Steroid Hormones Chemical Classification of Hormones are chemical









- Slides: 40

Steroid Hormones

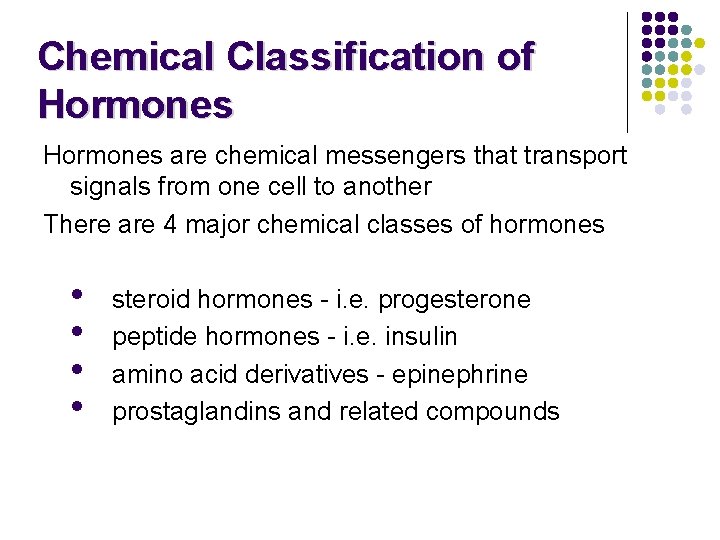
Chemical Classification of Hormones are chemical messengers that transport signals from one cell to another There are 4 major chemical classes of hormones • • steroid hormones - i. e. progesterone peptide hormones - i. e. insulin amino acid derivatives - epinephrine prostaglandins and related compounds

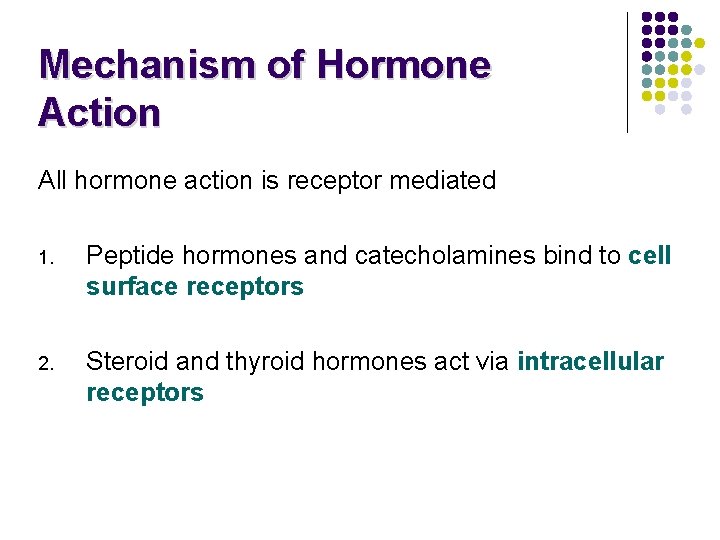
Mechanism of Hormone Action All hormone action is receptor mediated 1. Peptide hormones and catecholamines bind to cell surface receptors 2. Steroid and thyroid hormones act via intracellular receptors

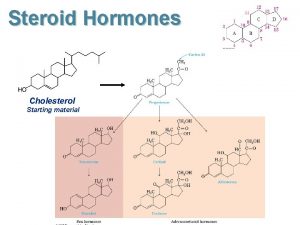
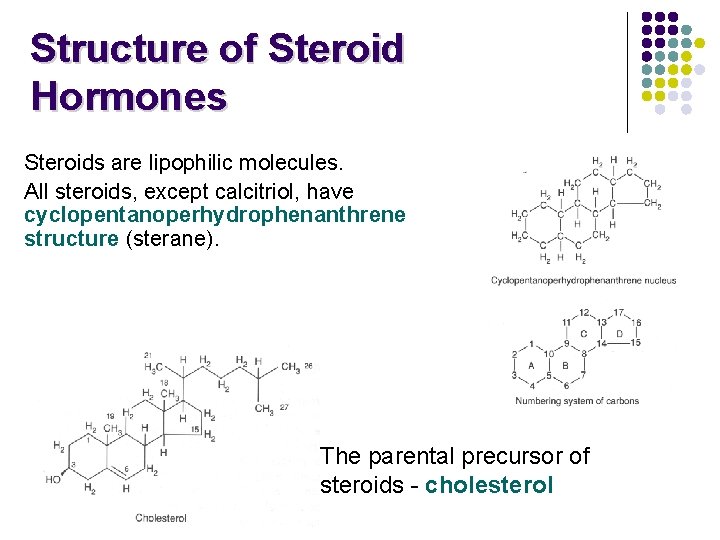
Structure of Steroid Hormones Steroids are lipophilic molecules. All steroids, except calcitriol, have cyclopentanoperhydrophenanthrene structure (sterane). The parental precursor of steroids - cholesterol

Steroid Hormone Classes Progestins Glucocorticoids Mineralocorticoids Androgens Estrogens Vitamin D

Steroid Hormones l l Are not packaged, but synthesized and immediately released. Are all derived from the same parent compound: cholesterol. Enzymes which produce steroid hormones from cholesterol are located in mitochondria and smooth ER. Steroids are lipid soluble and thus are freely permeable to membranes so are not stored in cells.

Steroid Hormones l l Are not water soluble so have to be carried in the blood complexed to specific binding globulins. Corticosteroid binding globulin carries cortisol. Sex steroid binding globulin carries testosterone and estradiol. In some cases a steroid is secreted by one cell and is converted to the active steroid by the target cell: (androgen is secreted by the gonad and converted into estrogen in the brain).

Steroid hormones l Steroid hormones play important roles in: - carbohydrate regulation (glucocorticoids) - mineral balance (mineralocorticoids) - reproductive functions (gonadal steroids) l Steroids also play roles in inflammatory responses, stress responses, bone metabolism, cardiovascular fitness, behavior, cognition, and mood.

