Please note these are the actual videorecorded proceedings







- Slides: 20

Please note, these are the actual video-recorded proceedings from the live CME event and may include the use of trade names and other raw, unedited content.

Local and Systemic Therapeutic Considerations for Patients with Newly Diagnosed Advanced Ovarian Cancer Angeles Alvarez Secord, MD, MHSc Duke Cancer Institute Research to Practice Symposium 2018 New Orleans

VERBAL DISCLOSURE • Clinical Trial Grants Astra. Zeneca Amgen Abb. Vie Astellas Pharma Inc Astex Bristol-Myers Squibb Company Boehringer Ingelheim Eisai Morphotek Endocyte Exelixis Inc Genentech Glaxo. Smith. Kline Incyte Merck Pharma. Mar Tesaro • Advisory Board Genentech Boehringer Ingelheim Clovis Tesaro Astra. Zeneca Janssen Alexion Myriad

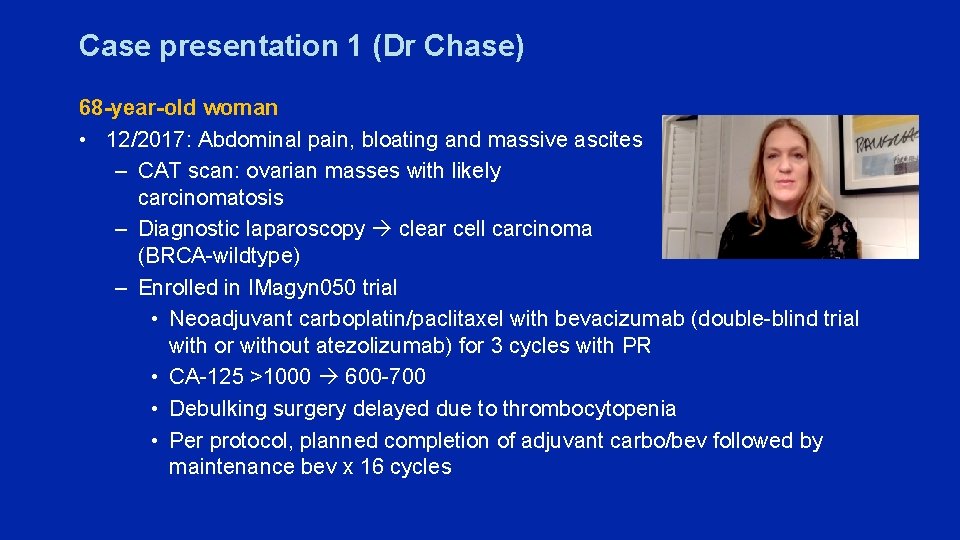
Case presentation 1 (Dr Chase) 68 -year-old woman • 12/2017: Abdominal pain, bloating and massive ascites – CAT scan: ovarian masses with likely carcinomatosis – Diagnostic laparoscopy clear cell carcinoma (BRCA-wildtype) – Enrolled in IMagyn 050 trial • Neoadjuvant carboplatin/paclitaxel with bevacizumab (double-blind trial with or without atezolizumab) for 3 cycles with PR • CA-125 >1000 600 -700 • Debulking surgery delayed due to thrombocytopenia • Per protocol, planned completion of adjuvant carbo/bev followed by maintenance bev x 16 cycles

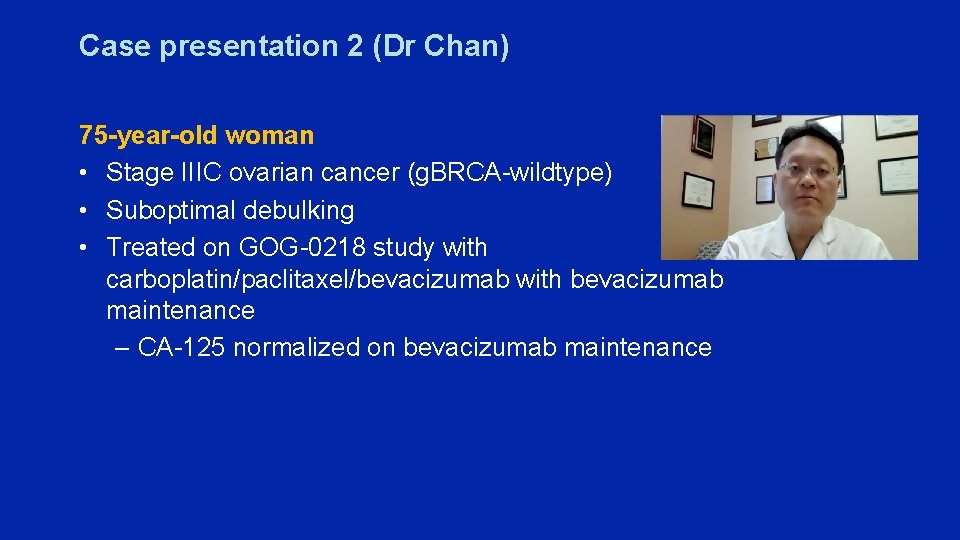
Case presentation 2 (Dr Chan) 75 -year-old woman • Stage IIIC ovarian cancer (g. BRCA-wildtype) • Suboptimal debulking • Treated on GOG-0218 study with carboplatin/paclitaxel/bevacizumab with bevacizumab maintenance – CA-125 normalized on bevacizumab maintenance

Case presentation 3 (Dr Chase) 60 -year-old woman • 1/2018: Known BRCA 1 germline mutation (delayed risk-reducing BSO) – US reveals complex ovarian mass, prompting further study • CAT scan: omental disease with carcinomatosis • CA-125 >1000 • 2/2018: Suboptimal surgical debulking + partial transverse colectomy – Ovarian masses, peritoneal carcinomatosis and large omental cake – Dehiscence wound VAC and drainage for abdominal incision

Advanced Ovarian Cancer Controversies • When should surgery be completed in front-line setting? – Primary debulking surgery vs neoadjuvant? • Optimal dosing and schedule? – Dose-dense weekly vs q 3 week – IP cisplatin dose? • Mode of adjuvant chemotherapy delivery? – IV, IP, HIPEC? • What is the role of bevacizumab in front-line therapy? – Will bevacizumab used in primary therapy effect therapeutic decisions at the time of recurrence?

