Primary Results of Testing platelet Reactivity In patients



- Slides: 23

Primary Results of Testing platelet Reactivity In patients under. Going elective stent placement on clopidogrel to Guide alternative th. Erapy with p. Rasugrel TRIGGER-PCI Study TCT 2011 Dietmar Trenk, Ph. D On behalf of the TRIGGER-PCI Investigators

Disclosures Consulting fees/honoraria: Eli Lilly, Daiichi Sankyo, sanofi -aventis, Astra. Zeneca Speaker Honoraria: Eli Lilly, Daiichi Sankyo, sanofiaventis, Astra. Zeneca Off-label use of prasugrel and Verify. Now P 2 Y 12 test will be discussed in this presentation

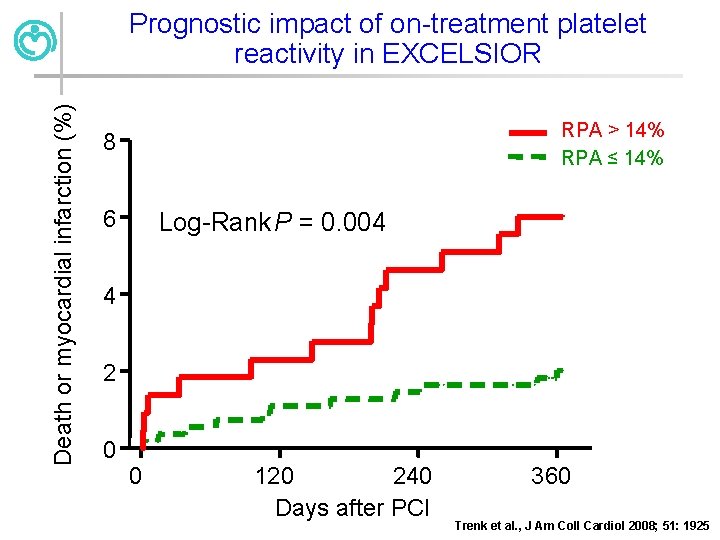
Death or myocardial infarction (%) Prognostic impact of on-treatment platelet reactivity in EXCELSIOR RPA > 14% RPA ≤ 14% 8 6 Log-Rank P = 0. 004 4 2 0 0 120 240 Days after PCI 360 Trenk et al. , J Am Coll Cardiol 2008; 51: 1925

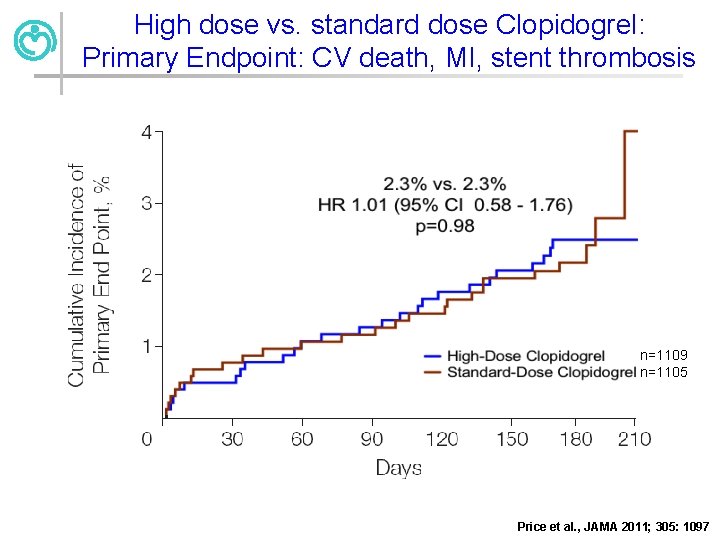
High dose vs. standard dose Clopidogrel: Primary Endpoint: CV death, MI, stent thrombosis n=1109 n=1105 Price et al. , JAMA 2011; 305: 1097