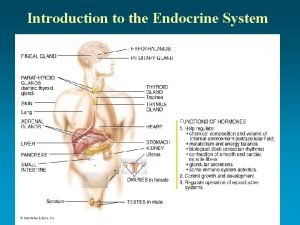
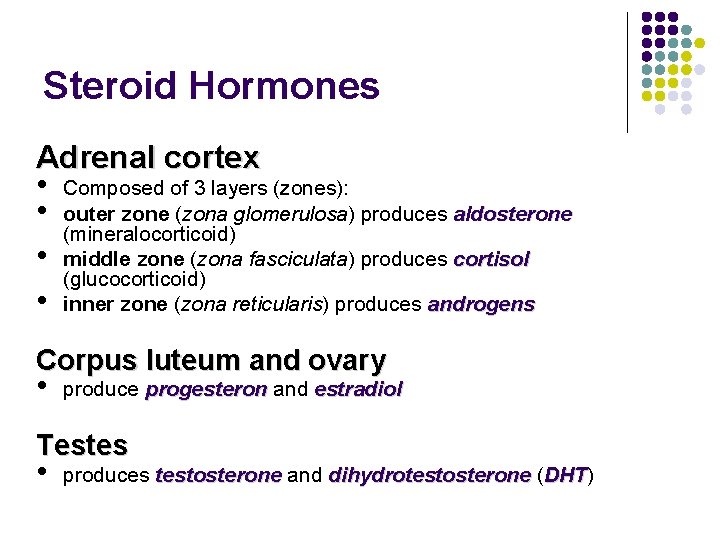
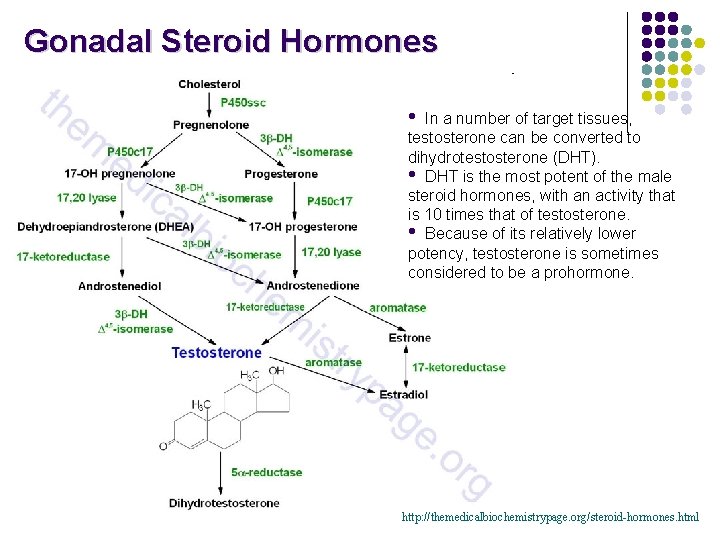
Steroid Hormones Adrenal cortex • Composed of 3 layers (zones): • outer zone (zona glomerulosa) produces aldosterone (mineralocorticoid) • middle zone (zona fasciculata) produces cortisol (glucocorticoid) • inner zone (zona reticularis) produces androgens Corpus luteum and ovary • produce progesteron and estradiol Testes • produces testosterone and dihydrotestosterone (DHT) DHT

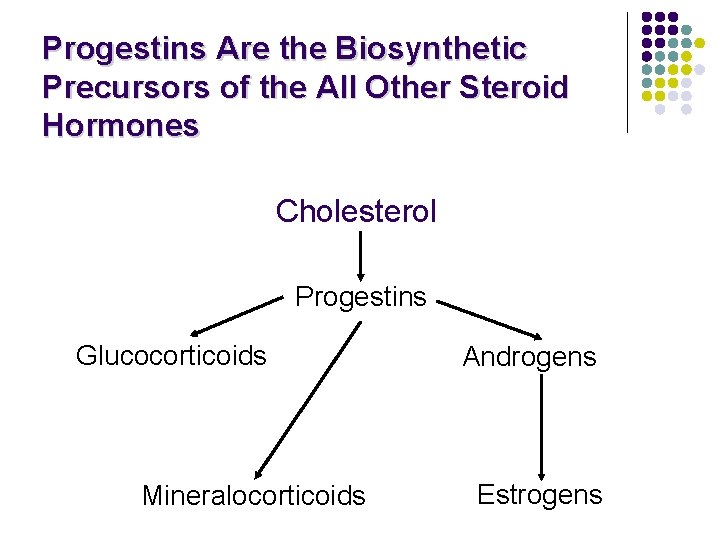
Progestins Are the Biosynthetic Precursors of the All Other Steroid Hormones Cholesterol Progestins Glucocorticoids Mineralocorticoids Androgens Estrogens

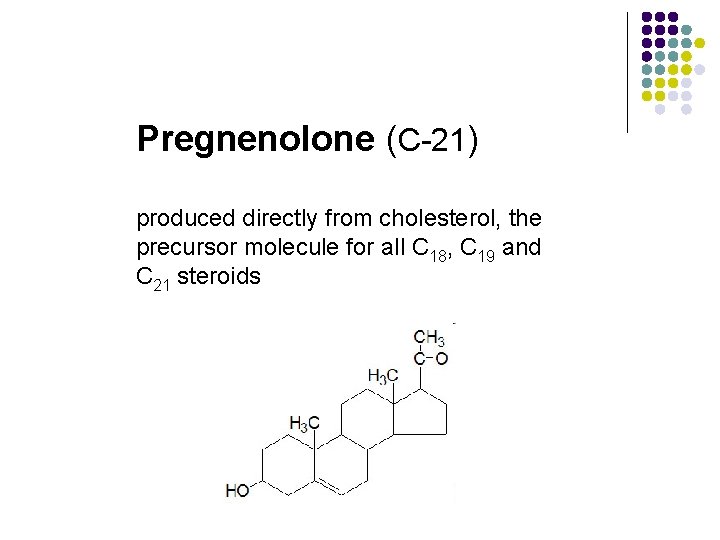
Pregnenolone (C-21) produced directly from cholesterol, the precursor molecule for all C 18, C 19 and C 21 steroids

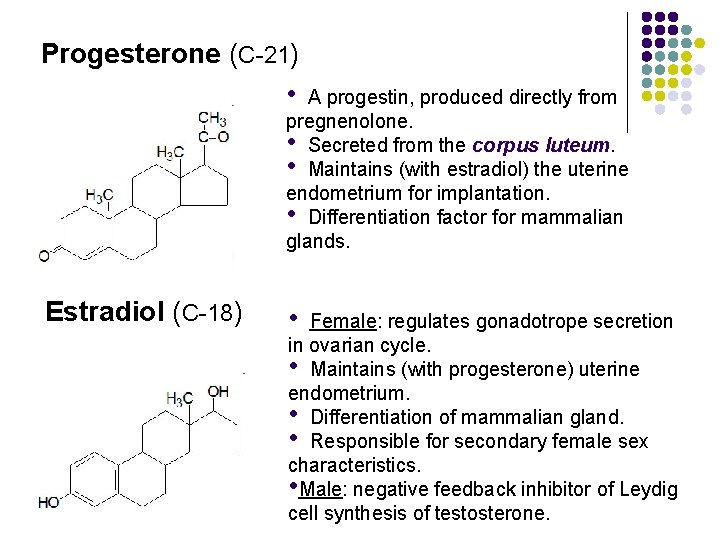
Progesterone (C-21) • A progestin, produced directly from pregnenolone. • Secreted from the corpus luteum. • Maintains (with estradiol) the uterine endometrium for implantation. • Differentiation factor for mammalian glands. Estradiol (C-18) • Female: regulates gonadotrope secretion in ovarian cycle. • Maintains (with progesterone) uterine endometrium. • Differentiation of mammalian gland. • Responsible for secondary female sex characteristics. • Male: negative feedback inhibitor of Leydig cell synthesis of testosterone.

