Practical organic chemistry Aldehyde ketones You may not








- Slides: 19

Practical organic chemistry Aldehyde & ketones

You may not know it, but you already have experience with aldehydes and ketones based on things you have likely smelled and tasted. the smell of vanilla, almond flavor , cinnamon and spearmint

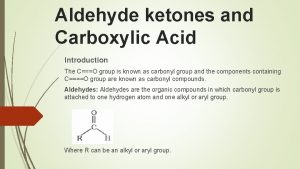
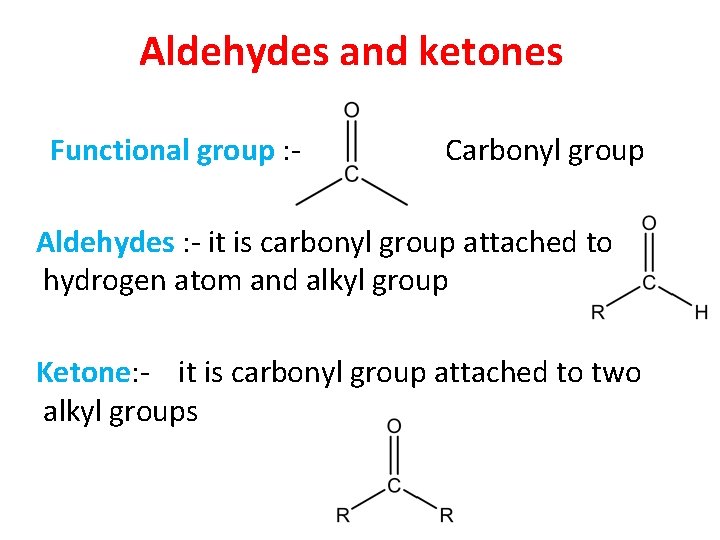
Aldehydes and ketones Functional group : - Carbonyl group Aldehydes : - it is carbonyl group attached to hydrogen atom and alkyl group Ketone: - it is carbonyl group attached to two alkyl groups

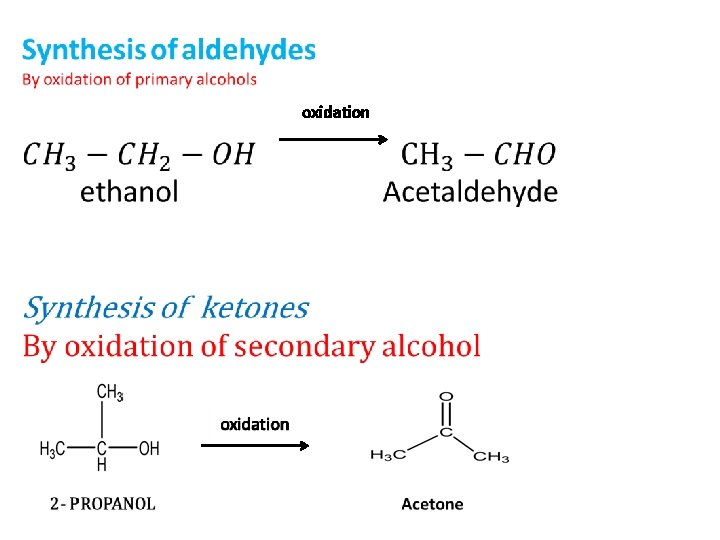
oxidation

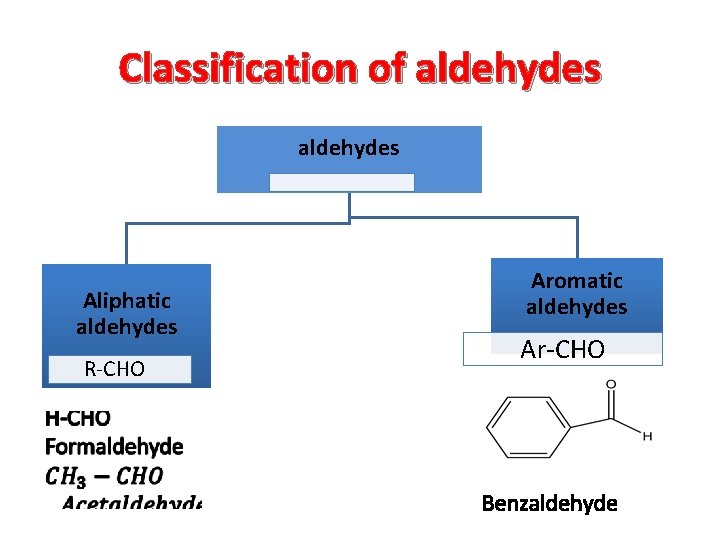
Classification of aldehydes Aliphatic aldehydes R-CHO Aromatic aldehydes Ar-CHO Benzaldehyde