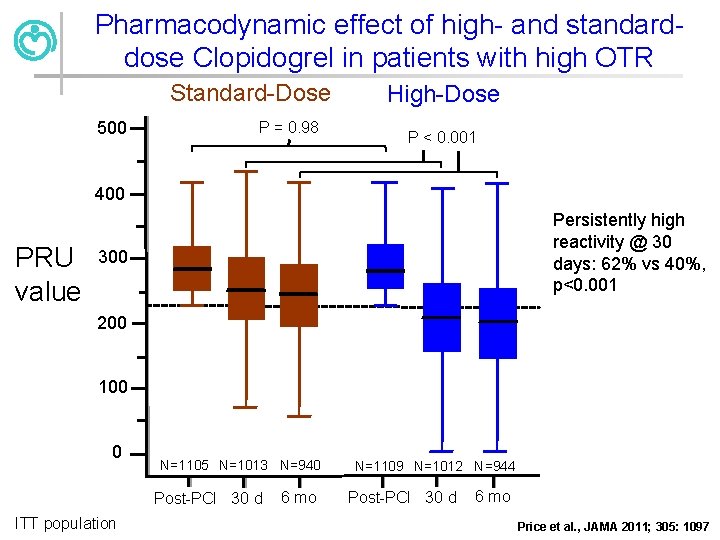
Pharmacodynamic effect of high- and standarddose Clopidogrel in patients with high OTR Standard-Dose 500 P = 0. 98 High-Dose P < 0. 001 400 PRU value Persistently high reactivity @ 30 days: 62% vs 40%, p<0. 001 300 200 100 0 N=1105 N=1013 N=940 Post-PCI 30 d ITT population 6 mo N=1109 N=1012 N=944 Post-PCI 30 d 6 mo Price et al. , JAMA 2011; 305: 1097

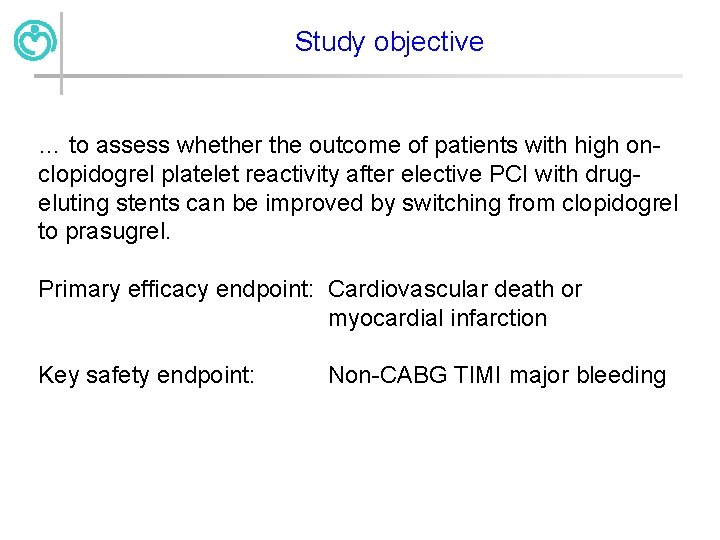
Study objective … to assess whether the outcome of patients with high onclopidogrel platelet reactivity after elective PCI with drugeluting stents can be improved by switching from clopidogrel to prasugrel. Primary efficacy endpoint: Cardiovascular death or myocardial infarction Key safety endpoint: Non-CABG TIMI major bleeding

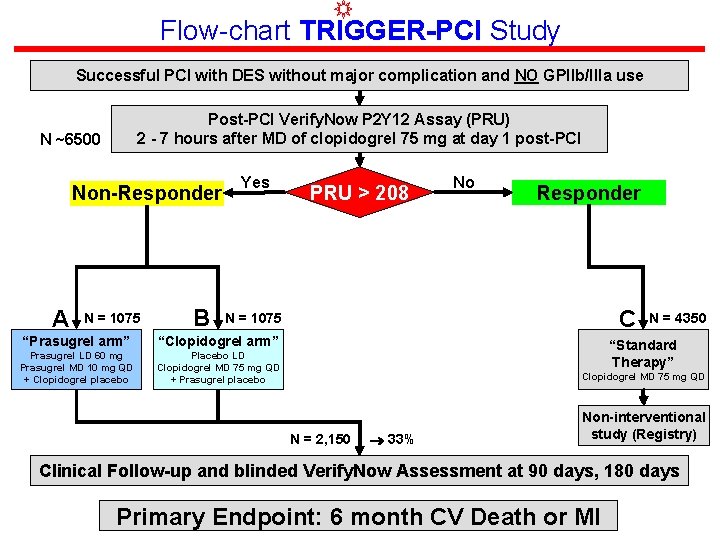
Flow-chart TRIGGER-PCI Study Successful PCI with DES without major complication and NO GPIIb/IIIa use Post-PCI Verify. Now P 2 Y 12 Assay (PRU) 2 - 7 hours after MD of clopidogrel 75 mg at day 1 post-PCI N ~6500 Non-Responder A N = 1075 B Yes PRU > 208 No Responder C N = 1075 “Prasugrel arm” “Clopidogrel arm” Prasugrel LD 60 mg Prasugrel MD 10 mg QD + Clopidogrel placebo Placebo LD Clopidogrel MD 75 mg QD + Prasugrel placebo N = 4350 “Standard Therapy” Clopidogrel MD 75 mg QD N = 2, 150 33% Non-interventional study (Registry) Clinical Follow-up and blinded Verify. Now Assessment at 90 days, 180 days Primary Endpoint: 6 month CV Death or MI

