Isolation of cell lines for in vitro culture










- Slides: 18


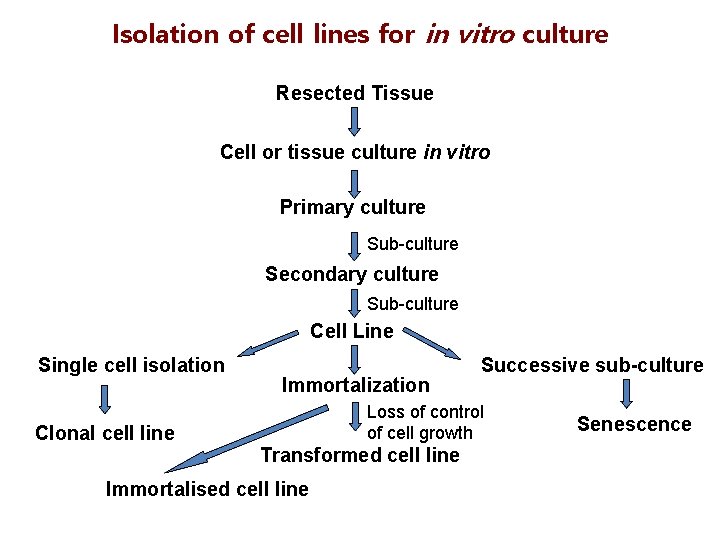
Isolation of cell lines for in vitro culture Resected Tissue Cell or tissue culture in vitro Primary culture Sub-culture Secondary culture Sub-culture Cell Line Single cell isolation Immortalization Successive sub-culture Loss of control of cell growth Clonal cell line Transformed cell line Immortalised cell line Senescence


in vitro에서 배양되는 세포의 종류 Secondary cultures • • • primary cell culture로부터 유도됨 Isolated by selection or cloning 여러 종류의 세포가 존재 제한된 배양능력 in vitro 분화된 표현형을 보유 부착이 잘 안됨- collagen-coated

in vitro에서 배양되는 세포의 종류 Continuous cultures • primary 또는 secondary culture • Immortalized: • Spontaneously (e. g. : spontaneous genetic mutation) • By transformation vectors (e. g. : viruses &/or plasmids) • • • 성장속도가 증가되어 배양액에서 계속적으로 배양 가능 균일한 세포 종류 배양기에 부착 잘됨 in vitro에서 계속적으로 배양가능 분화된 표현형 (Differentiated phenotype): • 암에서 유도된 세포의 성질을 가짐 • 유전적으로 불안정

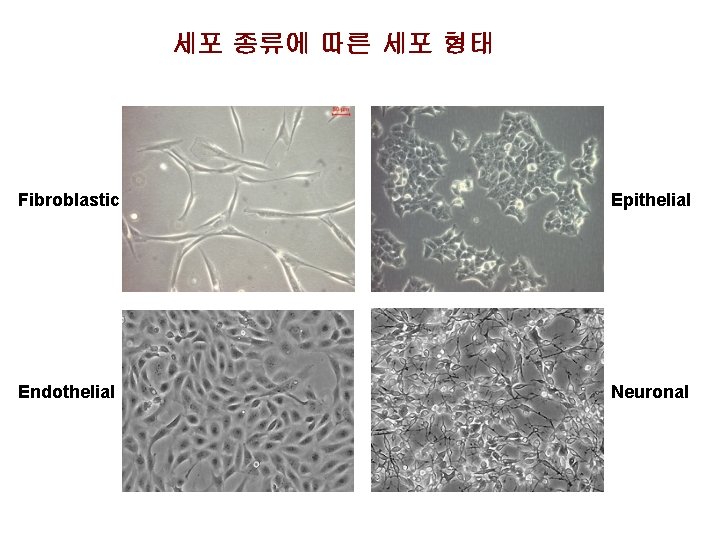
세포 종류에 따른 세포 형태 Fibroblastic Epithelial Endothelial Neuronal

세포 배양 환경 (in vitro) 세포성장에 필요한 성분 • Substrate 또는 liquid (cell culture flask or scaffold material) chemically modified plastic or coated with ECM proteins suspension culture • 영양분 (Nutrients): culture media • 환경 (5% CO 2, temperature 37 o. C, humidity) Oxygen tension maintained at atmospheric but can be varied • 위생적 환경 (aseptic technique, 항생제 and 항진균제) Mycoplasma tested

