WHO Prequalification and API Requirements Maryam MEHMANDOUST Prequalification


































- Slides: 46

WHO Prequalification and API Requirements Maryam MEHMANDOUST Prequalification of Medicines Programme QSM / EMP / HSS Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

Glossary l API Active Pharmaceutical Ingredient (interchangeable with drug substance or active substance) l APIMF Active Pharmaceutical Ingredient Master File l ARV Antiretroviral l Co. S (CEP) Certificate of Suitability l EDQM European Directorate for Quality of Medicines and Health. Care l Eo. I Expression of Interest l EMEA European Medicines Agency l FPP Finished Pharmaceutical Product l GMP Good Manufacturing Practices l ICH International Conference on Harmonization l Int. Ph. International Pharmacopoeia l JP Japanese Pharmacopoeia l Ph. European Pharmacopoeia l PQ Prequalification l PQIF Pharmaceutical Quality Information form l RH Reproductive Health l TB Tuberculosis l USFDA US Food and Drug Administration l USP United States Pharmacopoeia 2 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

UN / WHO Prequalification Medicines Programme l Action plan of UN since 2001 aiming to facilitate access to priority medicinal products l Revision of the procedure in October 2008 – Categories: HIV/AIDS, Malaria, Tuberculosis, Reproductive Health, Influenza – Potentially other categories of products possible, if there is the need – To ensure quality, efficacy and safety of medicines procured using international funds (e. g. GFTAM, UNITAID) – Products meeting WHO recommended Quality Standards to be included in the list of Prequalified products – Inclusion in the list does imply any approval by WHO of the products and manufacturing sites- this is the sole prerogative of National Authorities 3 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

Prequalification Medicines Programme Principles of Quality assessment procedure Ø General understanding of the production and quality control activities of the manufacturer Ø Assessment of product data and information on safety, efficacy and quality Ø Assessment of manufacturing sites for consistency in production, quality control of starting materials and FPPS through compliance with GMP Ø Assessment of clinical testing units or CROs for compliance with GCP and GLP Ø Reliance on the information supplied by national DRAs Ø Random sampling and testing Ø Handling of complaints and recalls Ø Monitoring of complaints from agencies and countries 4 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

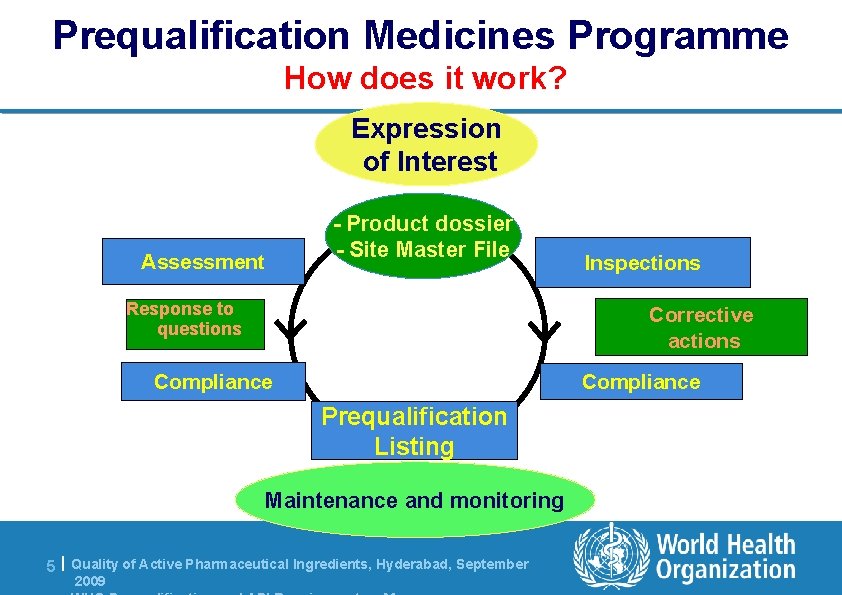
Prequalification Medicines Programme How does it work? Expression of Interest - Product dossier - Site Master File Assessment Response to questions Corrective actions Compliance Prequalification Listing Maintenance and monitoring 5 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009 Inspections

Prequalification Medicines Programme TB drugs in the latest (9 th) Expression of Interest l Rifampicin (rifampin) Ph. Eur. , USP, BP, Ph. Int. l Ethambutol 2 HCl Ph. Eur. , USP, BP, Ph. Int. , JP l Pyrazinamide Ph. Eur. , USP, BP, Ph. Int. , JP l Isoniazid Ph. Eur. , USP, BP, Ph. Int. , JP l Streptomycin sulfate Ph. Eur. , USP, BP, Ph. Int. l Amikacin Ph. Eur. , USP, BP, Ph. Int. , JP l Kanamycin Ph. Eur. , USP, BP l Capreomycin USP, Ph. Int. l Cycloserine USP, JP l Ethionamide Ph. Eur. , USP, BP, Ph. Int. , JP l Ofloxacin Ph. Eur. , USP l Levofloxacin Draft USP l Moxifloxacin Ph. Eur. , USP, BP l Prothionamide Ph. Int. , JP l p-Aminosalicylic acid (and sodium salt) Ph. Eur. , USP 6 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

