Practice Parameter Use of Epidural Steroid Injections to




































- Slides: 43

Practice Parameter: Use of Epidural Steroid Injections to Treat Radicular Lumbosacral Pain (An Evidence-Based Review) American Academy of Neurology (AAN) Quality Standards Subcommittee C Armon, MD; CE Argoff, MD; J Samuels, MD; M Backonja, MD American Academy of Neurology © 2007

The AAN develops these presentation slides as educational tools for neurologists and other health care practitioners. You may download and retain a single copy for your personal use. Please contact guidelines@aan. com to learn about options for sharing this content beyond your personal use.

Presentation Objectives • To identify key issues in the use of epidural steroid injections to treat radicular lumbosacral pain • To make evidence-based recommendations American Academy of Neurology © 2007

Overview • • • Background Gaps in care AAN guideline process Analysis of evidence Summary Recommendations for future research American Academy of Neurology © 2007

Background • Chronic back pain and its associated disabilities represent an important and increasing health problem. • Estimated costs of treatments for spinal pain for 1990 was at least $13 billion, growing by 7% each year. American Academy of Neurology © 2007

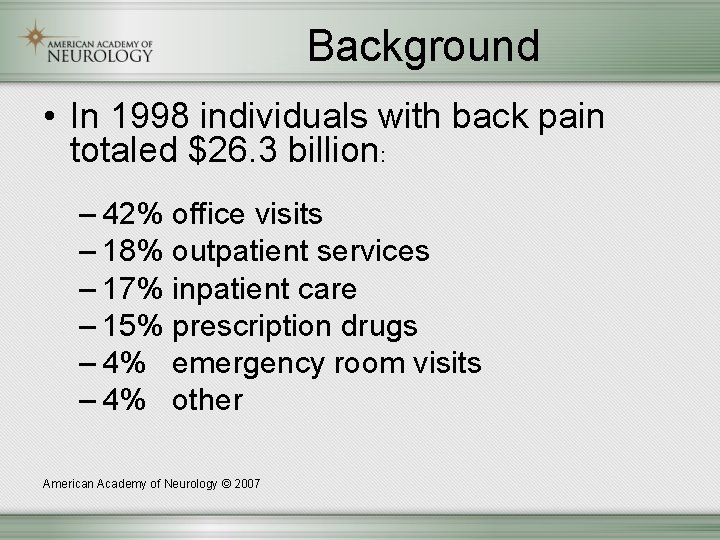
Background • In 1998 individuals with back pain totaled $26. 3 billion: – 42% office visits – 18% outpatient services – 17% inpatient care – 15% prescription drugs – 4% emergency room visits – 4% other American Academy of Neurology © 2007

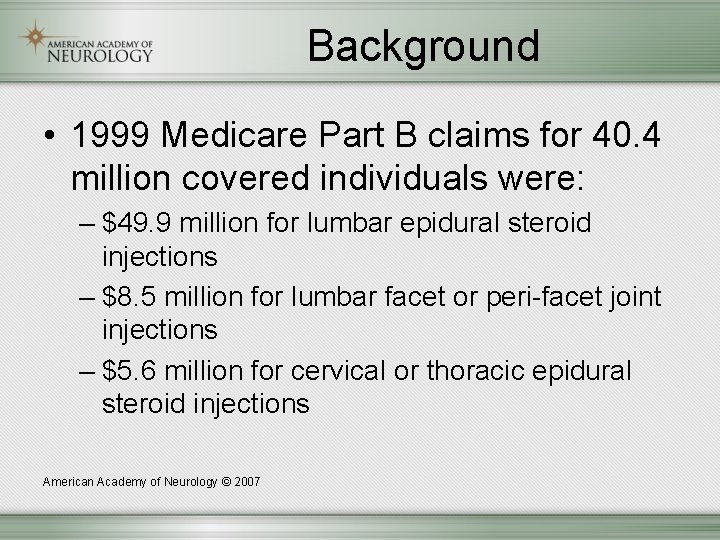
Background • 1999 Medicare Part B claims for 40. 4 million covered individuals were: – $49. 9 million for lumbar epidural steroid injections – $8. 5 million for lumbar facet or peri-facet joint injections – $5. 6 million for cervical or thoracic epidural steroid injections American Academy of Neurology © 2007