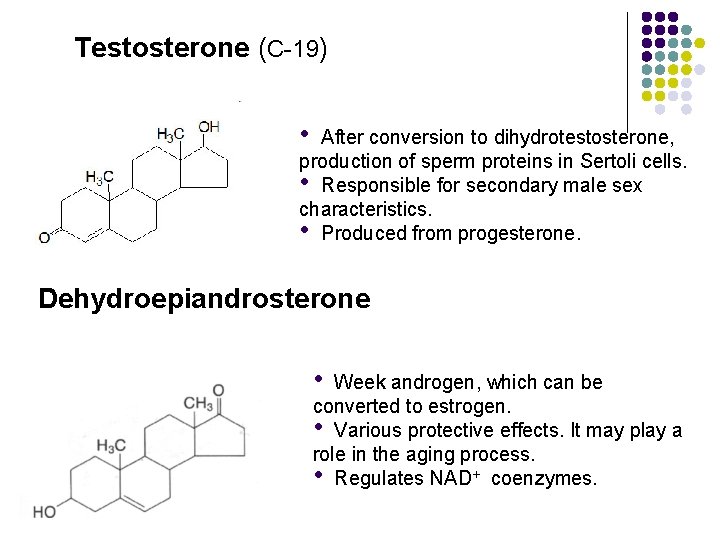
Testosterone (C-19) • After conversion to dihydrotestosterone, production of sperm proteins in Sertoli cells. • Responsible for secondary male sex characteristics. • Produced from progesterone. Dehydroepiandrosterone • Week androgen, which can be converted to estrogen. • Various protective effects. It may play a role in the aging process. • Regulates NAD+ coenzymes.

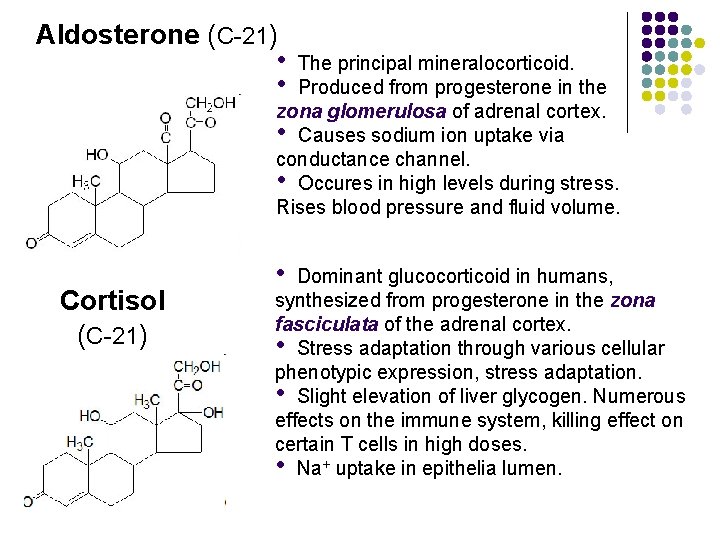
Aldosterone (C-21) • • The principal mineralocorticoid. Produced from progesterone in the zona glomerulosa of adrenal cortex. • Causes sodium ion uptake via conductance channel. • Occures in high levels during stress. Rises blood pressure and fluid volume. Cortisol (C-21) • Dominant glucocorticoid in humans, synthesized from progesterone in the zona fasciculata of the adrenal cortex. • Stress adaptation through various cellular phenotypic expression, stress adaptation. • Slight elevation of liver glycogen. Numerous effects on the immune system, killing effect on certain T cells in high doses. • Na+ uptake in epithelia lumen.

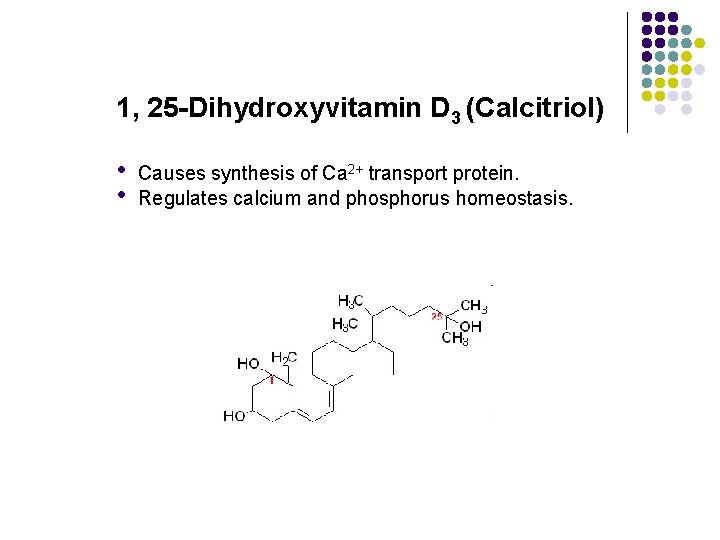
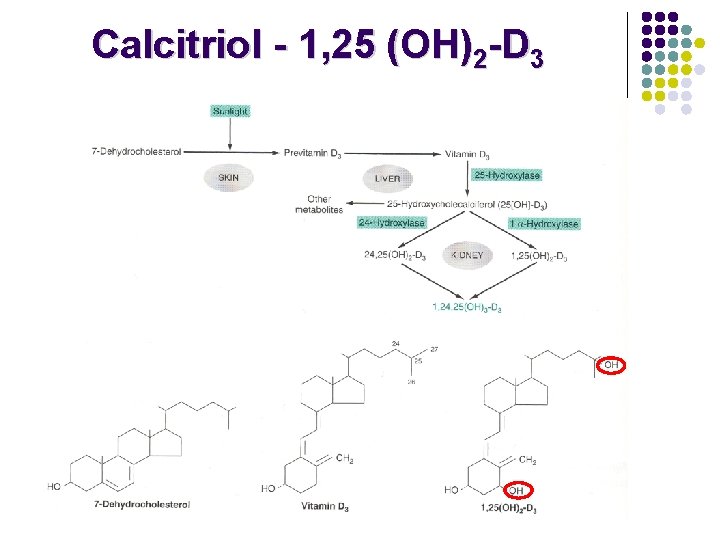
1, 25 -Dihydroxyvitamin D 3 (Calcitriol) • • Causes synthesis of Ca 2+ transport protein. Regulates calcium and phosphorus homeostasis.