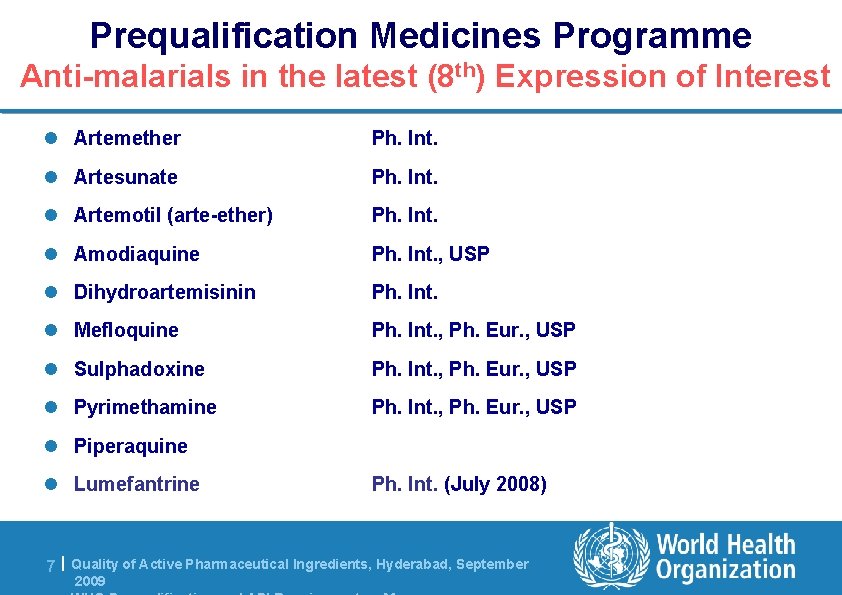
Prequalification Medicines Programme Anti-malarials in the latest (8 th) Expression of Interest l Artemether Ph. Int. l Artesunate Ph. Int. l Artemotil (arte-ether) Ph. Int. l Amodiaquine Ph. Int. , USP l Dihydroartemisinin Ph. Int. l Mefloquine Ph. Int. , Ph. Eur. , USP l Sulphadoxine Ph. Int. , Ph. Eur. , USP l Pyrimethamine Ph. Int. , Ph. Eur. , USP l Piperaquine l Lumefantrine Ph. Int. (July 2008) 7 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

Prequalification Medicines Programme ARVs in the latest (9 th) Expression of Interest l Abacavir Ph. Int. l Didanosine Ph. Int. , Ph. Eur. , USP l Efavirenz Ph. Int. l Emtricitabine Ph. Int. (February 2009) l Indinavir Ph. Int. , USP, Ph. Eur. l lamivudine Ph. Eur. , USP, BP, Ph. Int. l Nelfinavir mesilate Ph. Int. l Nevirapine Ph. Int. , USP, Ph. Eur. l Stavudine Ph. Eur. , USP, BP, Ph. Int. l Saquinavir mesilate Ph. Int. , USP l Ritonavir Ph. Int. , USP, Ph. Eur. l Zidovudine Ph. Int. , USP, Ph. Eur. , BP l Tenofovir, Lopinavir, Atazanavir Non-pharmacopoeial 8 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

Reproductive Health in the latest (4 th) EOI Antivirals 2 nd EOI Reproductive Health l Ethinylestradiol Ph. Int. , Ph. Eur. , USP, BP l Estradiol derivatives USP (cypronate), Ph. Eur. , USP (valerate) l Etonogestrel Non pharmacopoeial l Desogestrel Ph. Eur. , USP l Levonorgestrel Ph. Int. , Ph. Eur. , USP, BP l Medroxyprogesterone acetate Ph. Int. , Ph. Eur. , USP, BP l Lynestrenol Ph. Eur. l Norethisterone Ph. Int. , Ph. Eur. l Norgestrel USP, Ph. Eur. l Oxytocin Ph. Int. , USP, draft Ph. Eur. l Mifepristone l Misoprostol Ph. Eur. Influenza-specific antiviral drugs l Oseltamivir Ph. Int. (December 2008), draft Ph. Eur. , draft USP l Zanamivir Non pharmacopoeial 9 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

Prequalification Medicines Programme Guidelines for the assessment of product dossiers/quality API l Guideline on Submission of Documentation for Prequalification of Multisource (Generic) Finished Pharmaceutical Products (FPPs) Used in the Treatment of HIV/AIDS, Malaria and Tuberculosis [Main Generic Guide, under revision], Section 2 on APIs l Supplement 2 - Extension of the WHO list of stable (not easily degradable APIs) l Guideline on Active Pharmaceutical Ingredient Master File (APIMF) Procedure (DMF type procedure) 10 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

Prequalification Medicines Programme Guidelines for the assessment of product dossiers/quality ICH guidelines are used when a quality aspect cannot be (fully) assessed by the WHO guidelines, for instance: – Q 3 A(R 2) Impurities in new drug substances – Q 3 C(R 3) Impurities: Guideline for residual solvents – Q 6 A Specifications: Test procedures and acceptance criteria for new drug substances and new drug products: chemical substances (with decision trees) – Q 7 GMP for active pharmaceutical ingredients 11 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

How to submit data on APIs in PQ product dossier- Section 2 Scientific data on the API can be submitted using following ways and order of preference l A valid Certificate of Suitability (Co. S) or CEP, issued by EDQM l An APIMF (Active Pharmaceutical Ingredient Master File) submitted by the API manufacturer, containing the whole information requested in section 2 and presented in CTD format (see APIMF guideline and separate presentation) l Complete submission of data requested in Section 2 of PQ product dossier 12 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

