action (LH receptor mutation causes a dominantly transmitted form of male-limited precocious puberty) – Autoimmune disorders (Graves’ disease: antibody interactions with the TSH receptor mimic TSH action, leading to hormone overproduction

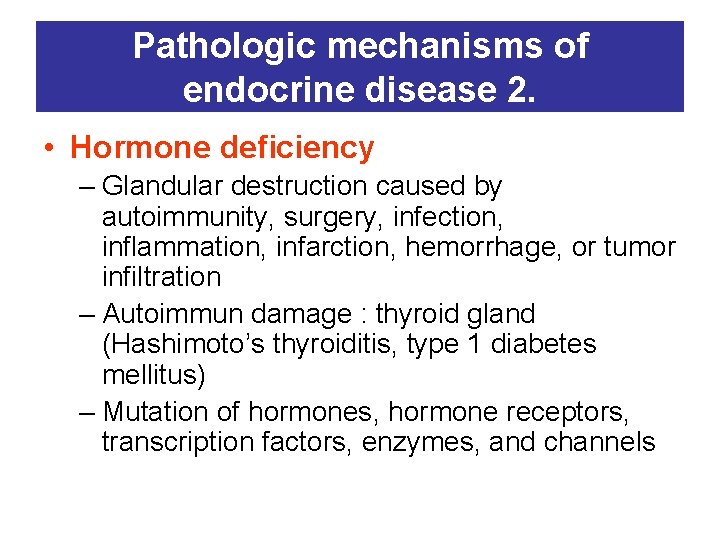
Pathologic mechanisms of endocrine disease 2. • Hormone deficiency – Glandular destruction caused by autoimmunity, surgery, infection, inflammation, infarction, hemorrhage, or tumor infiltration – Autoimmun damage : thyroid gland (Hashimoto’s thyroiditis, type 1 diabetes mellitus) – Mutation of hormones, hormone receptors, transcription factors, enzymes, and channels

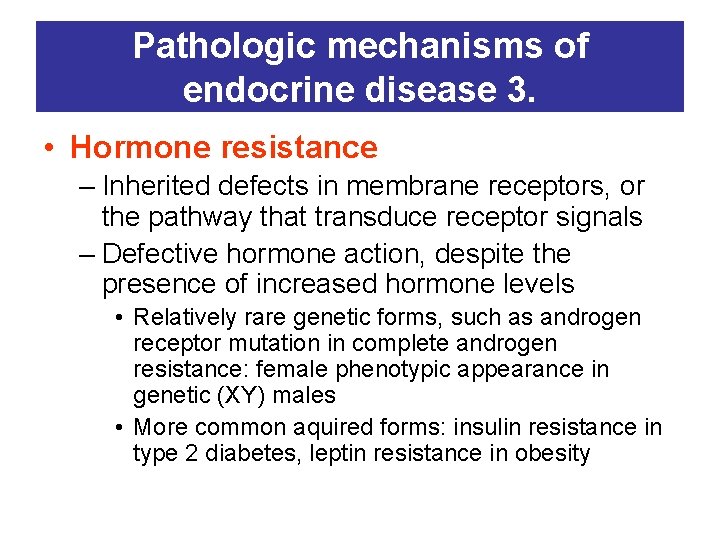
Pathologic mechanisms of endocrine disease 3. • Hormone resistance – Inherited defects in membrane receptors, or the pathway that transduce receptor signals – Defective hormone action, despite the presence of increased hormone levels • Relatively rare genetic forms, such as androgen receptor mutation in complete androgen resistance: female phenotypic appearance in genetic (XY) males • More common aquired forms: insulin resistance in type 2 diabetes, leptin resistance in obesity

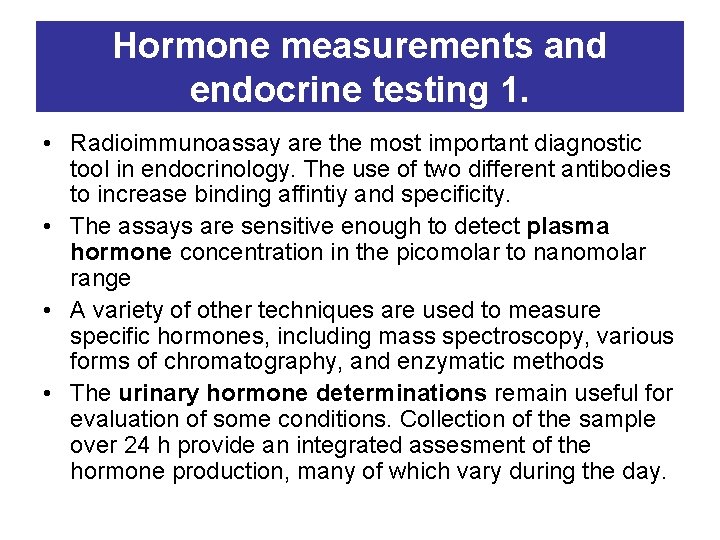
Hormone measurements and endocrine testing 1. • Radioimmunoassay are the most important diagnostic tool in endocrinology. The use of two different antibodies to increase binding affintiy and specificity. • The assays are sensitive enough to detect plasma hormone concentration in the picomolar to nanomolar range • A variety of other techniques are used to measure specific hormones, including mass spectroscopy, various forms of chromatography, and enzymatic methods • The urinary hormone determinations remain useful for evaluation of some conditions. Collection of the sample over 24 h provide an integrated assesment of the hormone production, many of which vary during the day.