Biosynthesis of Steroid Hormones l l l Peptide hormones are encoded by specific genes; steroid hormones are synthesized from the enzymaticaly modified cholesterol. Thus, there is no gene which encodes individual hormone. The regulation of steroidogenesis involves control of the enzymes which modify cholesterol into the steroid hormone of interest.

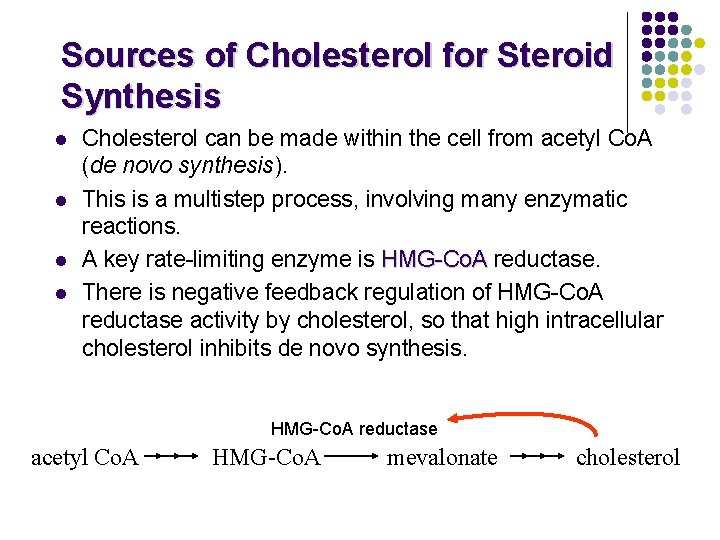
Sources of Cholesterol for Steroid Synthesis l l Cholesterol can be made within the cell from acetyl Co. A (de novo synthesis). This is a multistep process, involving many enzymatic reactions. A key rate-limiting enzyme is HMG-Co. A reductase. There is negative feedback regulation of HMG-Co. A reductase activity by cholesterol, so that high intracellular cholesterol inhibits de novo synthesis. HMG-Co. A reductase acetyl Co. A HMG-Co. A mevalonate cholesterol

Sources of Cholesterol for Steroid Synthesis l Cholesterol is also taken up by the cell in the form of low density lipoprotein (LDL). - LDL is a complex composed of cholesterol, phospholipids, triglycerides, and proteins (proteins and phospholipids make LDL soluble in blood). - LDL is taken into cells via LDL receptors, and broken down into esterified cholesterol, and then free cholesterol:

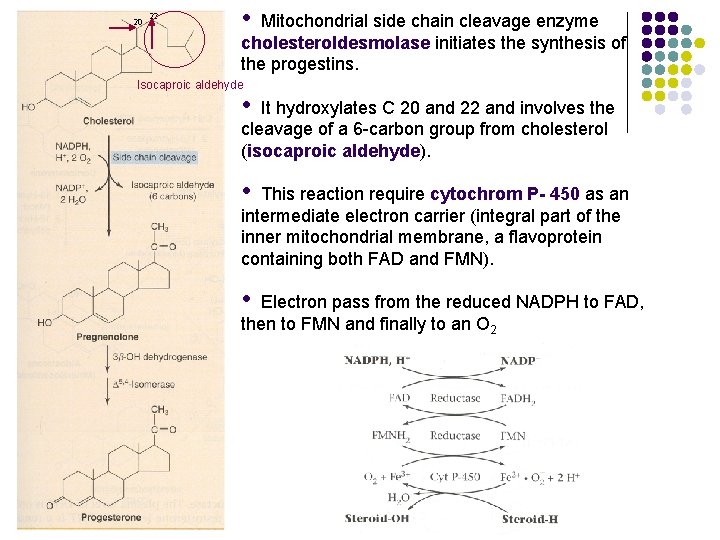
20 22 • Mitochondrial side chain cleavage enzyme cholesteroldesmolase initiates the synthesis of the progestins. Isocaproic aldehyde • It hydroxylates C 20 and 22 and involves the cleavage of a 6 -carbon group from cholesterol (isocaproic aldehyde). • This reaction require cytochrom P- 450 as an intermediate electron carrier (integral part of the inner mitochondrial membrane, a flavoprotein containing both FAD and FMN). • Electron pass from the reduced NADPH to FAD, then to FMN and finally to an O 2

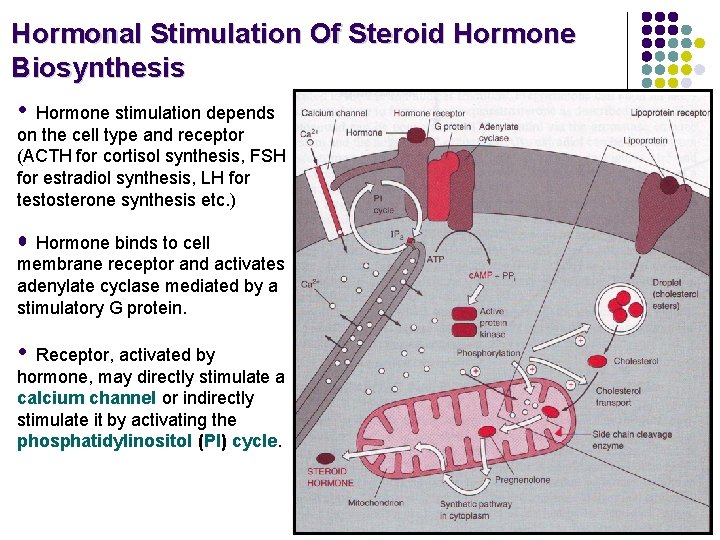
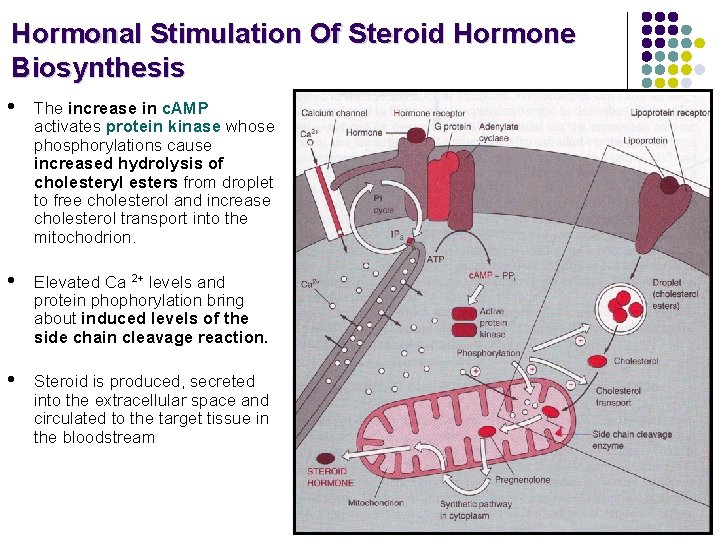
Hormonal Stimulation Of Steroid Hormone Biosynthesis • Hormone stimulation depends on the cell type and receptor (ACTH for cortisol synthesis, FSH for estradiol synthesis, LH for testosterone synthesis etc. ) • Hormone binds to cell membrane receptor and activates adenylate cyclase mediated by a stimulatory G protein. • Receptor, activated by hormone, may directly stimulate a calcium channel or indirectly stimulate it by activating the phosphatidylinositol (PI) cycle.