CEP Option What to provide in the PQ dossier? l A copy of the latest version of CEP with all appendices + access box duly filled out l Information which may not be covered by the CEP – Physicochemical characteristics and any relevant testing – Container closure system (if not mentioned on the CEP) – Stability data (if no re-test period on the CEP) l Results of batch analysis (for attributes listed in the monograph + any additional tests listed on the CEP + sterility if applicable) l In case of sterile substances (e. g. Medroxyprogesterone acetate) When there is no subsequent sterilization for the FPP – Sterilization process as specified on the CEP – Validation data 13 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

APIMF Option / Procedural aspects l Procedure implemented in PQ since January 2007, www. who. int/Prequal/ l Adopted by the WHO Expert Committee on Specifications for Pharmaceutical Preparations in October 2007 and recently published in WHO Technical Report Series (TRS) 948 as Annex 4. l Inspired from the Active Substance Master File (ASMF)/DMF procedure in use in EU, CPMP/QWP/227/02 Rev 2. l Scope only open to APIs # US and Canada DMFs (Drug Master File) – Applicable to all types of chemical APIs: pharmacopoeial and nonpharmacopoeial – Biological APIs out of scope of the APIMF procedure (like in EU) 14 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

APIMF Option / Procedural aspects l Mainly used when the API manufacturer is different from the FPP manufacturer but possible when API and FPP manufacturers are the same l Allows to protect valuable confidential "know-how" of the API manufacturer l While giving the whole information on manufacture of the API to the WHO PQ team of assessors l While giving a part of the information to the applicant to Prequalification/ FPP manufacturer l The APIMF procedure is not mandatory l For Prequalification, – Permits to avoid multiplication of assessments of one API from one source /centralised handling – Lightens the workload of manufacturers (reduces number of deficiencies sent to manufacturers) 15 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

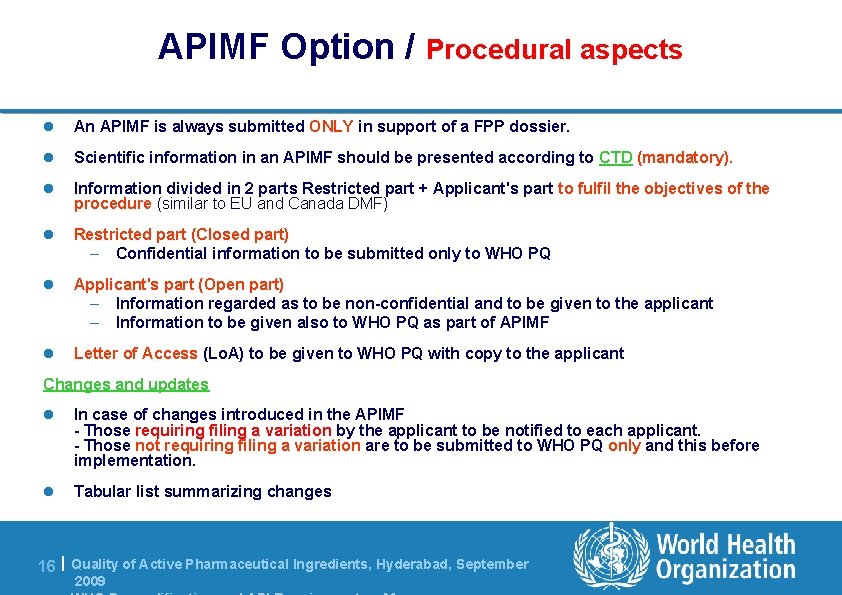
APIMF Option / Procedural aspects l An APIMF is always submitted ONLY in support of a FPP dossier. l Scientific information in an APIMF should be presented according to CTD (mandatory). l Information divided in 2 parts Restricted part + Applicant's part to fulfil the objectives of the procedure (similar to EU and Canada DMF) l Restricted part (Closed part) – Confidential information to be submitted only to WHO PQ l Applicant's part (Open part) – Information regarded as to be non-confidential and to be given to the applicant – Information to be given also to WHO PQ as part of APIMF l Letter of Access (Lo. A) to be given to WHO PQ with copy to the applicant Changes and updates l In case of changes introduced in the APIMF - Those requiring filing a variation by the applicant to be notified to each applicant. - Those not requiring filing a variation are to be submitted to WHO PQ only and this before implementation. l Tabular list summarizing changes 16 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