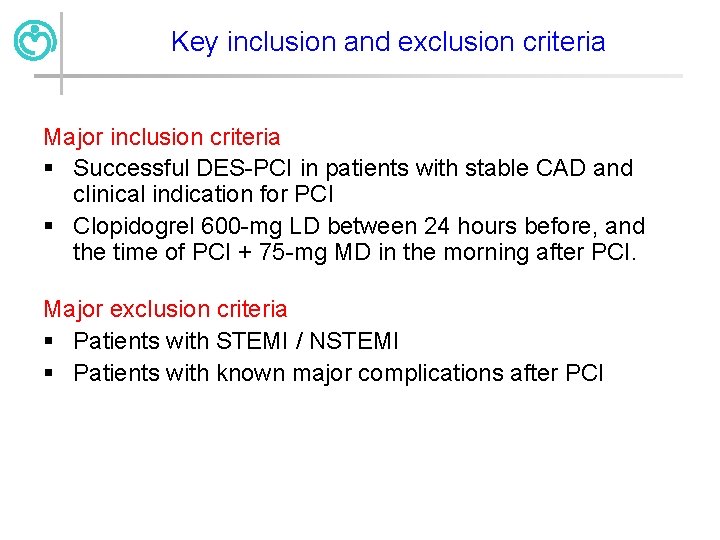
Key inclusion and exclusion criteria Major inclusion criteria § Successful DES-PCI in patients with stable CAD and clinical indication for PCI § Clopidogrel 600 -mg LD between 24 hours before, and the time of PCI + 75 -mg MD in the morning after PCI. Major exclusion criteria § Patients with STEMI / NSTEMI § Patients with known major complications after PCI

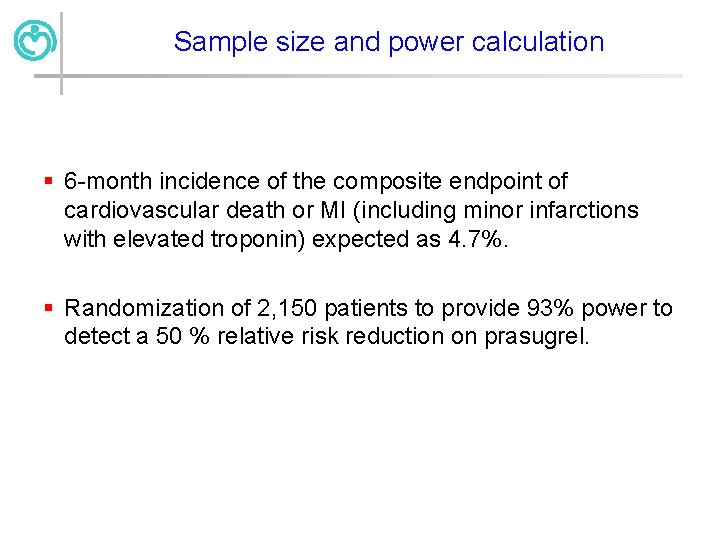
Sample size and power calculation § 6 -month incidence of the composite endpoint of cardiovascular death or MI (including minor infarctions with elevated troponin) expected as 4. 7%. § Randomization of 2, 150 patients to provide 93% power to detect a 50 % relative risk reduction on prasugrel.