Hormonal Stimulation Of Steroid Hormone Biosynthesis • The increase in c. AMP activates protein kinase whose phosphorylations cause increased hydrolysis of cholesteryl esters from droplet to free cholesterol and increase cholesterol transport into the mitochodrion. • Elevated Ca 2+ levels and protein phophorylation bring about induced levels of the side chain cleavage reaction. • Steroid is produced, secreted into the extracellular space and circulated to the target tissue in the bloodstream

Biosynthesis of Steroid Hormones l Critical step is the cell activity in mobilizing cholesterol stored in a droplets, transport of cholesterol to mitochondrion. l The rate-limiting step is the rate of cholesterol side chain cleavage in mitochondrion by enzymes known as the cytochrome P 450 side chain cleavage enzyme complex.

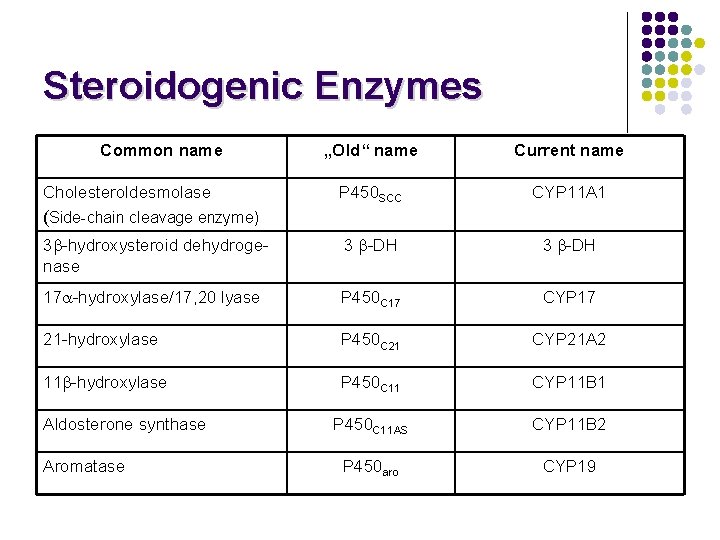
Steroidogenic Enzymes Common name „Old“ name Current name P 450 SCC CYP 11 A 1 3 -hydroxysteroid dehydrogenase 3 -DH 17 -hydroxylase/17, 20 lyase P 450 C 17 CYP 17 21 -hydroxylase P 450 C 21 CYP 21 A 2 11 -hydroxylase P 450 C 11 CYP 11 B 1 P 450 C 11 AS CYP 11 B 2 P 450 aro CYP 19 Cholesteroldesmolase (Side-chain cleavage enzyme) Aldosterone synthase Aromatase

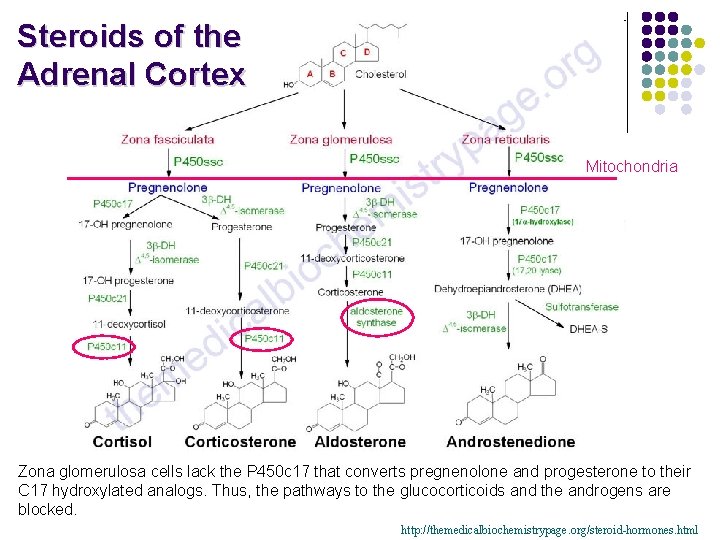
Steroids of the Adrenal Cortex Mitochondria Zona glomerulosa cells lack the P 450 c 17 that converts pregnenolone and progesterone to their C 17 hydroxylated analogs. Thus, the pathways to the glucocorticoids and the androgens are blocked. http: //themedicalbiochemistrypage. org/steroid-hormones. html

Steroid Hormones of the Gonades • Hormones that affect the development of the reproductive organs and sexual characteristics.

Testes Leydig cells produce: • Testosterone Sertoli cells produce: • • • dihydrotestosterone (DHT) – but most of conversion of testosterone to DHT occurs outside the testes. 17 - -estradiol – a small amount of testosterone is also converted into estradiol by aromatization (inhibits testosterone synthesis) inhibin – polypeptide hormone, which inhibits FSH releasing FSH binds to the Sertoli cells and stimulates the synthesis of androgen-binding protein (ABP). ABP binds testosterone (produced by Leydig cells) and transports it to the site of spermatogenesis.