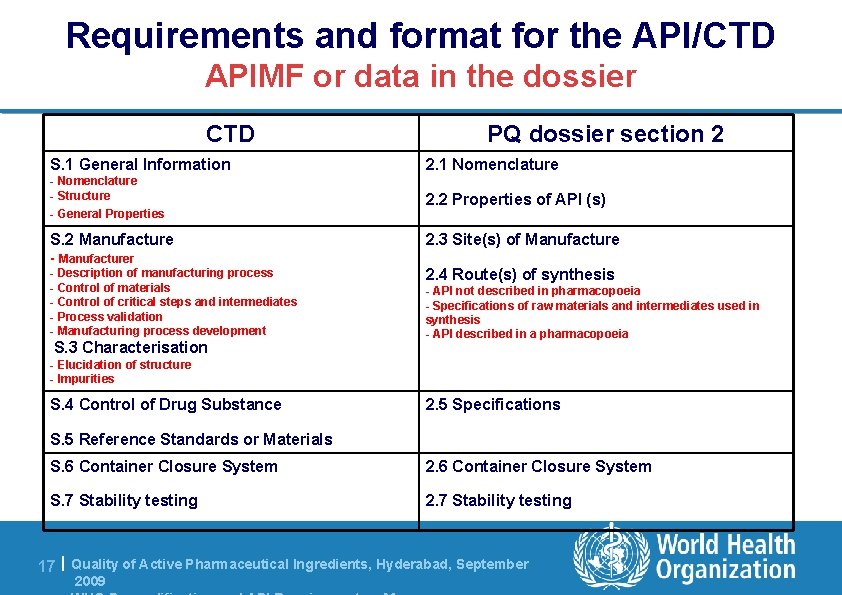
Requirements and format for the API/CTD APIMF or data in the dossier CTD PQ dossier section 2 S. 1 General Information 2. 1 Nomenclature - Structure 2. 2 Properties of API (s) - General Properties S. 2 Manufacture - Manufacturer - Description of manufacturing process - Control of materials - Control of critical steps and intermediates - Process validation - Manufacturing process development S. 3 Characterisation 2. 3 Site(s) of Manufacture 2. 4 Route(s) of synthesis - API not described in pharmacopoeia - Specifications of raw materials and intermediates used in synthesis - API described in a pharmacopoeia - Elucidation of structure - Impurities S. 4 Control of Drug Substance 2. 5 Specifications S. 5 Reference Standards or Materials S. 6 Container Closure System 2. 6 Container Closure System S. 7 Stability testing 2. 7 Stability testing 17 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

Requirements and format API Section/ CTD l S. 1. General information l S. 2. Manufacture – Manufacturer(s), description of the manufacturing process, control of materials, control of critical steps and intermediates, process validation, manufacturing process development) l S. 3. Characterisation l S. 4. Control of API l S. 5. Reference standards or Materials l S. 6. Container closure system l S. 7. Stability testing 18 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

3. 2. S. 2. Manufacturer(s) - Name, address and responsibility of each manufacturer, including contractors, and each proposed manufacturing site or facility involved in the manufacturing chain including specific steps such as milling or micronisation - Actual manufacturing sites with indication of unit, plot, block (if any) - Same information as above for alternative sites GMP compliance certificate for each site of production of API (if available), A valid manufacturing authorization for the production of APIs Manufacturing process should be performed according to the GMP for APIs ICH Q 7: Good manufacturing practice for active pharmaceutical ingredients 19 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

3. 2. S. 2. Manufacture Description of manufacturing process and process controls l A flow diagram of the process and a scheme of synthesis l Detailed description of the synthesis step-by-step indicating materials, reagents and solvents used and critical steps identified by the manufacturer l Description of the synthesis should go sufficiently back to well-characterized starting materials (see under control of materials) l Scale of manufacture: typical batch size and the maximum batch size (the range) l Last solvent of purification and crystallisation always to be controlled l Attention to concordance between the flow sheet of the synthesis and description of the process l Alternate processes (if any) can be accepted ONLY if there is no change in the final specifications of the API and in its impurity profile l In such case, description of the alternate process should be with the same level of details than the main process l If alternate process may lead to different specifications / impurity profile, then separate APIMF should be filed 20 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

3. 2. S. 2. Manufacture Description of manufacturing process and process controls l Mention clearly if reprocessing and reworking steps are performed or not. If performed, they should be clearly described with justification. l Attention to different impurity profile which may result from reworking. l Recovery of materials, if any, should be described in details with the step they are introduced in the process. l Recovery operations should be adequately controlled so impurity levels do not increase over time. l For recovery of solvents, it is important to know if there is any processing to improve the quality of the recovered solvent and if recovered solvents comes from manufacturing process of the specific API or can come from other processes. l Regarding recycling of filtrates ( mother liquors), information should be available on maximum holding times of mother liquors and maximum number of times the material can be recycled. Data on impurity levels should be provided to justify recycling of filtrates. l If there is no recovery of materials, this should be clarified in the dossier. l Blending of batches of API to obtain batches of lager size is acceptable only if batches are individually tested against specifications and found to meet specifications of the final API. 21 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

3. 2. S. 2. Manufacture Control of Materials Starting material of the API Most of manufacturers justify the choice of their starting material as per ICH Q 7 definition, i. e. : a raw material, intermediate, or an API that is used in production of an API and that is incorporated as a significant structural fragment into the structure of the API. It can be an article of commerce, purchased from another supplier or manufactured in-house. This definition is not sufficient from an assessment point of view - Significant structural fragment incorporated into the structure of the API can be a very complex molecule - Commercial availability is not sufficient justification in itself 22 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

3. 2. S. 2. Manufacture Control of Materials Starting material of the API Starting material for synthesis in the dossier may precede the ICH Q 7 "API starting material" by several steps in the synthetic process (Pharmaceutical sciences- Questions and answers, Therapeutic Products Directorate, Health Canada, May 2007) l If the structure of the starting material is too complex and synthesis too short, the proposed starting material should be rather considered as the "key intermediate". The real starting material as a synthetic precursor- one or more synthetic steps prior to the final API intermediate should be asked for. l Exception to the above, when the API starting material is covered by a CEP, l Acids, bases, salts, esters and similar derivatives of the API are not considered final intermediates, l The starting material should be incorporated as a significant structural fragment into the structure of the final API, l The starting material should be well characterized, its structure fully elucidated, l The starting material should have well defined specifications including one or more specific identity tests, tests and limits for the assay, specified and unspecified impurities and total impurities. 23 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

