Prequalification of in vitro diagnostics Irena Prat Diagnostics

















- Slides: 25

Prequalification of in vitro diagnostics Irena Prat, Diagnostics assessment Group Lead Prequalification Team World Health Organization April 2018

Presentation overview 1 • Landscape, market trends and WHO work in IVDs QA 2 • Introduction to the Prequalification of IVDs 3 • Access to the global market and procurement figures 2

The current IVDs landscape Every year, hundreds of millions of US dollars’ worth of IVDs and medicines are purchased for distribution in resource-limited countries IVDs play a critical role in ensuring blood safety, surveillance, diagnosis and treatment initiation and monitoring IVDs save lives, reduce suffering and improve health, but only when they are of good quality, safe, effective, available, affordable, acceptable and properly used

WHO's work in IVDs area: background SDGs WHA resolutions WHO's impact goals UHC WHO's 6 core functions

IVDs QA activities within WHO has been assessing IVDs performance and operational characteristics since 1988 : • HIV assays since 1988 • Hepatitis B assays since 2000 • Hepatitis C assays since 2000 • Syphilis assays since 2001 • Chagas assays since 2002 • Malaria assays since 2002 • CD 4 technologies ad-hoc in 1996 & 2003

Changing IVDs global market trends Globalised industry sectors with outsourced production Rapid emergence of new technologies Increasing expectations on quality, safety and performance Increasing pressure on human resources and increased workload for regulators Easy to operate tests/methods facilitate near patient testing, hard-to-reach populations, non-lab environments

WHO's response to changing market trends 2008: Shift from test kit evaluations to prequalification of IVDs : • More stringent approach • Alignment with global standards for assuring quality of IVDs Through a rigorous process identify IVDs that meet quality, safety and performance standards

Prequalification of IVDs: aim, scope and impact The aim of PQDx is to promote and facilitate access to safe, appropriate and affordable IVDs of good quality HIV Malaria Focus is placed on IVDs for priority diseases and their suitability for use in resource-limited settings The findings of PQDx are used to provide independent technical information on safety, quality and performance of IVDs, principally to other UN agencies but also to WHO Member States and other interested organizations The PQDx status, in conjunction with other procurement criteria, is used by UN agencies, WHO Member States and other interested organizations to guide their procurement of IVDs Hepatitis C Hepatitis B HPV G 6 PD Cholera

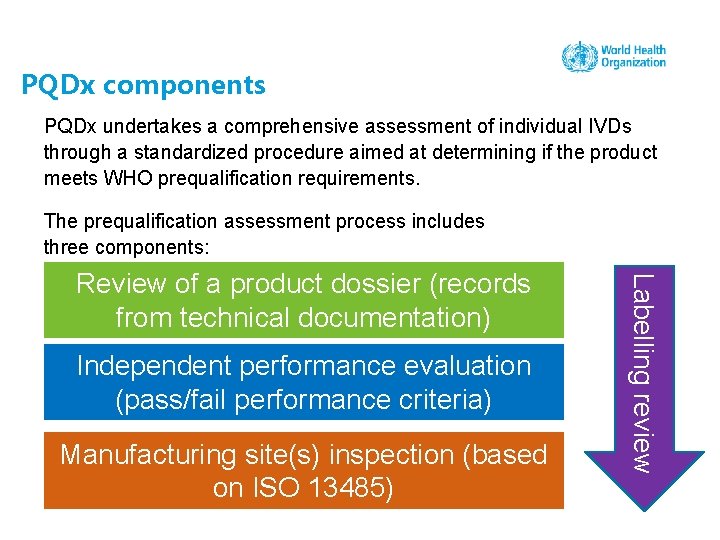
PQDx components PQDx undertakes a comprehensive assessment of individual IVDs through a standardized procedure aimed at determining if the product meets WHO prequalification requirements. The prequalification assessment process includes three components: Independent performance evaluation (pass/fail performance criteria) Manufacturing site(s) inspection (based on ISO 13485) Labelling review Review of a product dossier (records from technical documentation)

Prequalification requirements Based on internationally accepted standards and guidance: WHO International Medical Device Regulators Forum (IMDRF) Global Harmoniza -tion Task Force (GHTF) International Organization for Standardiza tion (ISO), Clinical and Laboratory Standards Institute (CLSI). International Electrotechn -ical Commission (IEC), 10

What does PQ do differently to IMDRF/GHTF PQ is aligned with internationally accepted practice BUT assess products' regulatory versions intended for the global market : Where a stringently reviewed versions exist, they are often not supplied to the global market • Ro. W versions differ from stringently assessed version in Mx site, QC, labelling, key suppliers, composition, intended use etc. Review aspects of particular relevance for resource-limited settings : Risk assessment, stability, flex studies, labelling, training and support network • Take into account environment and user skills • Reflect risks related to the use in LMIC