Gonadal Steroid Hormones • In a number of target tissues, testosterone can be converted to dihydrotestosterone (DHT). • DHT is the most potent of the male steroid hormones, with an activity that is 10 times that of testosterone. • Because of its relatively lower potency, testosterone is sometimes considered to be a prohormone. http: //themedicalbiochemistrypage. org/steroid-hormones. html

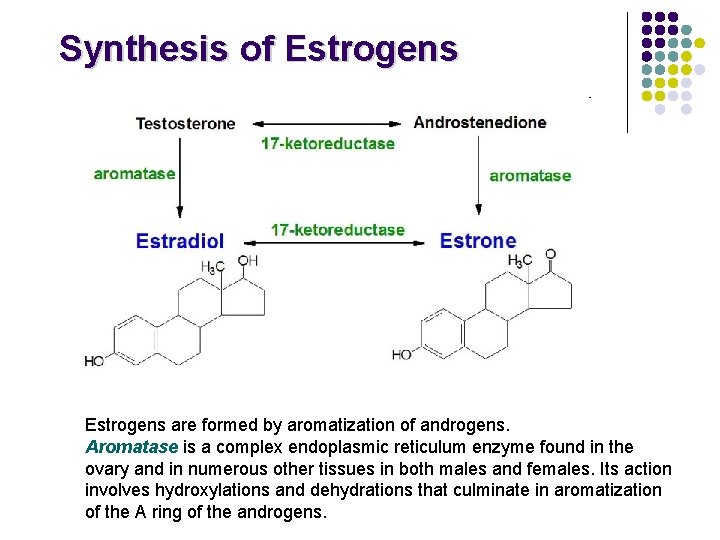
Synthesis of Estrogens are formed by aromatization of androgens. Aromatase is a complex endoplasmic reticulum enzyme found in the ovary and in numerous other tissues in both males and females. Its action involves hydroxylations and dehydrations that culminate in aromatization of the A ring of the androgens.

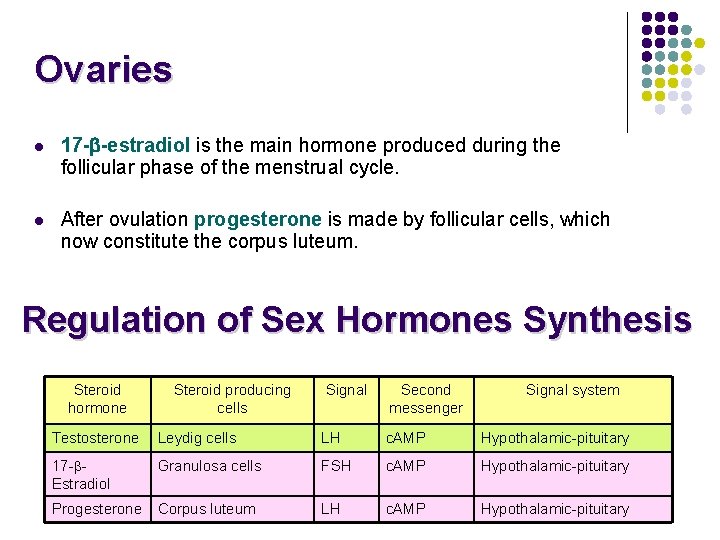
Ovaries l 17 - -estradiol is the main hormone produced during the follicular phase of the menstrual cycle. l After ovulation progesterone is made by follicular cells, which now constitute the corpus luteum. Regulation of Sex Hormones Synthesis Steroid hormone Steroid producing cells Signal Second messenger Signal system Testosterone Leydig cells LH c. AMP Hypothalamic-pituitary 17 - Estradiol Granulosa cells FSH c. AMP Hypothalamic-pituitary Progesterone Corpus luteum LH c. AMP Hypothalamic-pituitary

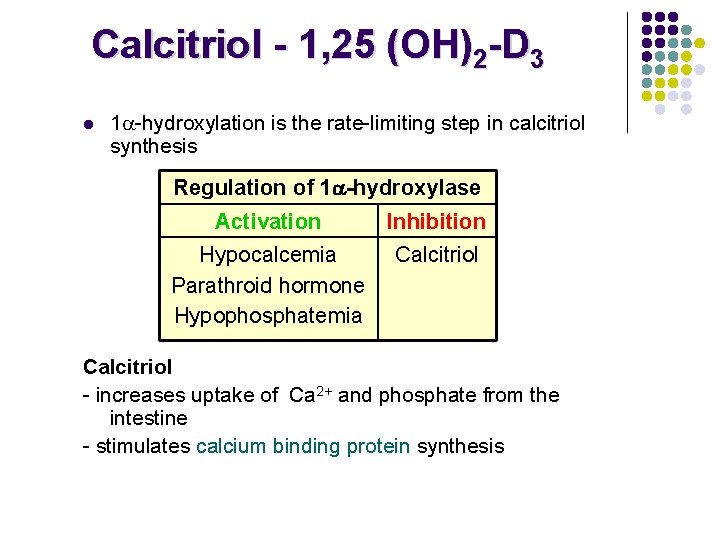
Calcitriol - 1, 25 (OH)2 -D 3

Calcitriol - 1, 25 (OH)2 -D 3 l 1 -hydroxylation is the rate-limiting step in calcitriol synthesis Regulation of 1 -hydroxylase Activation Inhibition Hypocalcemia Parathroid hormone Hypophosphatemia Calcitriol - increases uptake of Ca 2+ and phosphate from the intestine - stimulates calcium binding protein synthesis

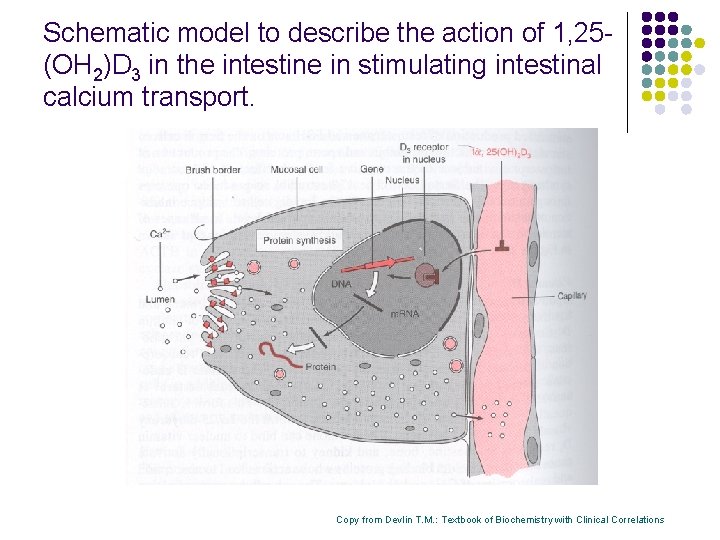
Schematic model to describe the action of 1, 25(OH 2)D 3 in the intestine in stimulating intestinal calcium transport. Copy from Devlin T. M. : Textbook of Biochemistry with Clinical Correlations