3. 2. S. 2. Manufacture Control of Materials Starting material of the API l In order to assess the presence of all potential impurities, a brief outline of the preparation of the starting material of the API beginning from simpler molecules should be presented including solvents and reagents in order to enable assessors to judge of the appropriateness of specifications of the starting material of the API. l If the APIMF holder is not in possession of the above information which may be considered confidential, then the starting material manufacturer/supplier can directly submit it to WHO PQ by referencing the APIMF number. l Indicate the name and address (preferably manufacturing site) of the starting material manufacturer. l If there are several starting material manufacturers, then clarify if the material obtained from different sources is prepared by the same route or different routes and if the specifications for the starting material can apply to the material sourced from all of them. 24 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

3. 2. S. 2. Manufacture Control of Materials Specifications of materials l Specifications for solvents and reagents - Solvents used in final stages require greater purity and control - Control of residual benzene in solvents such as toluene, etc l Recovered solvents: specifications l Quantitative and qualitative composition of denatured solvents l Recovered materials: specifications l Any material used in the process which may be of biological origin, viral and/or TSE safety aspects should be addressed. Declaration on use/non use of material of biological origin. 25 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

3. 2. S. 2. Manufacture Controls of critical steps and Intermediates l Specifications for isolated intermediates l In-process controls l Identification of critical steps (examples) • Steps where significant impurities are removed or introduced, • Steps where an essential molecular structural element such as chiral centres are introduced • Steps where a major chemical transformation is performed • Steps having an impact on solid-state properties and homogeneity of the API (relevant for solid dosage forms) 26 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

3. 2. S. 3. Characterization Elucidation of structure and other characteristics l Confirmation of structure based on synthetic route and spectral analyses l Pharmacopoeial APIs: comparison of spectral data between the pharmacopoeial reference standard and the test product l Non pharmacopoeial API: evidence of structure should be brought by elemental analysis, IR, NMR (proton and carbon), UV, mass with interpretation of spectra, Xray and so on. - Unequivocal proof of configuration of chiral centres (if applicable) and geometric isomerism (if applicable) e. g. by single X-ray crystal 27 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

3. 2. S. 3. Characterization Elucidation of structure and other characteristics l Particle size distribution for poorly soluble API, routine testing to be justified according to ICH Q 6 A l Polymorphism – Discussion on polymorphic forms (if applicable) – Availability of analytical methods to distinguish between the forms – Demonstration of consistency of production – Routine control necessary or not, to be justified under 3. 2. S. 4. according to ICH Q 6 A 28 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

3. 2. S. 3. Characterization Impurities l Discussion on potential and actual impurities – Organic impurities • Non pharmacopoeial APIs (name and origin of impurity, availability of actual samples for test procedures and structural analysis, suitability of the proposed analytical methods, batch results, justified limits given ICH Q 3 A identification and qualification thresholds) • Pharmacopoeial APIs (whether the process actually leads to impurities described in individual monograph, whether new impurities may be present in comparison to those described in the monograph) – Principle: different routes of synthesis may lead to different impurity profile – If new impurities present, same requirements as above for non pharmacopoeial APIs – Residual solvents • Q 3 C (R 3) and EU note for guidance CPMP/QWP/450/03 • Limit for TEA NMT 320 ppm (option 1) or 3. 2 mg/day intake as per EDQM document PA/PH/ CEP (04) 1 4 R 29 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

3. 2. S. 3. Characterization Impurities l Discussion on potential and actual impurities (Cont. ) – Metal catalysts • Residues of metal catalysts to be monitored and acceptance criteria set according to relevant guidelines e. g. EU note CPMP/SWP/QWP/4446/00 – Genotoxic impurities • Absence of known, established highly toxic impurities used in the process or formed as by-product is to be discussed. • Otherwise, suitable limits for their control are to be proposed. • Limits to be justified by appropriate reference to specific available guidances (EU CPMP/SWP/5199/02 or USFDA draft December 2008) or to experimental safety data or to published data in peer-reviewed journals. • L. Müller et al. article (Elsevier, 2006) can be used to have examples of known alerting structures. 30 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

3. 2. S. 4. Control of the API Specifications Recognised Pharmacopoeias by WHO PQ are Ph. Int. , Ph. Eur. , (BP) and USP APIs described in a pharmacopoeia Requirements of the monograph claimed apply + those of general monographs and chapters of that pharmacopoeia (if applicable) Øe. g. for Ph. Eur. : requirements of individual monograph + general monograph (2034)+ general chapter residual solvents (5. 4. ) + general monograph (1483) on products with TSE risk, . . all together apply Pharmacopoeial monograph available but the applicant/manufacturer claims in-house standards, these can be acceptable only if they are demonstrated as to be at least equivalent or more stringent than the pharmacopoeial monograph. Non pharmacopoeial APIs Justification according to ICH Q 6 A 31 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

3. 2. S. 4. Control of the API Analytical procedures l When a pharmacopoeial related substances method is used, demonstration should be done that the method is actually suitable for determination of impurities of the manufacturer's specific route of synthesis. l Use of a pharmacopoeial method for related substances is not mandatory, an in-house method can be accepted. l In case an in-house method is used, the presence or absence of impurities listed in the transparency list of the pharmacopoeial monograph should be checked and their relevance for the manufacturer's specific route of synthesis has to be discussed. Batch analyses l Results of at least 3 consecutive recent primary batches from each site Primary batches should be at least of pilot size, 10% of maximum commercial batch size at time of acceptance of the APIMF 32 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