Hormone measurements and endocrine testing 2. • The normal range for most hormone is relatively broad, varying by a factor of two to tenfold. The correct normative database is essential part of interpreting hormone tests. • For many endocrine systems, much information can be gained from basal hormone testing, when different components of the endocrine axis are assessed simultaneously. – – Testosterone and LH TSH and free thyroxine Parathormone and serum calcium ACTH and cortisol

Hormone measurements and endocrine testing 3. • It is not uncommon, however, for baseline hormone levels associated with pathologic endocrine conditions to overlap with the normal hormone range. In this circumstance, dynamic testing is useful for further separate the two gropus: – Suppression in case of suspected hyperfunction – Stimulation in the setting of suspected hypofunction

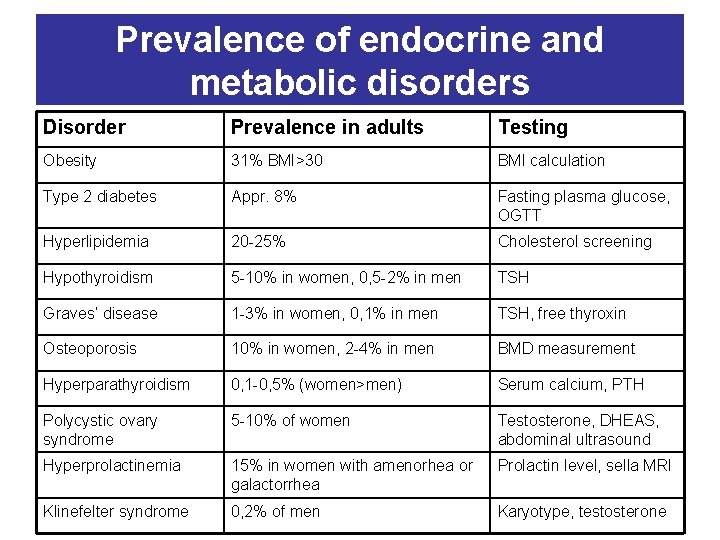
Prevalence of endocrine and metabolic disorders Disorder Prevalence in adults Testing Obesity 31% BMI>30 BMI calculation Type 2 diabetes Appr. 8% Fasting plasma glucose, OGTT Hyperlipidemia 20 -25% Cholesterol screening Hypothyroidism 5 -10% in women, 0, 5 -2% in men TSH Graves’ disease 1 -3% in women, 0, 1% in men TSH, free thyroxin Osteoporosis 10% in women, 2 -4% in men BMD measurement Hyperparathyroidism 0, 1 -0, 5% (women>men) Serum calcium, PTH Polycystic ovary syndrome 5 -10% of women Testosterone, DHEAS, abdominal ultrasound Hyperprolactinemia 15% in women with amenorhea or galactorrhea Prolactin level, sella MRI Klinefelter syndrome 0, 2% of men Karyotype, testosterone
 Endocrinology of pregnancy
Endocrinology of pregnancy Endocrinology of pregnancy
Endocrinology of pregnancy Park nicollet pediatric endocrinology
Park nicollet pediatric endocrinology Contoh root+prefix, suffix
Contoh root+prefix, suffix Reproductive biology and endocrinology
Reproductive biology and endocrinology Reproductive endocrinology near campbell
Reproductive endocrinology near campbell Endocrinology
Endocrinology Semmelweis
Semmelweis Agnes csaki semmelweis
Agnes csaki semmelweis Semmelweis university faculty of medicine
Semmelweis university faculty of medicine Doktori iskola sote
Doktori iskola sote Itc semmelweis
Itc semmelweis Grundsubstanz
Grundsubstanz Khidai
Khidai Orthodontics semmelweis
Orthodontics semmelweis Semmelweis
Semmelweis Semmelweis egyetem konzerváló fogászati klinika budapest
Semmelweis egyetem konzerváló fogászati klinika budapest Semmelweis egyetem szemészeti klinika budapest
Semmelweis egyetem szemészeti klinika budapest Doktori iskola semmelweis
Doktori iskola semmelweis Nasal vestibule
Nasal vestibule Semmelweis
Semmelweis Erasmus plus semmelweis
Erasmus plus semmelweis Itc semmelweis seka
Itc semmelweis seka Different between plasma and serum
Different between plasma and serum Ectomesenchyma
Ectomesenchyma Semmelweis
Semmelweis