세포 배양 환경 (in vitro) Basal Media (배지) • Maintain p. H and osmolarity (260 -320 m. Osm/L). • Provide nutrients and energy source. Basal Media의 구성성분 무기염 • 삼투압 유지 (osmolarity) • 세포막전위 유지 (Na+, K+, Ca 2+) • 세포부착과 조효소 공급 p. H Indicator – Phenol Red • 세포최적성장 p. H 7. 4 Buffers (Bicarbonate and HEPES) • Bicarbonate buffered media requires CO 2 atmosphere • HEPES Strong chemical buffer range p. H 7. 2 – 7. 6 (does not require CO 2) Glucose • 에너지 공급

세포 배양 환경 (in vitro) Components of Basal Media Keto acids (oxalacetate and pyruvate) • Intermediate in Glycolysis/Krebs cycle • Keto acids added to the media as additional energy source • Maintain maximum cell metabolism Carbohydrates • Energy source • Glucose and galactose • Low (1 g/L) and high (4. 5 g/L) concentrations of sugars in basal media Vitamins • Precursors for numerous co-factors • B group vitamins necessary for cell growth and proliferation • Common vitamins found in basal media is riboflavin, thiamine and biotin Trace Elements • Zinc, copper, selenium and tricarboxylic acid intermediates

세포 배양 환경 (in vitro) Supplements L-glutamine • Essential amino acid (not synthesised by the cell) • Energy source (citric acid cycle), used in protein synthesis • Unstable in liquid media - added as a supplement Non-essential amino acids (NEAA) • Usually added to basic media compositions • Energy source, used in protein synthesis • May reduce metabolic burden on cells Growth Factors and Hormones (e. g. : insulin) • Stimulate glucose transport and utilisation • Uptake of amino acids • Maintenance of differentiation Antibiotics and Antimycotics • Penicillin, streptomycin, gentamicin, amphotericin B • Reduce the risk of bacterial and fungal contamination • Cells can become antibiotic resistant – changing phenotype • Preferably avoided in long term culture

세포 배양 환경 (in vitro) Fetal Calf/Bovine Serum (FCS & FBS) • • Growth factors and hormones Aids cell attachment Binds and neutralise toxins Long history of use • • Infectious agents (prions) Variable composition Expensive Regulatory issues (to minimise risk) Heat Inactivation (56 o. C for 30 mins) – why? • Destruction of complement and immunoglobulins • Destruction of some viruses (also gamma irradiated serum) Care! Overdoing it can damage growth factors, hormones & vitamins and affect cell growth

실험실에서 세포 배양하는 법 Revive frozen cell population Isolate from tissue Containment level 2 cell culture laboratory Maintain in culture (aseptic technique) Typical cell culture flask Sub-culture (passaging) Count cells Cryopreservation ‘Mr Frosty’ Used to freeze cells

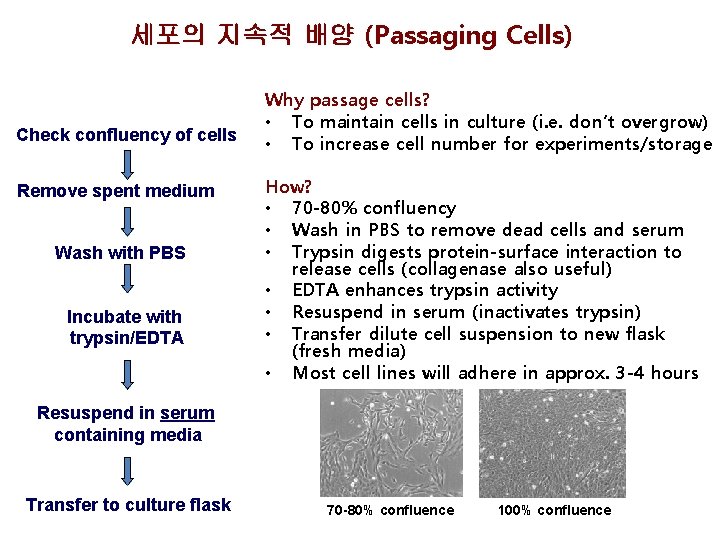
세포의 지속적 배양 (Passaging Cells) Check confluency of cells Remove spent medium Wash with PBS Incubate with trypsin/EDTA Why passage cells? • To maintain cells in culture (i. e. don’t overgrow) • To increase cell number for experiments/storage How? • 70 -80% confluency • Wash in PBS to remove dead cells and serum • Trypsin digests protein-surface interaction to release cells (collagenase also useful) • EDTA enhances trypsin activity • Resuspend in serum (inactivates trypsin) • Transfer dilute cell suspension to new flask (fresh media) • Most cell lines will adhere in approx. 3 -4 hours Resuspend in serum containing media Transfer to culture flask 70 -80% confluence 100% confluence