Leveraging stringent approvals – Abridged PQ assessment Avoid duplication where evidence of compliance with quality, safety and performance requirements exists Recognised stringent assessments: • European Union: Annex II, List A • US Food and Drug Administration: Class III The regulatory version submitted for WHO prequalification has undergone prior stringent regulatory review by a GHTF founding member; • Health Canada: Class IV OR • Therapeutic Goods Administration, Australia : Class 4 • Ministry of Health, Labour and Welfare, Japan: Class III The regulatory version submitted for WHO prequalification is different from the version that underwent regulatory review by a GHTF founding member, but there are no substantial differences 12

Prequalification decision and procurement eligibility § Final prequalification outcome depends on: • Results of dossier assessment • Results of inspection(s) • Meeting the acceptance criteria for the laboratory evaluation § WHO PQDx Public Report is posted on WHO website and product is added to the list of WHO prequalified products § Product is then eligible for WHO and UN and other donors procurement

Post - Prequalification Activities Post - Prequalification Commitments to PQ Change Reporting Annual Reporting Product lifecycle PMS / vigilance

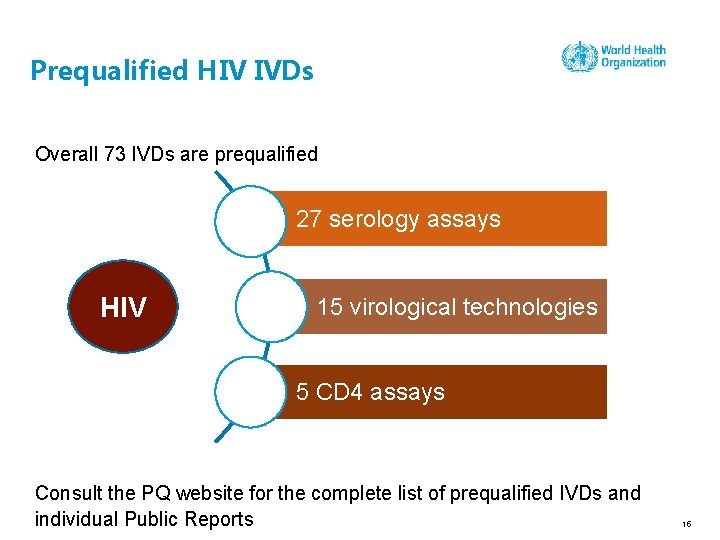
Prequalified HIV IVDs Overall 73 IVDs are prequalified 27 serology assays HIV 15 virological technologies 5 CD 4 assays Consult the PQ website for the complete list of prequalified IVDs and individual Public Reports 15

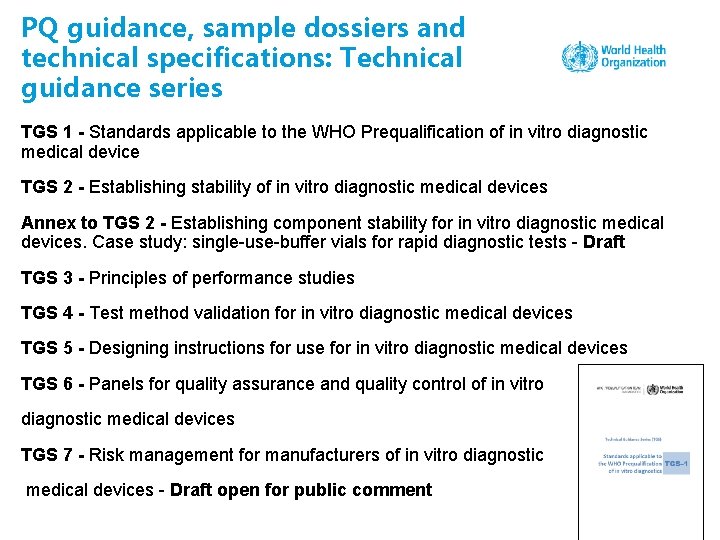
PQ guidance, sample dossiers and technical specifications: Technical guidance series TGS 1 - Standards applicable to the WHO Prequalification of in vitro diagnostic medical device TGS 2 - Establishing stability of in vitro diagnostic medical devices Annex to TGS 2 - Establishing component stability for in vitro diagnostic medical devices. Case study: single-use-buffer vials for rapid diagnostic tests - Draft TGS 3 - Principles of performance studies TGS 4 - Test method validation for in vitro diagnostic medical devices TGS 5 - Designing instructions for use for in vitro diagnostic medical devices TGS 6 - Panels for quality assurance and quality control of in vitro diagnostic medical devices TGS 7 - Risk management for manufacturers of in vitro diagnostic medical devices - Draft open for public comment