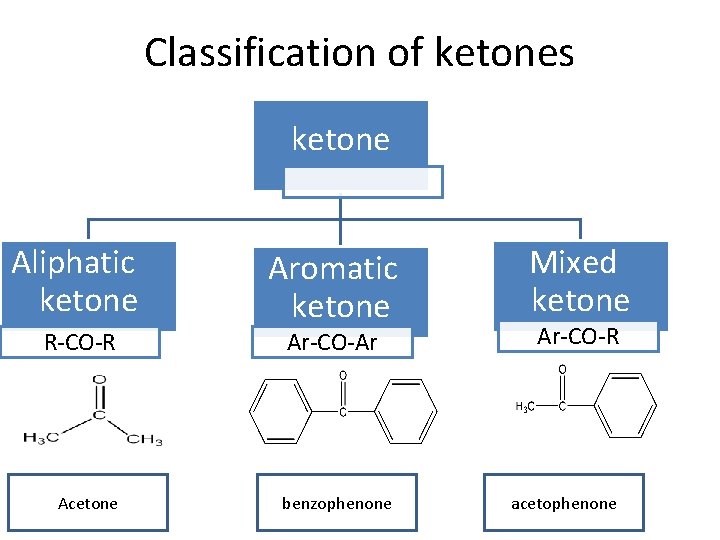
Classification of ketones ketone Aliphatic ketone R-CO-R Acetone Aromatic ketone Ar-CO-Ar benzophenone Mixed ketone Ar-CO-R acetophenone

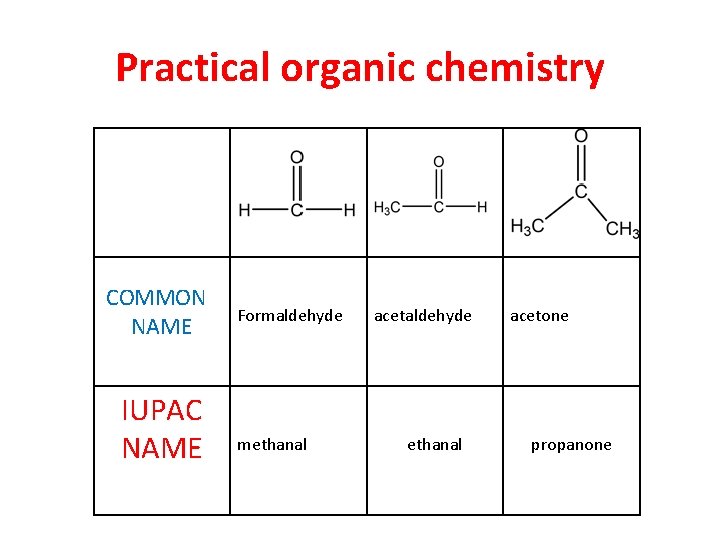
Practical organic chemistry COMMON NAME IUPAC NAME Formaldehyde methanal acetaldehyde ethanal acetone propanone

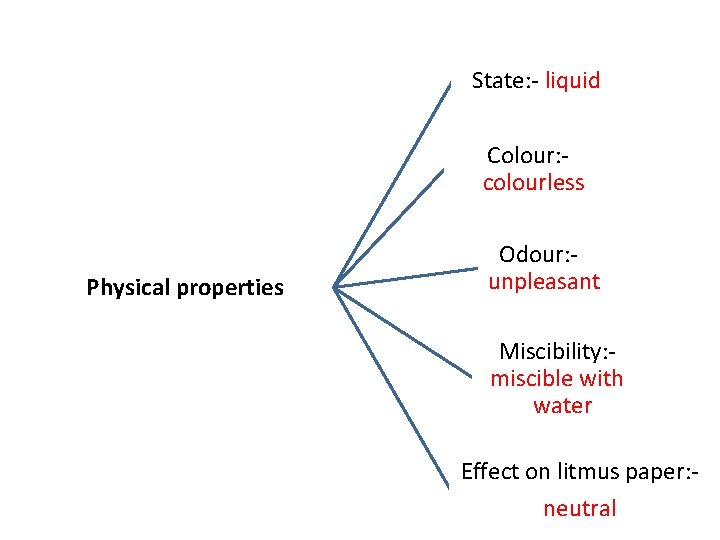
State: - liquid Colour: colourless Physical properties Odour: unpleasant Miscibility: miscible with water Effect on litmus paper: neutral