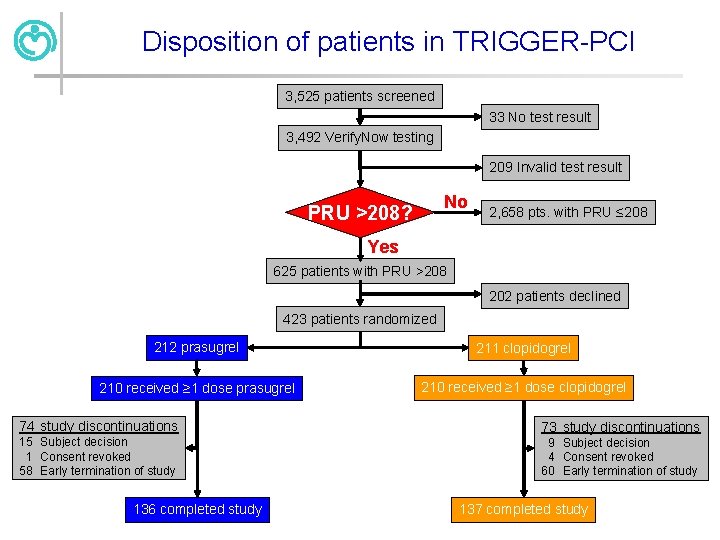
Disposition of patients in TRIGGER-PCI 3, 525 patients screened 33 No test result 3, 492 Verify. Now testing 209 Invalid test result No PRU >208? 2, 658 pts. with PRU ≤ 208 Yes 625 patients with PRU >208 202 patients declined 423 patients randomized 212 prasugrel 211 clopidogrel 210 received ≥ 1 dose prasugrel 210 received ≥ 1 dose clopidogrel 74 study discontinuations 73 study discontinuations 15 Subject decision 1 Consent revoked 58 Early termination of study 9 Subject decision 4 Consent revoked 60 Early termination of study 136 completed study 137 completed study

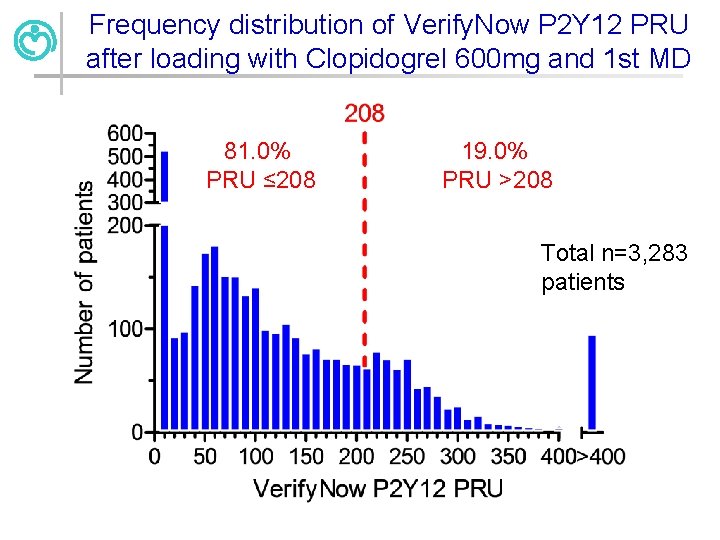
Frequency distribution of Verify. Now P 2 Y 12 PRU after loading with Clopidogrel 600 mg and 1 st MD 81. 0% PRU ≤ 208 19. 0% PRU >208 Total n=3, 283 patients

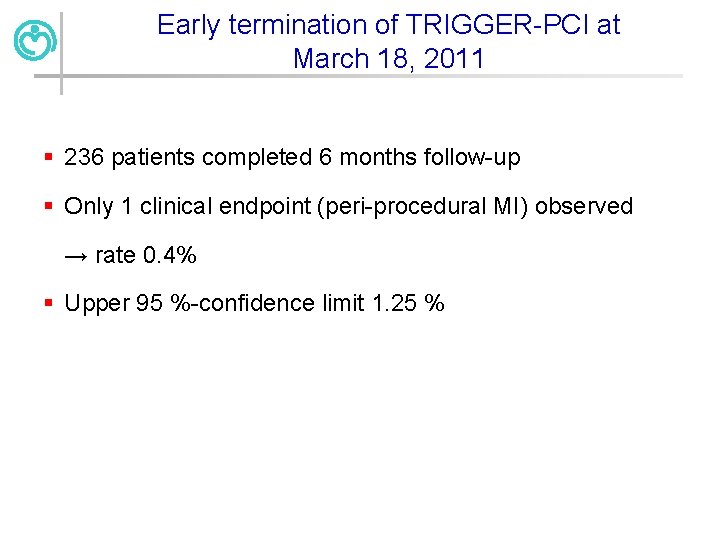
Early termination of TRIGGER-PCI at March 18, 2011 § 236 patients completed 6 months follow-up § Only 1 clinical endpoint (peri-procedural MI) observed → rate 0. 4% § Upper 95 %-confidence limit 1. 25 %