3. 2. S. 4. Control of the API Justification of specifications l inclusion OR omission of certain main/ critical tests and acceptance criteria, l any difference with pharmacopoeial limits if wider, l any modification of pharmacopoeial tests, l Specifications of non pharmacopoeial APIs to be justified as per ICH Q 6 A 33 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

3. 2. S. 5. Reference Standards or Materials § For pharmacopoeial APIs: use an official Reference Standard. § Working standard to be qualified against the official RS § For non pharmacopoeial APIs § A primary and/or a working standard are to be established § Description of how this RS/WS has been set in terms of Identity (full structural analyses) and Assay The purity must be determined using an absolute procedure as follows: 100% minus organic impurities (determined by an absolute assay procedure) minus inorganic impurities, minus water content and minus residual solvents. Certificate of Analysis + instructions for storage and duration of use 34 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

3. 2. S. 7. Stability testing WHO guideline on stability testing of APIs and FPPs, TRS 953, 2009, annex 2 § Forced degradation studies in stress condition – Help to know about the intrinsic stability of the API – Help to know about the degradation pathways and degradation products formed – Help to know whether the analytical method is suitable to determine degradation products/ and whether it is stability-indicating It is possible not to perform stress testing if the information can be found in scientific literature 35 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

3. 2. S. 7. Stability testing Forced degradation studies in stress condition l Requirement: 1 API batch l Stress Conditions - temperature, in 10° increments above accelerated (i. e. 50°C, 60°C …) - humidity (75% or greater) - oxidation and photolysis, where appropriate - susceptibility of the API to hydrolysis across a justified range of p. H values when in solution or suspension - photostability testing: generally as per Q 1 B, Omission of photostability testing can be justified if pharmacopoeial monograph for the API states, "Protect from light" 36 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

3. 2. S. 7. Stability testing Regulatory stability testing ü serves to define a re-test period for the API ü to recommend a storage condition Definition of re-test period Period of time during which the API is expected to remain within its specifications and can be used in the manufacture of a given product (without control prior to manufacture of Drug Product) in condition that the API has been stored under defined conditions If a re-test period cannot be defined, The API is to be tested before manufacture of each lot of drug product 37 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

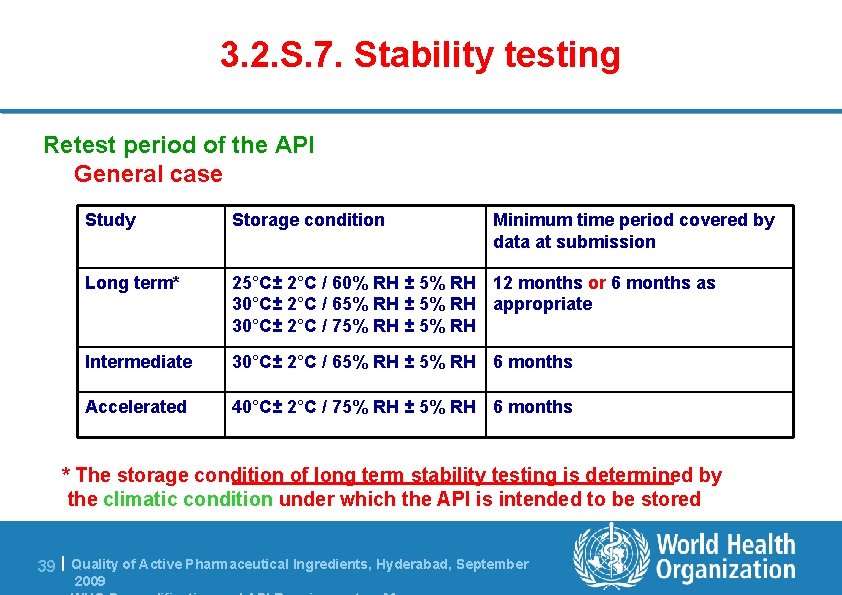
3. 2. S. 7. Stability testing Re-test period of the API l Selection of batches (at least 3 primary) – Primary batch of API should be at least pilot scale – Manufactured by the same synthesis as production batches and the same manufacturing procedure simulating the final process l Same packaging than that proposed for storage /distribution l Parameters to be tested: those susceptible to change during storage such as assay, degradation products, physico-chemical characteristics if relevant l Analytical methods same as release OR if different, should be validated and demonstrated to be stability indicating 38 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

3. 2. S. 7. Stability testing Retest period of the API General case Study Storage condition Minimum time period covered by data at submission Long term* 25°C± 2°C / 60% RH ± 5% RH 12 months or 6 months as 30°C± 2°C / 65% RH ± 5% RH appropriate 30°C± 2°C / 75% RH ± 5% RH Intermediate 30°C± 2°C / 65% RH ± 5% RH 6 months Accelerated 40°C± 2°C / 75% RH ± 5% RH 6 months * The storage condition of long term stability testing is determined by the climatic condition under which the API is intended to be stored 39 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