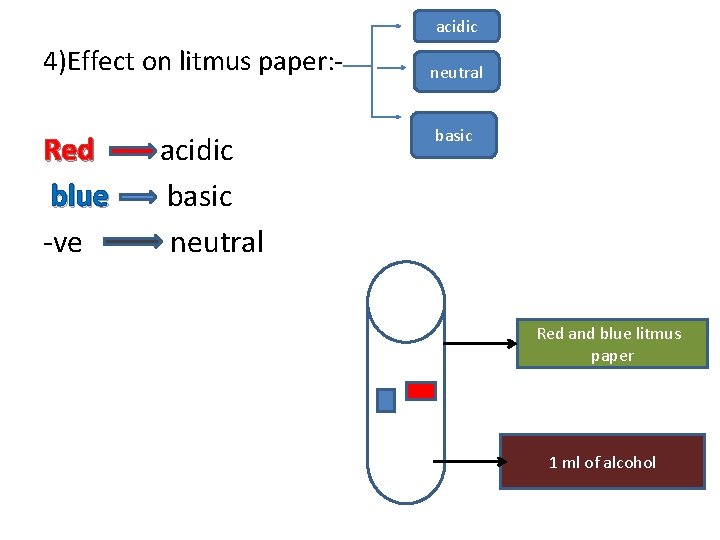
acidic 4)Effect on litmus paper: - Red blue -ve acidic basic neutral basic Red and blue litmus paper 1 ml of alcohol

Practical organic chemistry 2 ) chemical properties



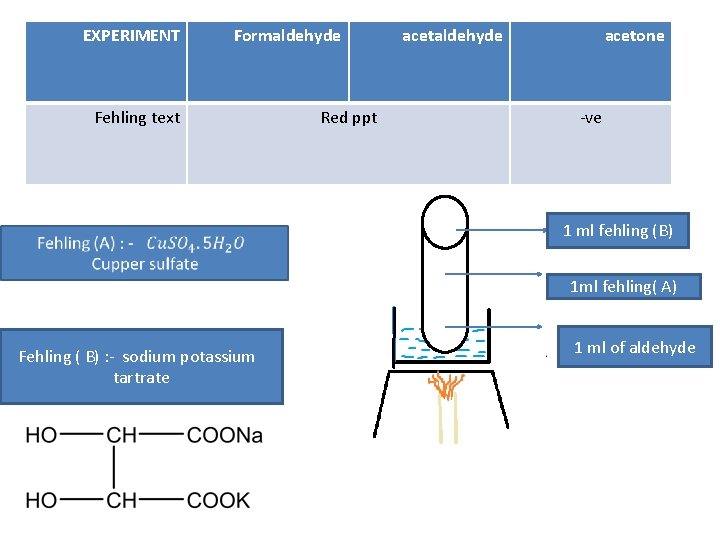
EXPERIMENT Formaldehyde Fehling text Red ppt acetaldehyde acetone -ve 1 ml fehling (B) 1 ml fehling( A) Fehling ( B) : - sodium potassium tartrate 1 ml of aldehyde






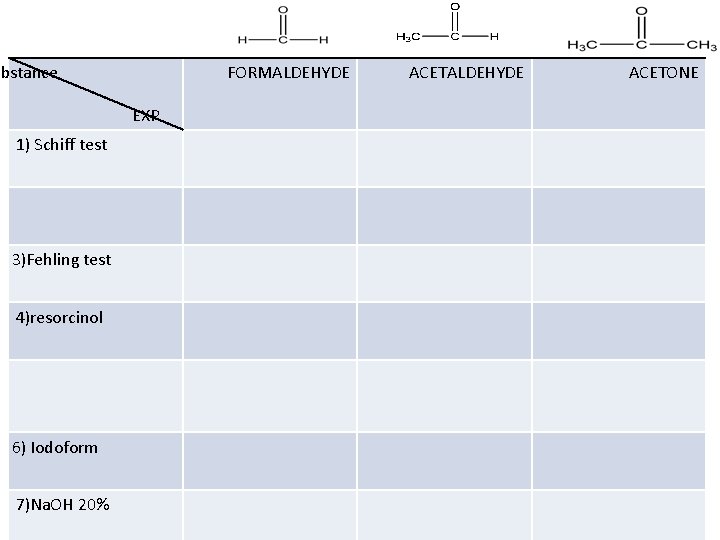
ubstance FORMALDEHYDE EXP 1) Schiff test 3)Fehling test 4)resorcinol 6) Iodoform 7)Na. OH 20% ACETALDEHYDE ACETONE
 Aldehyde and ketones
Aldehyde and ketones Ib chemistry organic chemistry
Ib chemistry organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Hci design patterns
Hci design patterns If you're not confused you're not paying attention
If you're not confused you're not paying attention If you can't measure it, you can't improve it
If you can't measure it, you can't improve it I will follow you wherever you ...........................
I will follow you wherever you ........................... Numbering carbon chains
Numbering carbon chains Canola oil
Canola oil Ester organic chemistry
Ester organic chemistry Structural formula vs displayed formula
Structural formula vs displayed formula Rearranged most stable carbocation is
Rearranged most stable carbocation is Ee organic chemistry
Ee organic chemistry Leveling effect organic chemistry
Leveling effect organic chemistry Pericyclic
Pericyclic David klein organic chemistry
David klein organic chemistry Organic chemistry 2nd edition klein
Organic chemistry 2nd edition klein What is the leveling effect organic chemistry
What is the leveling effect organic chemistry What functional group is ch3
What functional group is ch3 Organic chemistry lab report format
Organic chemistry lab report format