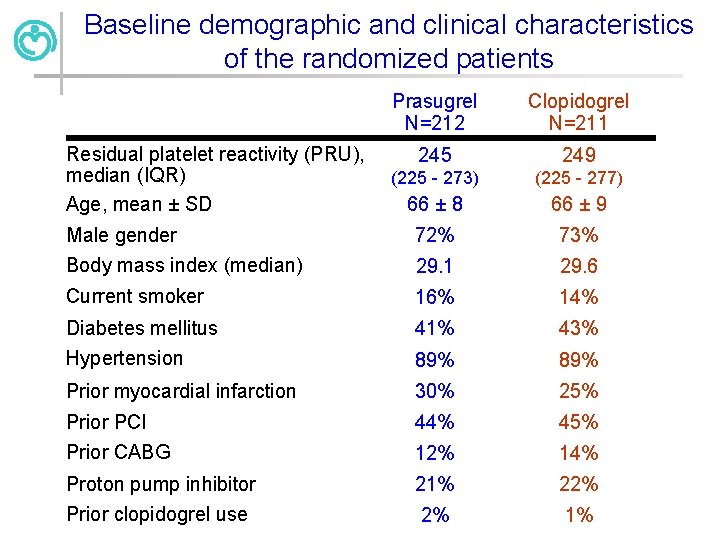
Baseline demographic and clinical characteristics of the randomized patients Prasugrel N=212 Clopidogrel N=211 245 249 (225 - 273) (225 - 277) 66 ± 8 66 ± 9 Male gender Body mass index (median) 72% 73% 29. 1 29. 6 Current smoker 16% 14% Diabetes mellitus 41% 43% Hypertension 89% Prior myocardial infarction 30% 25% Prior PCI 44% 45% Prior CABG 12% 14% Proton pump inhibitor 21% 22% Prior clopidogrel use 2% 1% Residual platelet reactivity (PRU), median (IQR) Age, mean ± SD

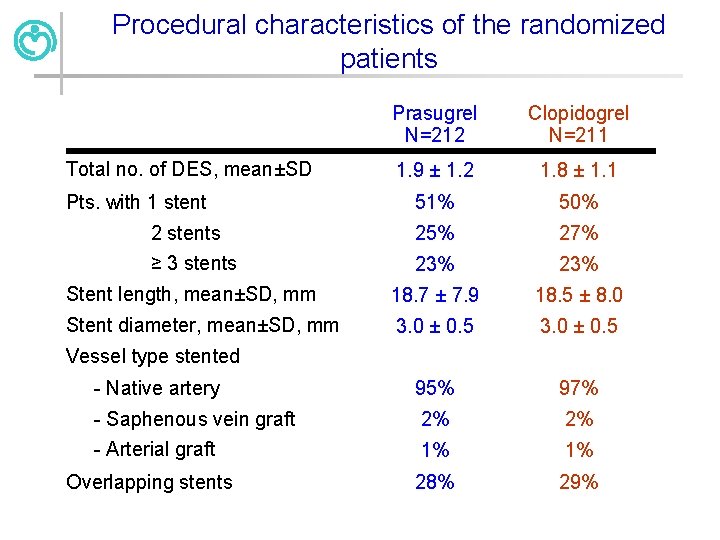
Procedural characteristics of the randomized patients Prasugrel N=212 Clopidogrel N=211 1. 9 ± 1. 2 1. 8 ± 1. 1 51% 50% 2 stents ≥ 3 stents 25% 27% 23% Stent length, mean±SD, mm 18. 7 ± 7. 9 18. 5 ± 8. 0 Stent diameter, mean±SD, mm 3. 0 ± 0. 5 - Native artery 95% 97% - Saphenous vein graft 2% 2% - Arterial graft 1% 1% Overlapping stents 28% 29% Total no. of DES, mean±SD Pts. with 1 stent Vessel type stented