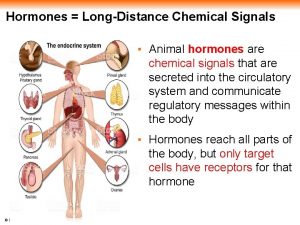
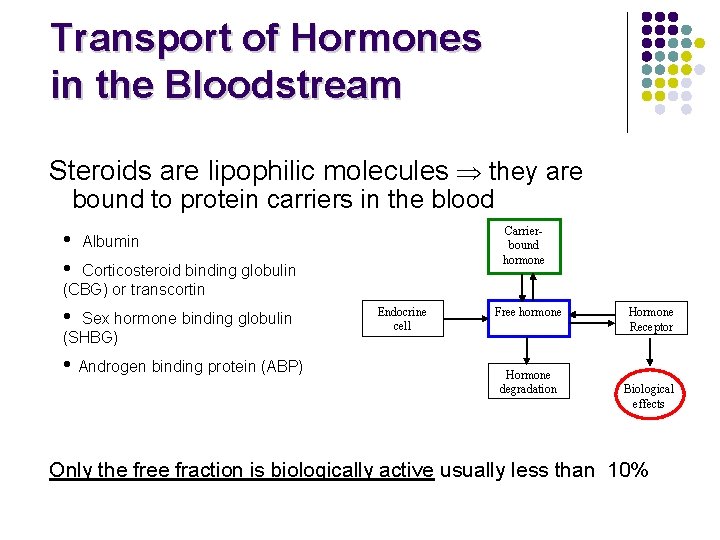
Transport of Hormones in the Bloodstream Steroids are lipophilic molecules they are bound to protein carriers in the blood • • Carrierbound hormone Albumin Corticosteroid binding globulin (CBG) or transcortin • Sex hormone binding globulin (SHBG) • Androgen binding protein (ABP) Endocrine cell Free hormone Hormone degradation Hormone Receptor Biological effects Only the free fraction is biologically active usually less than 10%

Hormone half life Steroids and thyroid hormone, which are bound to plasma proteins, have a long half life (~ hours) Peptides and catecholamines are watersoluble, they are transported dissolved in plasma generally have a very short half life (~ seconds to minutes)

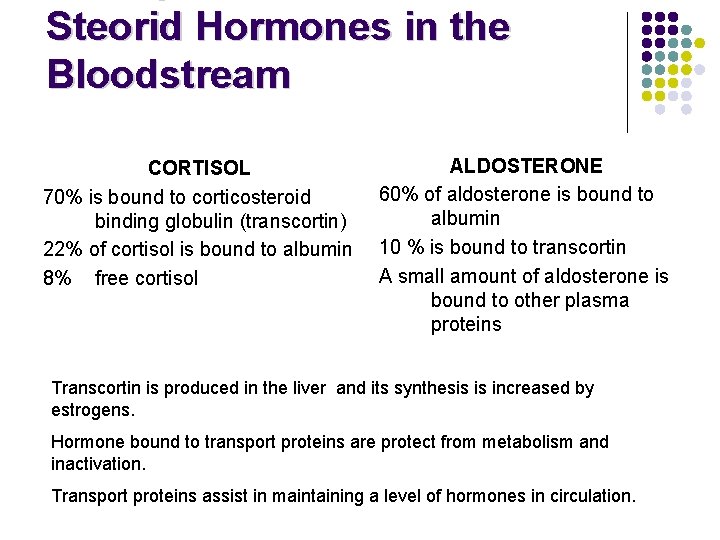
Steorid Hormones in the Bloodstream CORTISOL 70% is bound to corticosteroid binding globulin (transcortin) 22% of cortisol is bound to albumin 8% free cortisol ALDOSTERONE 60% of aldosterone is bound to albumin 10 % is bound to transcortin A small amount of aldosterone is bound to other plasma proteins Transcortin is produced in the liver and its synthesis is increased by estrogens. Hormone bound to transport proteins are protect from metabolism and inactivation. Transport proteins assist in maintaining a level of hormones in circulation.

Transport of Sex Hormones in the Bloodstream Testosterone & estradiol bind to sex hormone binding globulin (SHBG). Progesterone binds to transcortin. Affinity of SHBG for testosterone is higher than for estradiol. • Before puberty - the level of SHBG is about the same in males and females. • At the puberty - there is a small decrease in the level of circulating SHBG in females and larger decrease in males, insuring relatively greater amount of the unbound, biologically active sex hormones. • • In adults, males have half of the amount of SHBG than females. Testosterone lowers SHBG levels in blood, whereas estradiol raises SHBG levels.

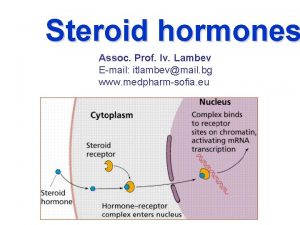
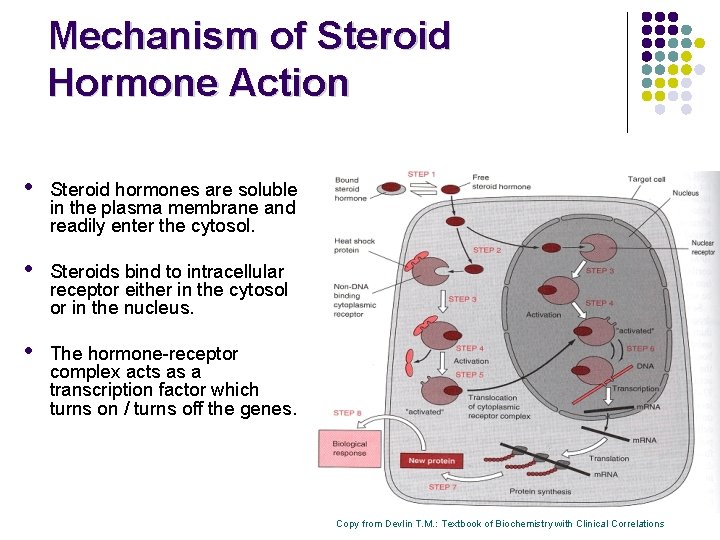
Mechanism of Steroid Hormone Action • Steroid hormones are soluble in the plasma membrane and readily enter the cytosol. • Steroids bind to intracellular receptor either in the cytosol or in the nucleus. • The hormone-receptor complex acts as a transcription factor which turns on / turns off the genes. Copy from Devlin T. M. : Textbook of Biochemistry with Clinical Correlations