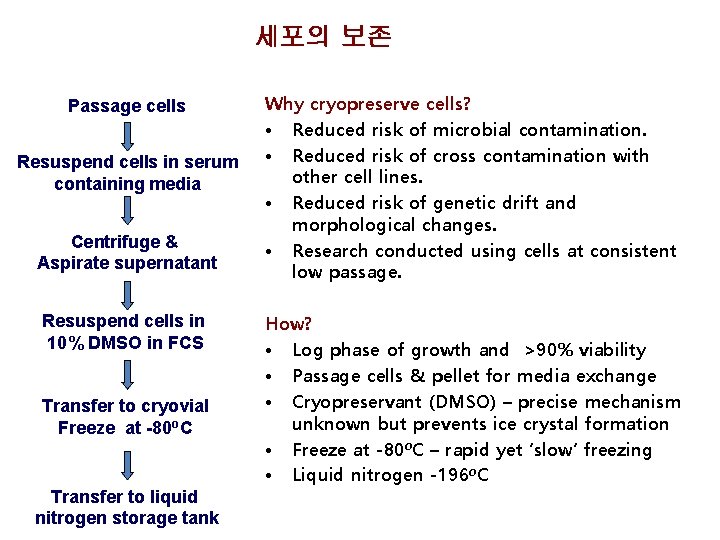
세포의 보존 Passage cells Resuspend cells in serum containing media Centrifuge & Aspirate supernatant Resuspend cells in 10% DMSO in FCS Transfer to cryovial Freeze at -80 o. C Transfer to liquid nitrogen storage tank Why cryopreserve cells? • Reduced risk of microbial contamination. • Reduced risk of cross contamination with other cell lines. • Reduced risk of genetic drift and morphological changes. • Research conducted using cells at consistent low passage. How? • Log phase of growth and >90% viability • Passage cells & pellet for media exchange • Cryopreservant (DMSO) – precise mechanism unknown but prevents ice crystal formation • Freeze at -80 o. C – rapid yet ‘slow’ freezing • Liquid nitrogen -196 o. C

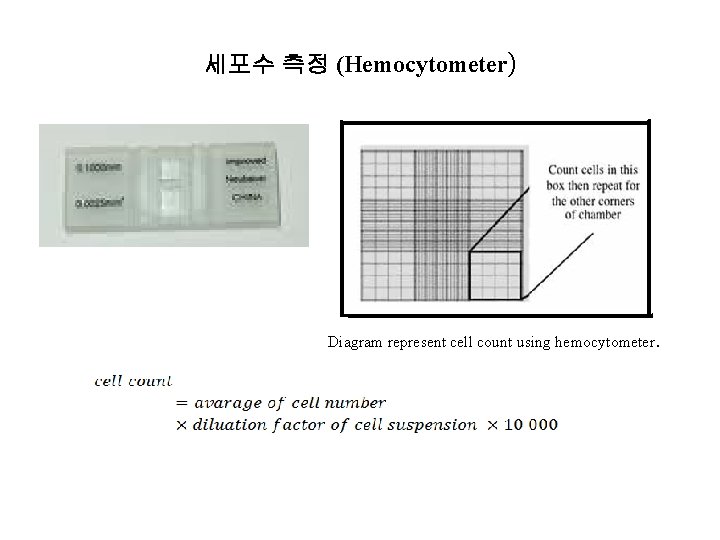
세포수 측정 (Hemocytometer) Diagram represent cell count using hemocytometer.