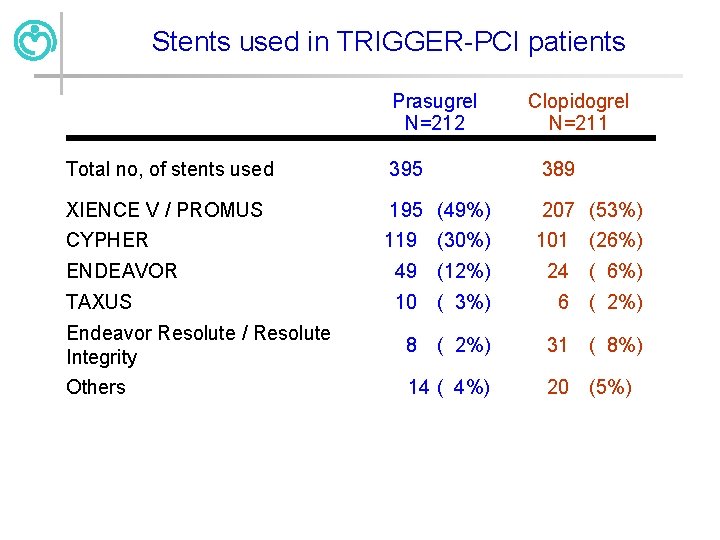
Stents used in TRIGGER-PCI patients Prasugrel N=212 Clopidogrel N=211 Total no, of stents used 395 389 XIENCE V / PROMUS 195 (49%) 207 (53%) CYPHER 119 (30%) 101 (26%) ENDEAVOR 49 (12%) 24 ( 6%) TAXUS 10 ( 3%) 6 ( 2%) Endeavor Resolute / Resolute Integrity 8 ( 2%) 31 ( 8%) Others 14 ( 4%) 20 (5%)

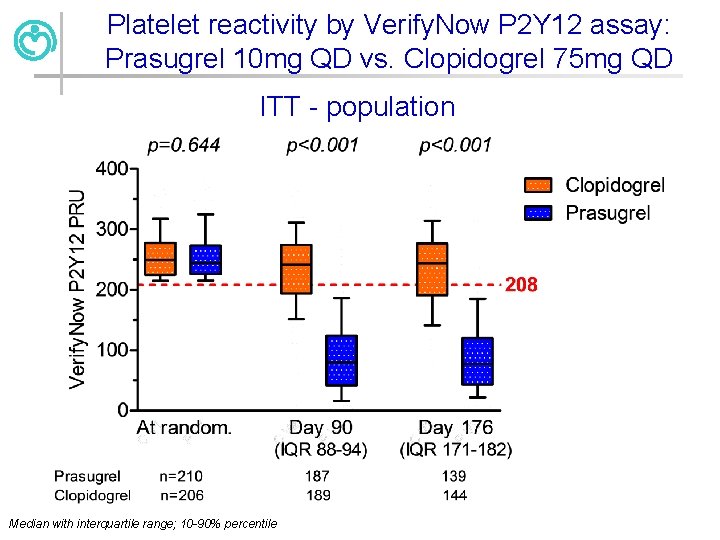
Platelet reactivity by Verify. Now P 2 Y 12 assay: Prasugrel 10 mg QD vs. Clopidogrel 75 mg QD ITT - population Median with interquartile range; 10 -90% percentile

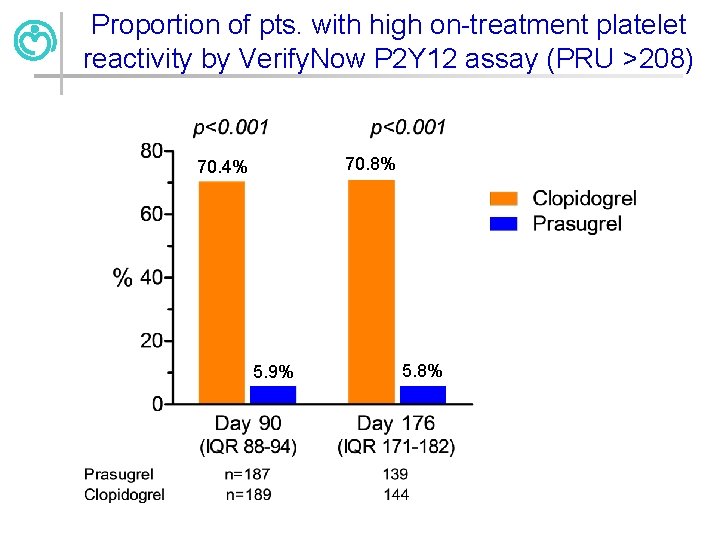
Proportion of pts. with high on-treatment platelet reactivity by Verify. Now P 2 Y 12 assay (PRU >208) 70. 8% 70. 4% 5. 9% 5. 8%