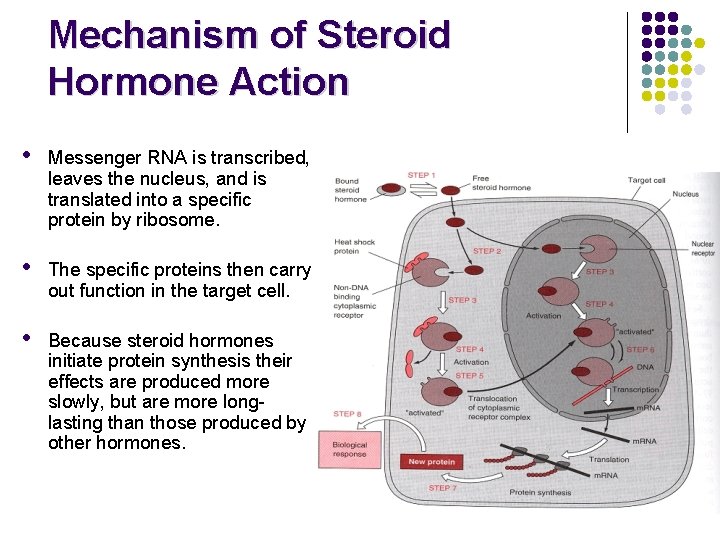
Mechanism of Steroid Hormone Action • Messenger RNA is transcribed, leaves the nucleus, and is translated into a specific protein by ribosome. • The specific proteins then carry out function in the target cell. • Because steroid hormones initiate protein synthesis their effects are produced more slowly, but are more longlasting than those produced by other hormones.

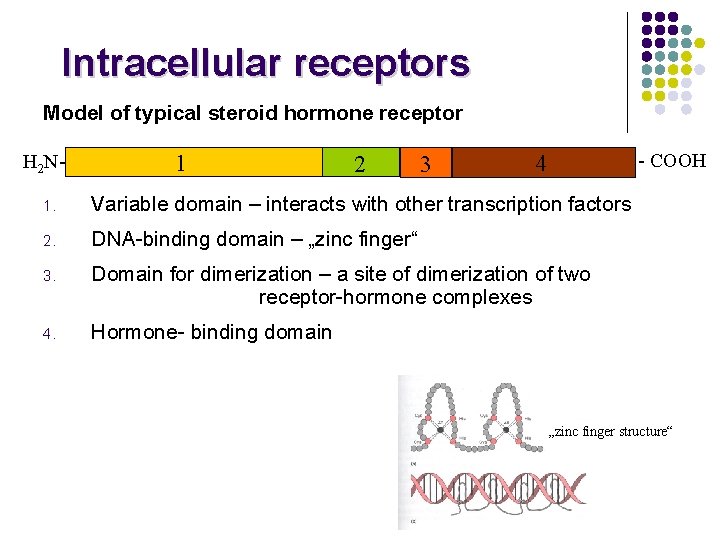
Intracellular receptors Model of typical steroid hormone receptor H 2 N- 1 2 3 - COOH 4 1. Variable domain – interacts with other transcription factors 2. DNA-binding domain – „zinc finger“ 3. Domain for dimerization – a site of dimerization of two receptor-hormone complexes 4. Hormone- binding domain „zinc finger structure“

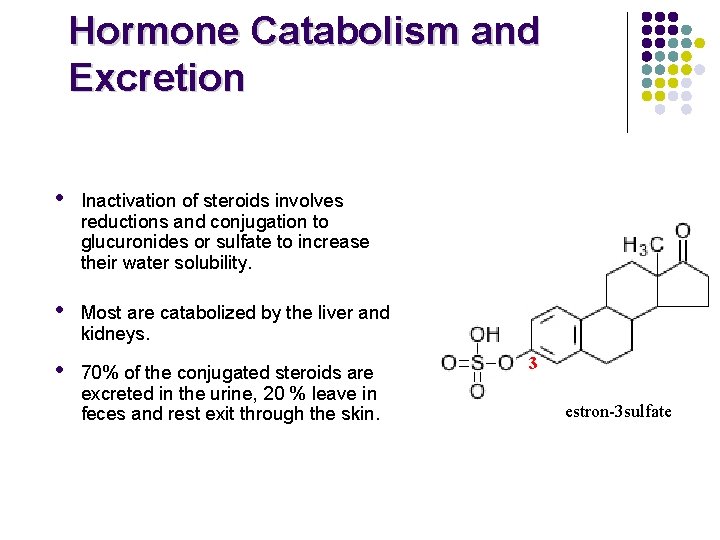
Hormone Catabolism and Excretion • Inactivation of steroids involves reductions and conjugation to glucuronides or sulfate to increase their water solubility. • Most are catabolized by the liver and kidneys. • 70% of the conjugated steroids are excreted in the urine, 20 % leave in feces and rest exit through the skin. 3 estron-3 sulfate
 Action of steroid hormones
Action of steroid hormones Insidan region jh
Insidan region jh Types of hormones
Types of hormones Steroid hormones are water soluble. true false
Steroid hormones are water soluble. true false Steroid classification
Steroid classification Endocrine system abbreviations
Endocrine system abbreviations Intracellular hormone binding
Intracellular hormone binding Steroid nucleus
Steroid nucleus Steroid eşdeğerlik tablosu
Steroid eşdeğerlik tablosu Halodrol
Halodrol Topicsl steroid withdrawal
Topicsl steroid withdrawal Steroid potency
Steroid potency Naturally occurring fatty acids
Naturally occurring fatty acids Steroid function
Steroid function Steroid muscle
Steroid muscle Sharon hulley
Sharon hulley Terpenes to steroids
Terpenes to steroids Steroid synthesis
Steroid synthesis Karnitin açil transferaz
Karnitin açil transferaz Steroid family tree
Steroid family tree Steroid myopathy
Steroid myopathy Kolestrol sentezi
Kolestrol sentezi Koprosterol
Koprosterol Identify the hormone that is not steroid
Identify the hormone that is not steroid Amino acid-based hormones
Amino acid-based hormones Plant biology ppt
Plant biology ppt Prolactin hormones
Prolactin hormones Kelenjar dan hormon
Kelenjar dan hormon Transportation of hormones
Transportation of hormones Prolactin target
Prolactin target Releasing inhibiting hormones
Releasing inhibiting hormones Lipid soluble hormones examples
Lipid soluble hormones examples Lipid soluble hormones examples
Lipid soluble hormones examples Gonadal hormones
Gonadal hormones Vasopresine
Vasopresine Bioflix activity homeostasis low blood glucose
Bioflix activity homeostasis low blood glucose Nontropic hormones
Nontropic hormones Amino acid-based hormones
Amino acid-based hormones Examples of amine hormones
Examples of amine hormones Amino acid-based hormones
Amino acid-based hormones Tropic hormones
Tropic hormones