Background • Low back pain may occur without or with radicular features (the latter sometime referred to as sciatica). • Reports of epidural corticosteroid injections to treat sciatica date back to the 1950’s. American Academy of Neurology © 2007

Background • A 2004 review by the Technology Assessment Committee of the Institute for Clinical Systems Improvement (ICSI) focused on fluoroscopically guided, transforaminal epidural steroid injections in radicular lumbar pain. ICSI’s evidence-based approach used a rating system different from AAN’s. American Academy of Neurology © 2007

Background • ICSI’s Technology Assessment concluded that there was insufficient evidence to comment on the efficacy of transforaminal epidural steroid injections in radicular lumbar pain. American Academy of Neurology © 2007

Gaps in Care • Effective treatment for low back pain and sciatica is difficult. • The natural history is one of improvement in the majority of sufferers. • Providers are unsure what to recommend for their patients. American Academy of Neurology © 2007

AAN Guideline Process Clinical Question Evidence Conclusions Recommendations American Academy of Neurology © 2007

Clinical Question • Question should address an area of quality concern, controversy, confusion, or variation in practice • Question must be answerable with sufficient scientific data – Potential to improve clinical care and patient outcomes American Academy of Neurology © 2007

Literature Search/Review: Rigorous, Comprehensive, Transparent Complete Search Review abstracts Review full text Select articles Relevant American Academy of Neurology © 2007

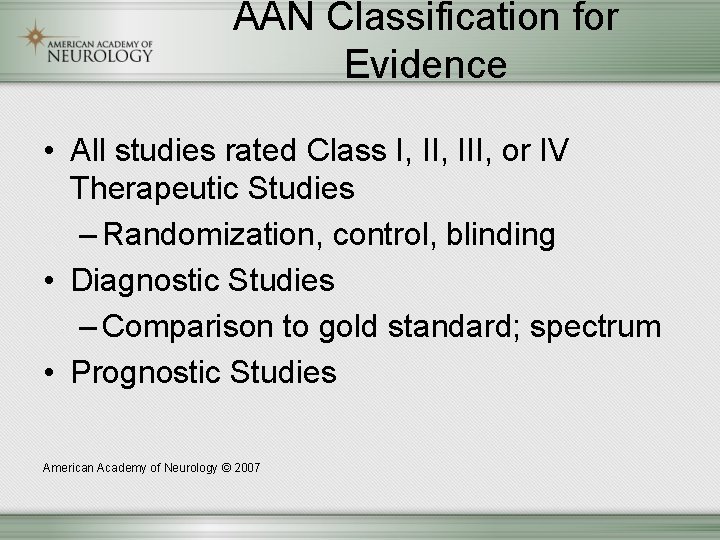
AAN Classification for Evidence • All studies rated Class I, III, or IV Therapeutic Studies – Randomization, control, blinding • Diagnostic Studies – Comparison to gold standard; spectrum • Prognostic Studies American Academy of Neurology © 2007

Classification of Evidence for Therapeutic Intervention Class 1 – Prospective, randomized, controlled clinical trials with masked outcome assessment, in a representative population. The following are required: a) primary outcome(s) is/are clearly defined, b) exclusion/inclusion criteria are clearly defined, c) adequate accounting for drop-outs and American Academy of Neurology © 2007

Classification of Evidence for Therapeutic Intervention cross-overs with numbers sufficiently low to make minimal potential for bias, d) relevant baseline characteristics are presented and substantially equivalent among treatment groups or there is appropriate statistical adjustment for differences. American Academy of Neurology © 2007

Classification of Evidence for Therapeutic Intervention Class II – Prospective matched group cohort study in a representative population with masked outcome assessment that meets a – d above OR a RCT in a representative population that lacks one criterion a – d. American Academy of Neurology © 2007