3. 2. S. 7. Stability testing Climatic zones and long term testing conditions l Long term stability testing conditions are determined by the climatic condition under which the API is intended to be stored. Zone I: temperate 21°C/45%RH Zone II: subtropical/mediterranean 25°C/60%RH Zone III: hot/dry 30°C/35%RH Zone VIa: hot/humid 30°C/65%RH Zone VIb: hot/very humid 30°C/75%RH 40 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

3. 2. S. 7. Stability testing - Testing frequency: every 3 months the first year, every 6 months the second year and then annually - For an API, “significant change” is failure to meet the specification for any parameter - Extrapolation: possible either according to WHO PQ supplement 2 or according to ICH Q 1 E Exception for NOT easily degradable APIs, see the list in Supplement 2 6 months long term data at submission (either ICH condition 25°C/60% RH OR 30°C/65% RH OR 30°C/75% RH) + 6 months accelerated ICH 40°C/75% RH A re-test period of 24 months can be accorded Stability study should be continued to cover the re-test period accorded commitment will be requested to submit complementary data 41 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

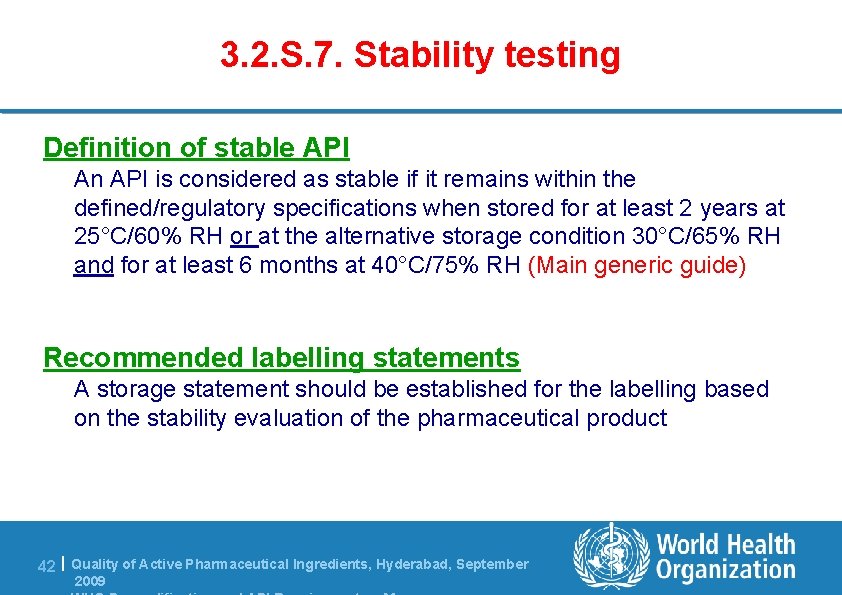
3. 2. S. 7. Stability testing Definition of stable API An API is considered as stable if it remains within the defined/regulatory specifications when stored for at least 2 years at 25°C/60% RH or at the alternative storage condition 30°C/65% RH and for at least 6 months at 40°C/75% RH (Main generic guide) 0°C / 75% RH. Recommended labelling statements A storage statement should be established for the labelling based on the stability evaluation of the pharmaceutical product 42 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

3. 2. S. 7. Stability testing Recommended labelling statements for APIs appendix 3 to WHO stability guideline Testing conditions under which stability of API is demonstrated Recommended labelling statement 25°C /60 % RH (long term) 40°C/ 75% RH (accelerated) Do not store above 25°C /60 % RH (long term) Do not store above 25°C 30°C /65 % RH (intermediate, failure of accelerated) 30°C /65 % RH (long term) OR 30°C /75 % RH (long term) 40°C/ 75% RH (accelerated) Do not store above 30°C 5°C ± 3°C Store in a refrigerator (2 to 8°C) -20°C± 5°C Store in a freezer 43 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

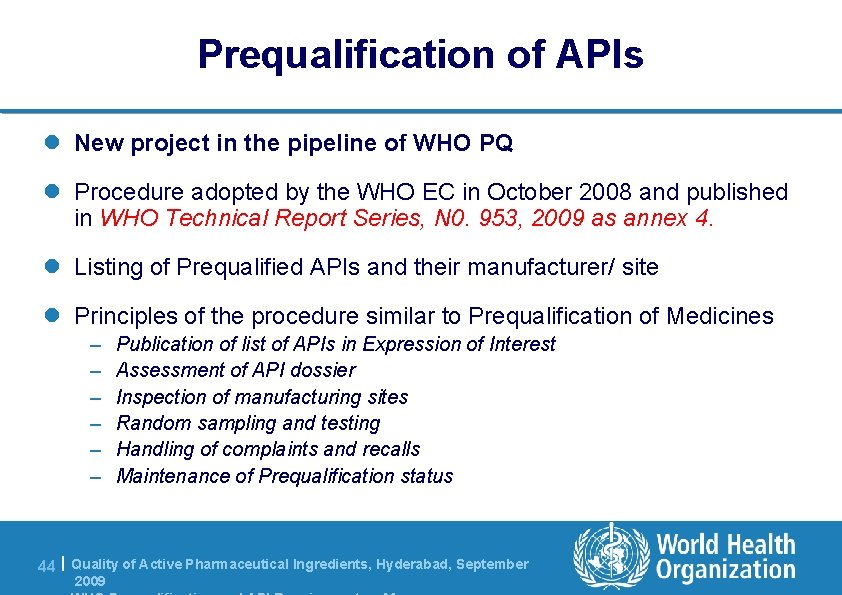
Prequalification of APIs l New project in the pipeline of WHO PQ l Procedure adopted by the WHO EC in October 2008 and published in WHO Technical Report Series, N 0. 953, 2009 as annex 4. l Listing of Prequalified APIs and their manufacturer/ site l Principles of the procedure similar to Prequalification of Medicines – – – Publication of list of APIs in Expression of Interest Assessment of API dossier Inspection of manufacturing sites Random sampling and testing Handling of complaints and recalls Maintenance of Prequalification status 44 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