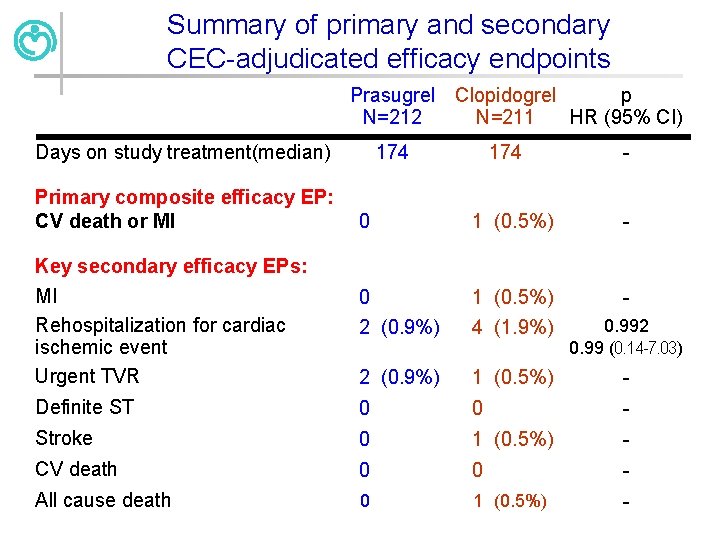
Summary of primary and secondary CEC-adjudicated efficacy endpoints Prasugrel N=212 Days on study treatment(median) Primary composite efficacy EP: CV death or MI Clopidogrel p N=211 HR (95% CI) 174 - 0 1 (0. 5%) - MI Rehospitalization for cardiac ischemic event Urgent TVR 0 1 (0. 5%) - 2 (0. 9%) 4 (1. 9%) 0. 992 0. 99 (0. 14 -7. 03) 2 (0. 9%) 1 (0. 5%) - Definite ST 0 0 - Stroke 0 1 (0. 5%) - CV death 0 0 - All cause death 0 1 (0. 5%) - Key secondary efficacy EPs:

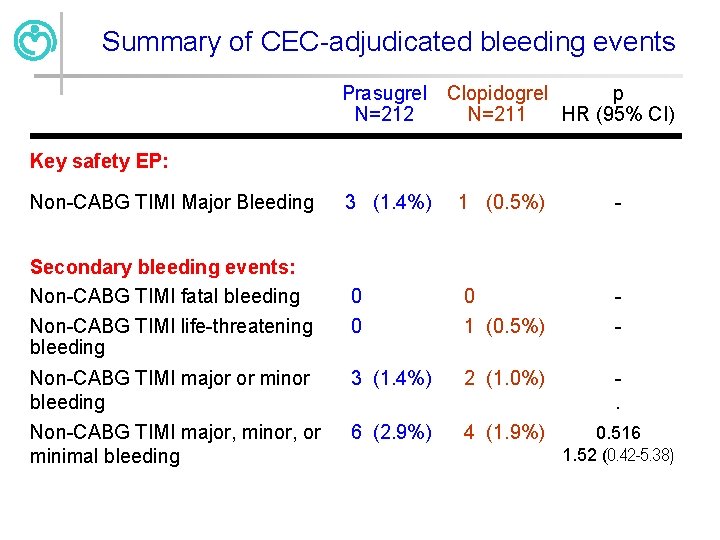
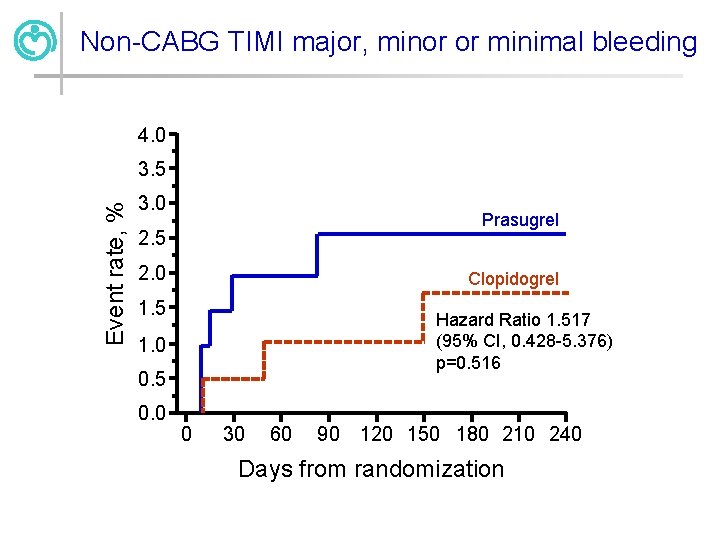
Summary of CEC-adjudicated bleeding events Prasugrel N=212 Clopidogrel p N=211 HR (95% CI) Key safety EP: Non-CABG TIMI Major Bleeding Secondary bleeding events: Non-CABG TIMI fatal bleeding Non-CABG TIMI life-threatening bleeding Non-CABG TIMI major or minor bleeding Non-CABG TIMI major, minor, or minimal bleeding 3 (1. 4%) 1 (0. 5%) - 0 0 0 1 (0. 5%) - 3 (1. 4%) 2 (1. 0%) . 6 (2. 9%) 4 (1. 9%) 0. 516 1. 52 (0. 42 -5. 38)