Classification of Evidence for Therapeutic Intervention Class III – All other controlled trials including well defined natural history controls or patients serving as own controls in a representative population, here outcome is independently assessed or independently derived by objective outcome measurement. * American Academy of Neurology © 2007

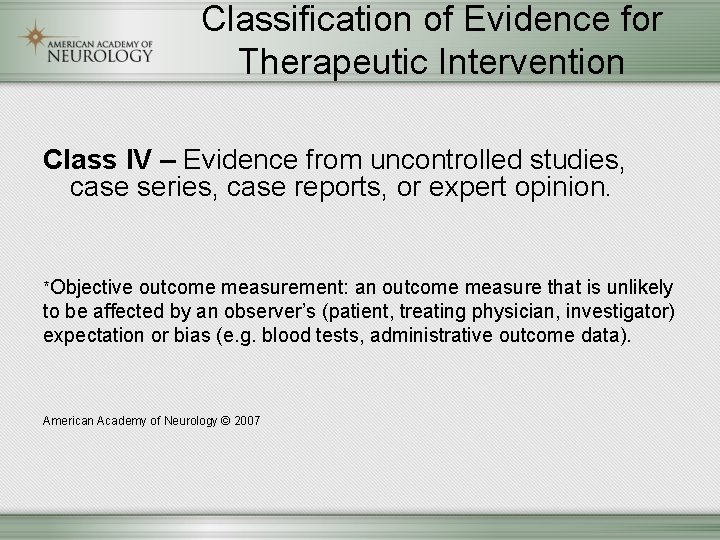
Classification of Evidence for Therapeutic Intervention Class IV – Evidence from uncontrolled studies, case series, case reports, or expert opinion. *Objective outcome measurement: an outcome measure that is unlikely to be affected by an observer’s (patient, treating physician, investigator) expectation or bias (e. g. blood tests, administrative outcome data). American Academy of Neurology © 2007

AAN Level of Recommendations • A = Established as effective, ineffective, or harmful for the given condition in the specified population • B = Probably effective, ineffective, or harmful for the given condition in the specified population • C = Possibly effective, ineffective, or harmful for the given condition in the specified population • U = Data is inadequate or conflicting; given current knowledge, treatment is unproven American Academy of Neurology © 2007

AAN Level of Recommendations • A = Requires two consistent Class I studies • B = Requires one Class I study or two consistent Class II studies • C = Requires one Class II study or two consistent Class III studies • U = Studies not meeting criteria for Class I through Class III American Academy of Neurology © 2007

Clinical Question • What is the evidence to support use of epidural steroid injections in radicular lumbosacral pain to produce pain relief? American Academy of Neurology © 2007

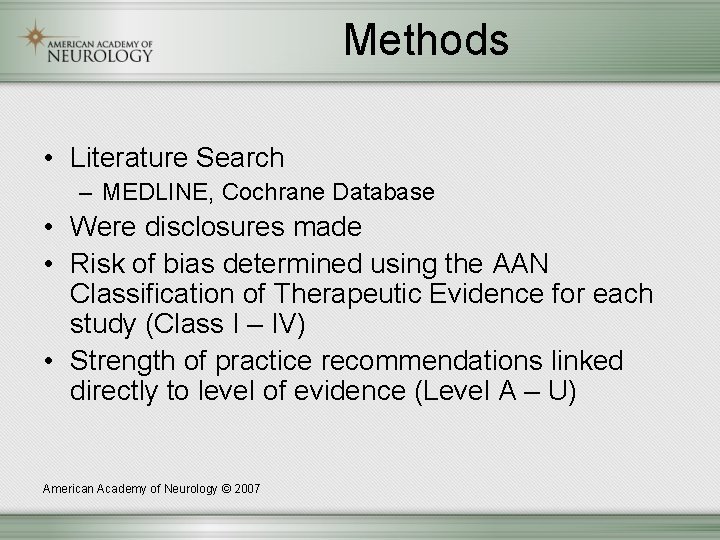
Methods • Literature Search – MEDLINE, Cochrane Database • Were disclosures made • Risk of bias determined using the AAN Classification of Therapeutic Evidence for each study (Class I – IV) • Strength of practice recommendations linked directly to level of evidence (Level A – U) American Academy of Neurology © 2007