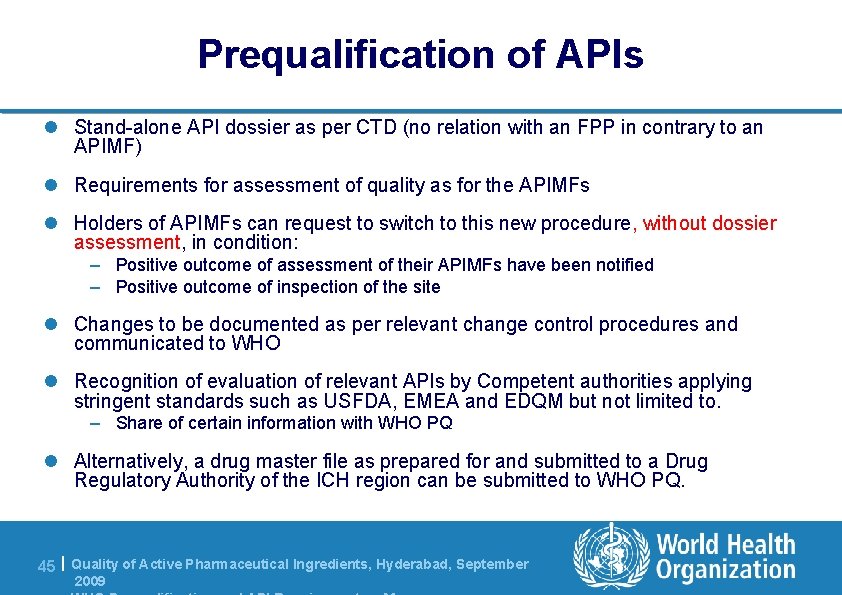
Prequalification of APIs l Stand-alone API dossier as per CTD (no relation with an FPP in contrary to an APIMF) l Requirements for assessment of quality as for the APIMFs l Holders of APIMFs can request to switch to this new procedure, without dossier assessment, in condition: – Positive outcome of assessment of their APIMFs have been notified – Positive outcome of inspection of the site l Changes to be documented as per relevant change control procedures and communicated to WHO l Recognition of evaluation of relevant APIs by Competent authorities applying stringent standards such as USFDA, EMEA and EDQM but not limited to. – Share of certain information with WHO PQ l Alternatively, a drug master file as prepared for and submitted to a Drug Regulatory Authority of the ICH region can be submitted to WHO PQ. 45 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009

Thank you for your attention 46 | Quality of Active Pharmaceutical Ingredients, Hyderabad, September 2009
 Indot prequalified contractors list
Indot prequalified contractors list Api monogram requirements
Api monogram requirements Sae ams2750
Sae ams2750 Josyl barchue
Josyl barchue Maryam merrikhpour
Maryam merrikhpour Maryam khodabakhsh
Maryam khodabakhsh Sptamaths
Sptamaths Djevojčica iz afganistana tema
Djevojčica iz afganistana tema Dr maryam syed
Dr maryam syed Fuel consumption rate
Fuel consumption rate Maryam majedi
Maryam majedi Maryam saeri
Maryam saeri Characters in parvana
Characters in parvana دکتر مریم زاهدی
دکتر مریم زاهدی Vad är basyta
Vad är basyta Dr. maryam zahedi clinic
Dr. maryam zahedi clinic Daneshgah esfahan
Daneshgah esfahan Iitb
Iitb Maryam nazir
Maryam nazir Maryam.h2009
Maryam.h2009 Dr maryam ebrahimi
Dr maryam ebrahimi Maryam merrikhpour
Maryam merrikhpour Idd cpd
Idd cpd Conventional/stylized decorative design
Conventional/stylized decorative design Functions of hospital pharmacy
Functions of hospital pharmacy Socio-organizational issues and stakeholder requirements
Socio-organizational issues and stakeholder requirements Warehouse health and safety
Warehouse health and safety Requirements modeling in system analysis and design
Requirements modeling in system analysis and design Micro-purchases and section 508 requirements
Micro-purchases and section 508 requirements Hardware and software requirements
Hardware and software requirements Use case narrative
Use case narrative Small n light
Small n light Kebutuhan dana untuk aktiva tetap
Kebutuhan dana untuk aktiva tetap Geotools api
Geotools api Kronos api documentation
Kronos api documentation Wan mbn mode set
Wan mbn mode set Jenis wesel kereta api
Jenis wesel kereta api Api maria
Api maria Knime api call
Knime api call Api common characteristics in unix
Api common characteristics in unix Usbank developer portal
Usbank developer portal Pear http_request
Pear http_request Digium phone api
Digium phone api Netflix api documentation
Netflix api documentation Openclinica api
Openclinica api Organigramma gruppo api
Organigramma gruppo api Whizard api
Whizard api





































