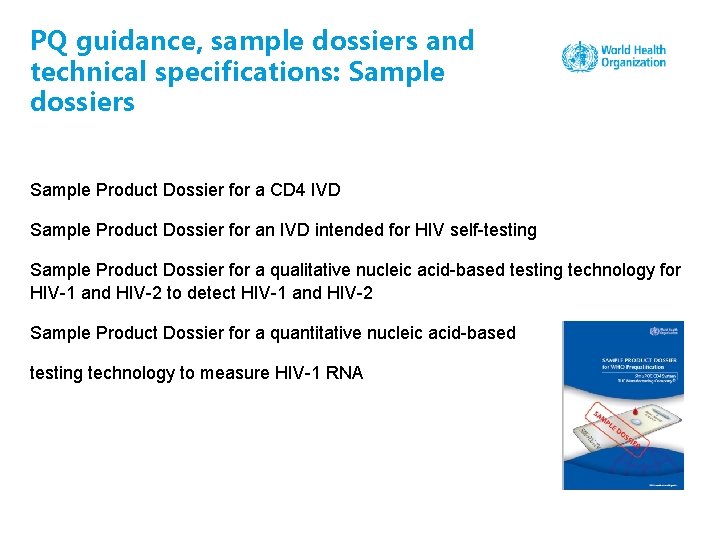
PQ guidance, sample dossiers and technical specifications: Sample dossiers Sample Product Dossier for a CD 4 IVD Sample Product Dossier for an IVD intended for HIV self-testing Sample Product Dossier for a qualitative nucleic acid-based testing technology for HIV-1 and HIV-2 to detect HIV-1 and HIV-2 Sample Product Dossier for a quantitative nucleic acid-based testing technology to measure HIV-1 RNA

PQ guidance, sample dossiers and technical specifications: Technical specifications series TSS 1 - Diagnostics Assessment: HIV rapid diagnostic tests for professional use and/or self-testing TSS 2 - Diagnostic Assessment: IVD to identify Glucose-6 -phosphate dehydrogenase (G 6 PD) activity TSS 3 - Diagnostic Assessment: Malaria rapid diagnostic tests TSS 4 - Diagnostic Assessment: IVDs used for the detection of highrisk Human Papillomavirus (HPV) types in cervical cancer screening TSS 5 - Diagnostic Assessment: Rapid diagnostic tests used for surveillance and detection of an outbreak of cholera

Prequalification fees and timelines PQ application fee: 12, 000 USD per product PQ change assessment fee: 3, 000 per change PQ financial model to embrace IVDs during 2018; public consultation on new fee structure Timelines for assessment: Total target assessment time for full assessment: 270 -350 days (WHO max time) + manufacturer time Total target time for abridged assessment: 100 -180 days (WHO max time) + manufacturer time

WHO Prequalification – access to the global market Prequalification listing linked to • UN agencies procurement (WHO, UNICEF, UNDP, UNFPA) • The Global Fund QA policy • MSF • Strong collaboration with USG (USAID, CDC) • National procurement / tendering specs Range of products from which procurers can select 20

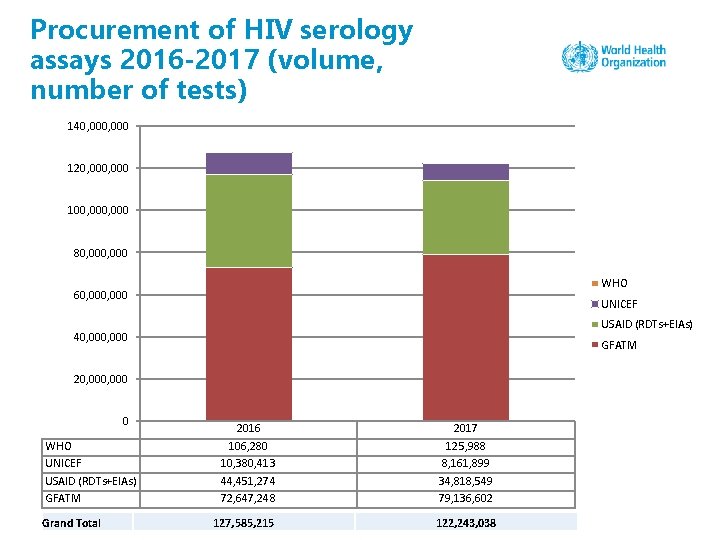
Procurement of HIV serology assays 2016 -2017 (volume, number of tests) 140, 000 120, 000 100, 000 80, 000 WHO 60, 000 UNICEF USAID (RDTs+EIAs) 40, 000 GFATM 20, 000 0 WHO UNICEF USAID (RDTs+EIAs) GFATM Grand Total 2016 106, 280 10, 380, 413 44, 451, 274 72, 647, 248 2017 125, 988 8, 161, 899 34, 818, 549 79, 136, 602 127, 585, 215 122, 243, 038