Literature Search/Review 37 articles 4 articles 2 ICSI review articles American Academy of Neurology © 2007 Inclusion criteria: -clear case definition -clear measure of outcome -use of a control group -randomization -at least double-blind study design -prospective study design -adequate statistical analysis

Literature Search/Review • Relevant studies graded as Class I – IV using the AAN Classification of Therapeutic Evidence • One study was Class III American Academy of Neurology © 2007

Literature Search/Review • One study was Class I for primary outcome variables at 3 months; was Class II for pain at earlier time points; and was Class III for other outcome measures: multiple analyses; small mean effect size • ICSI review – One study was Class III American Academy of Neurology © 2007

Evidence-Based Guideline Recommendations Good evidence supports Epidural steroid injections for radicular lumbosacral pain have shown no impact on average impairment of function; need for surgery; or long-term pain relief beyond 3 months. Their routine use for these indications Is not recommended (Level B – Class I – III). American Academy of Neurology © 2007

Recommendations Weak evidence supports Epidural steroid injections may result in some improvement in radicular lumbosacral pain when determined between 2 and 6 weeks following the injection, compared to control treatment (Level C, Class I – III). American Academy of Neurology © 2007

Recommendations Insufficient evidence exists To support or refute data on use of epidural steroid injections to treat cervical radicular pain. No recommendation can be made (Level U). American Academy of Neurology © 2007

Principal Findings in Clinical Perspective • Amelioration of Pain – The findings of 4 high quality studies are consistent, showing the following efficacy pattern compared to a control group: • No efficacy at 24 hours • Some efficacy at 2 to 6 weeks • No difference or rebound worsening at 3 and 6 mo. • No difference at one year American Academy of Neurology © 2007

Principal Findings in Clinical Perspective • These results support the individual perception of benefit from epidural steroids expressed in terms of short-term symptomatic relief. • The average effect difference (advantage of steroids over control treatment) was small, usually falling short of the value proposed as a clinically meaningful average difference – 15 mm on the 100 mm visual analogue pain scale. American Academy of Neurology © 2007

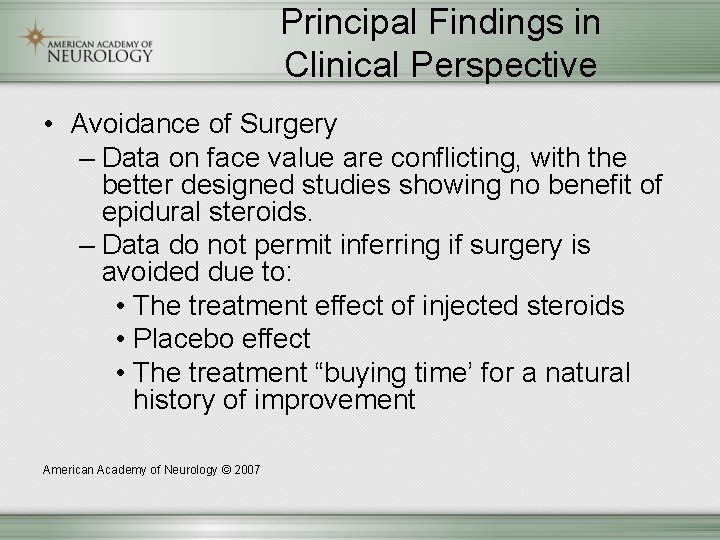
Principal Findings in Clinical Perspective • Avoidance of Surgery – Data on face value are conflicting, with the better designed studies showing no benefit of epidural steroids. – Data do not permit inferring if surgery is avoided due to: • The treatment effect of injected steroids • Placebo effect • The treatment “buying time’ for a natural history of improvement American Academy of Neurology © 2007

Principle Findings in Clinical Perspective • Data do not address how epidural steroid injections might compare to other treatment modalities and the role of patient and provider characteristics, including temperament and pain tolerance in selecting among various treatment options. • The recommendations gave greater weight to the data from the better designed studies showing that epidural steroid injections did not result in less surgery. American Academy of Neurology © 2007