Non-CABG TIMI major, minor or minimal bleeding 4. 0 Event rate, % 3. 5 3. 0 Prasugrel 2. 5 2. 0 Clopidogrel 1. 5 Hazard Ratio 1. 517 (95% CI, 0. 428 -5. 376) p=0. 516 1. 0 0. 5 0. 0 0 30 60 90 120 150 180 210 240 Days from randomization

Summary and conclusion: Effectiveness of Prasugrel vs Clopidogrel after elective PCI § High on-clopidogrel platelet reactivity (>208 PRU by Verify. Now P 2 Y 12 test) was observed less frequently than expected. § Compared with standard-dose clopidogrel 75 mg QD, prasugrel 10 mg QD substantially decreased platelet reactivity in patients with high on-clopidogrel platelet reactivity after elective PCI. § Given the low event rate in elective PCI patients without peri-procedural complications it was not possible to assess the risk – benefit ratio with prasugrel treatment. Therefore, the study was terminated prematurely for futility.

Study organization Trial Leadership: Franz-Josef Neumann (Chair), Dietmar Trenk, Adnan Kastrati, Meinrad Gawaz, Gregg W. Stone (US PI), Dominick J. Angiolillo, Joseph A. Jakubowski Sponsor: Eli Lilly and Company, Daiichi Sankyo Co. , Ltd. Data Center and Site Management: Quintiles Data Safety and Monitoring Board: Hanjörg Just (chair), Jochen Senges, Kurt Ulm

Principal Investigators F. -J. Neumann (Bad Krozingen, GER) R. Zimmermann (Pforzheim, GER) G. Schuler (Leipzig, GER) E. Dogu (Bremen, GER) M. Gawaz (Tuebingen, GER) M. Bergmann (Hamburg, GER) A. Kastrati (Munich, GER) C. Toma (Pittsburgh, PA, US) G. Richardt (Bad Segeberg, GER) S. V. Manoukian (Nashville, TN, US) J. Yu (Bad Berka, GER) T. J. Gluckman (Portland, OR, US) W. Jung (Villingen-Schwenningen, GER) D. J. Spriggs (Clearwater, FL, US) B. Witzenbichler (Berlin, GER) D. J. Angiolillo (Jacksonville, FL, US) H. Heuer (Dortmund, GER) J. C. Merritt (Rome, GA, US) S. Genth-Zotz (Mainz, GER) M. Haude (Neuss, GER) B. Goldmann (Hamburg, GER) V. Schaechinger (Fulda, GER) M. Moser (Freiburg, GER) H. Schuehlen (Berlin, GER) U. Sechtem (Stuttgart, GER) D. A. Purdy (Rapid City, SD, US) D. Boese (Essen, GER) S. Puri (Moline, IL, US) H. Mudra (Munich, GER) K. Garatt (New York, NY, US)
 13 blood coagulation factors names
13 blood coagulation factors names Clifford consulting and research
Clifford consulting and research Manual wbc count
Manual wbc count Sol gel zone platelet
Sol gel zone platelet Platelet clumper
Platelet clumper Polychromasia
Polychromasia Process of platelet plug formation
Process of platelet plug formation Platelet satellitosis
Platelet satellitosis Snowplow effect in blood smear
Snowplow effect in blood smear Circulatory compromise
Circulatory compromise Peripheral blood smear platelet count
Peripheral blood smear platelet count Cryoprecipetate
Cryoprecipetate Cryoprecipitate
Cryoprecipitate Cbc platelet count low
Cbc platelet count low Rees and ecker method procedure drawing
Rees and ecker method procedure drawing Fresh frozen plasma contents
Fresh frozen plasma contents Lines of zahn is seen in coralline thrombus
Lines of zahn is seen in coralline thrombus Types of cbc
Types of cbc Platelet aggregation test
Platelet aggregation test In seconds
In seconds Platelet aggregation test
Platelet aggregation test Platelet
Platelet Platelet
Platelet Whats a platelet transfusion
Whats a platelet transfusion