Total expenditure (US$) HIV serology assays by agency 120, 000 100, 000 80, 000 WHO PSM 60, 000 UNICEF RMI GFATM 40, 000 20, 000 0 2016 Row Labels GFATM RMI UNICEF PSM WHO Grand Total 2016 60, 720, 301 38, 371, 596 9, 573, 002 514, 287 84, 821 109, 264, 006. 95 2017 70, 385, 311 31, 096, 191 9, 085, 227 202, 792 121, 439 110, 890, 960. 19

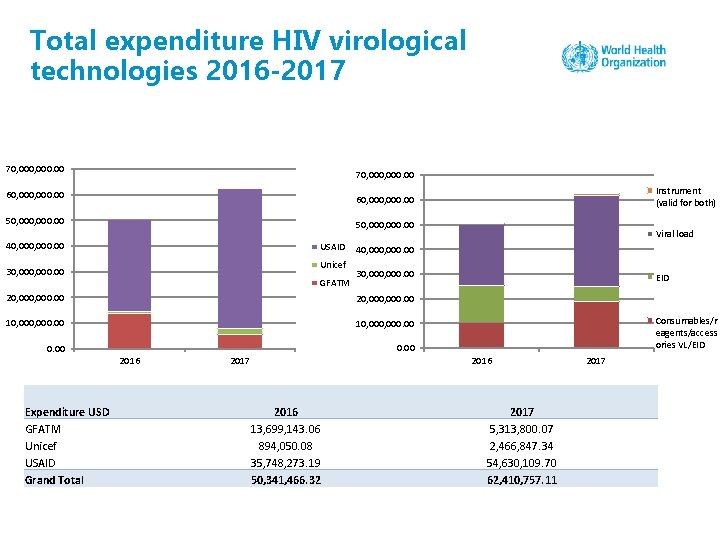
Total expenditure HIV virological technologies 2016 -2017 70, 000, 000. 00 60, 000, 000. 00 50, 000. 00 40, 000. 00 USAID Unicef 30, 000. 00 GFATM 30, 000. 00 20, 000, 000. 00 10, 000. 00 2016 Viral load 40, 000. 00 20, 000. 00 Expenditure USD GFATM Unicef USAID Grand Total Instrument (valid for both) 60, 000. 00 EID Consumables/r eagents/access ories VL/EID 2016 2017 2016 13, 699, 143. 06 894, 050. 08 35, 748, 273. 19 50, 341, 466. 32 2017 5, 313, 800. 07 2, 466, 847. 34 54, 630, 109. 70 62, 410, 757. 11 2017

Essential diagnostics list List under development Ongoing discussions with experts SAGE-IVD meeting to be held in Geneva 16 -20 April 2018

Thank you Any questions? Email to diagnostics@who. int 25
 Felice shieh
Felice shieh Doing business with indot
Doing business with indot Iman siadat
Iman siadat Vitro data center
Vitro data center Pembuahan in vitro
Pembuahan in vitro Absorption intestinale
Absorption intestinale Ukupni ige referentne vrednosti
Ukupni ige referentne vrednosti Procedure for isolation of cells for in vitro culture
Procedure for isolation of cells for in vitro culture Belign
Belign Poesia de arturo prat
Poesia de arturo prat Foveal
Foveal Concepto de correo electrónico
Concepto de correo electrónico Ponikowska irena
Ponikowska irena Monika sobolewska córka ireny jarockiej
Monika sobolewska córka ireny jarockiej Irena sendler premios
Irena sendler premios Irena cajner mraović
Irena cajner mraović Irena černič
Irena černič Irena davidovic
Irena davidovic Irena
Irena Irena horek
Irena horek Irena cajner mraović
Irena cajner mraović Uvod u razvojnu ekonomiju
Uvod u razvojnu ekonomiju Irena stanisława sendler mga nagawa
Irena stanisława sendler mga nagawa Nedelea irena
Nedelea irena Irena 2015
Irena 2015 Reflektiranje primjeri
Reflektiranje primjeri