Principal Findings in Clinical Perspective • An uncontrolled study with partial follow-up of treated patients has identified factors that predict poor outcome: • Greater number of previous treatments for pain • More medications taken • Pain not necessarily increased by activity • Pain increased by coughing American Academy of Neurology © 2007

Principal Findings in Clinical Perspective • The same uncontrolled study with partial follow-up of treated patients has identified factors that predict no benefit 1 year after treatment: • Pain does not interfere with activities • Unemployment due to pain • Normal straight leg raising tests before treatment American Academy of Neurology © 2007

Limitations • Review is limited by its inability to compare all techniques and all treatment approaches. • Review did not assess issues of frequency of injection or dosage. • Review did not evaluate operator experience • Limited ability to generalize the findings. • Focus on pain relief, guided by the chief indication for which epidural steroid injections are used, is a limitation. American Academy of Neurology © 2007

Future Research • Further studies of the efficacy of epidural steroids for radicular lumbosacral pain should be well-designed, meeting specific criteria (see full guideline). • Studies of use of epidural steroids to treat radicular cervical pain should also be welldesigned, meeting similar criteria. American Academy of Neurology © 2007

Future Research • Principal questions that need to be answered follow: – What is the degree of efficacy, expressed in terms of magnitude of effect, duration of effect, and percent of patients who achieve clinically meaningful improvement, in comparison to alternative treatments? American Academy of Neurology © 2007

Future Research • Using a controlled design, are there predictors of lack of efficacy or poor efficacy? • How many treatments are appropriate, and at what intervals? • How frequent are complications, and what are they? American Academy of Neurology © 2007

Future Research • Initially, it will be necessary to standardize some of the variables reflected in the questions in Table 1 (see full guideline) such as a specific technical approach, the minimal competency of the treating physician, and utilization of additional therapies. American Academy of Neurology © 2007

To access the full guideline please visit: AAN. com/Guidelines Published in Neurology, March 6, 2007 68: 723 -729

Questions or Comments? American Academy of Neurology © 2007
 Chalzion
Chalzion Tationil side effects
Tationil side effects Masseter injections
Masseter injections Jawline fillers injections in dubai
Jawline fillers injections in dubai Whitening injection dubai
Whitening injection dubai Asa msa psa
Asa msa psa Syringe
Syringe Skin whitening injection in dubai
Skin whitening injection in dubai Subq injection
Subq injection Akinosi block
Akinosi block Circular flow of income injections and withdrawals
Circular flow of income injections and withdrawals Epidural space
Epidural space Epidural block level chart
Epidural block level chart Fon
Fon Epidural anesthesia drugs
Epidural anesthesia drugs Epidural space brain
Epidural space brain Epidural catheter markings measurement
Epidural catheter markings measurement New england journal of medicine
New england journal of medicine Spinal anesthesia structures pierced
Spinal anesthesia structures pierced Pefras
Pefras Epidural slide
Epidural slide Difference between epidural and spinal
Difference between epidural and spinal Hematoma epidural arteria
Hematoma epidural arteria Fungsi spinal needle
Fungsi spinal needle Stilletes
Stilletes Epidural blood patch steps
Epidural blood patch steps Epidural
Epidural Dura cutting needle
Dura cutting needle Disconnected epidural catheter guideline
Disconnected epidural catheter guideline Kibas triadı
Kibas triadı Subdural epidural subarachnoid
Subdural epidural subarachnoid Types of hormones
Types of hormones Steroid nucleus
Steroid nucleus Hormones chemical classification
Hormones chemical classification Steroid eşdeğerlik tablosu
Steroid eşdeğerlik tablosu Gaspari nutrition steroids
Gaspari nutrition steroids Topicsl steroid withdrawal
Topicsl steroid withdrawal Steroid potency
Steroid potency Cephalin structure
Cephalin structure Classification of steroids
Classification of steroids Steroid muscle
Steroid muscle Sharon hulley
Sharon hulley Steroid
Steroid Steroid synthesis
Steroid synthesis