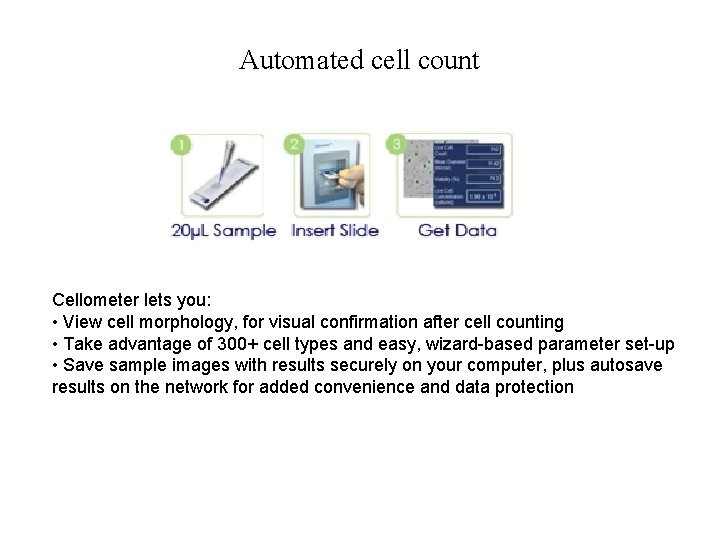
Automated cell count Cellometer lets you: • View cell morphology, for visual confirmation after cell counting • Take advantage of 300+ cell types and easy, wizard-based parameter set-up • Save sample images with results securely on your computer, plus autosave results on the network for added convenience and data protection

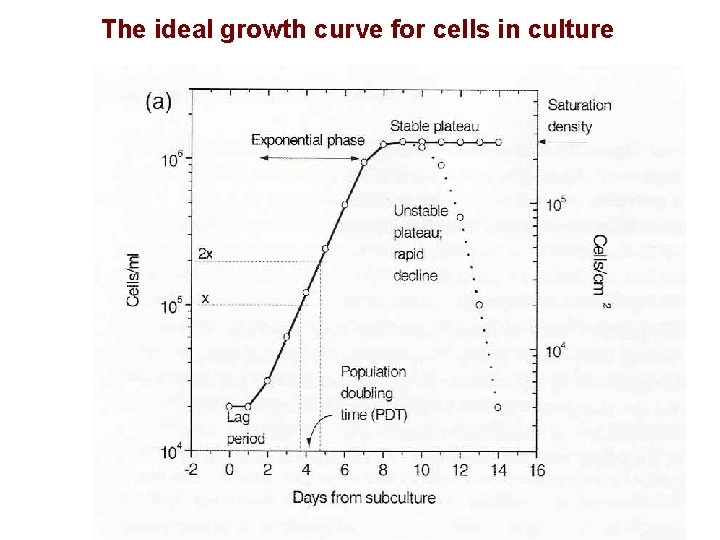
The ideal growth curve for cells in culture

오염 (contamination) A cell culture contaminant can be defined as some element in the culture system that is undesirable because of its possible adverse effects on either the system or its use. 1 -Chemical Contamination Media Incubator Serum water 2 -Biological Contamination Bacteria and yeast Viruses Mycoplasmas Cross-contamination by other cell culture How Can Cell Culture Contamination Be Controlled?
 Procedure for isolation of cells for in vitro culture
Procedure for isolation of cells for in vitro culture Apparail digestif
Apparail digestif University of pittsburgh
University of pittsburgh Pembuahan in vitro
Pembuahan in vitro In vivo in vitro značenje
In vivo in vitro značenje Sap netweaver portal vitro
Sap netweaver portal vitro In vitro diagnostics
In vitro diagnostics Stroke culture
Stroke culture Isolation of pure cultures
Isolation of pure cultures Examples of popular culture
Examples of popular culture Sociologists define a symbol as
Sociologists define a symbol as Batch culture vs continuous culture
Batch culture vs continuous culture Continuous culture and batch culture
Continuous culture and batch culture Collectivistic cultures
Collectivistic cultures Difference between american and indian culture
Difference between american and indian culture Stab culture and stroke culture
Stab culture and stroke culture Folk culture and popular culture venn diagram
Folk culture and popular culture venn diagram Sub culture vs counter culture
Sub culture vs counter culture Folk culture and popular culture venn diagram
Folk culture and popular culture venn diagram