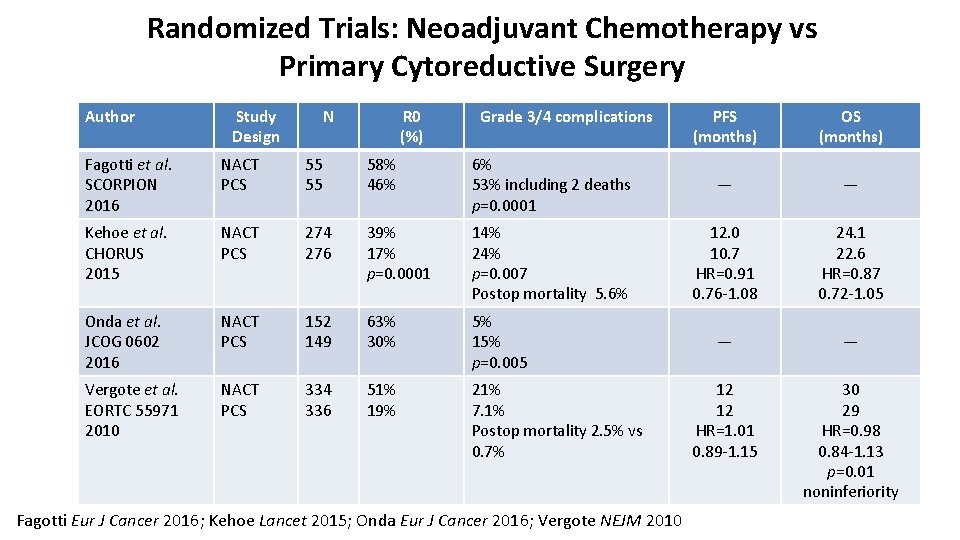
Randomized Trials: Neoadjuvant Chemotherapy vs Primary Cytoreductive Surgery Author Study Design Fagotti et al. SCORPION 2016 NACT PCS Kehoe et al. CHORUS 2015 N R 0 (%) Grade 3/4 complications PFS (months) OS (months) 55 55 58% 46% 6% 53% including 2 deaths p=0. 0001 — — NACT PCS· 274 276 39% 17% p=0. 0001 14% 24% p=0. 007 Postop mortality 5. 6% 12. 0 10. 7 HR=0. 91 0. 76 -1. 08 24. 1 22. 6 HR=0. 87 0. 72 -1. 05 Onda et al. JCOG 0602 2016 NACT PCS 152 149 63% 30% 5% 15% p=0. 005 — — Vergote et al. EORTC 55971 2010 NACT PCS 334 336 51% 19% 21% 7. 1% Postop mortality 2. 5% vs 0. 7% 12 12 HR=1. 01 0. 89 -1. 15 30 29 HR=0. 98 0. 84 -1. 13 p=0. 01 noninferiority · Fagotti Eur J Cancer 2016; Kehoe Lancet 2015; Onda Eur J Cancer 2016; Vergote NEJM 2010

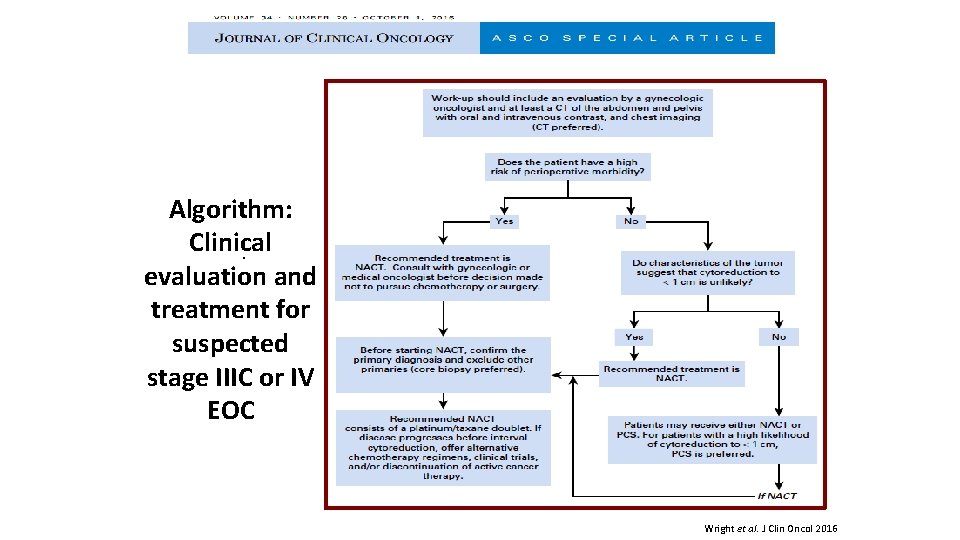
Algorithm: · Clinical · evaluation and treatment for suspected stage IIIC or IV EOC Wright et al. J Clin Oncol 2016

Algorithm: · Clinical · evaluation and treatment for suspected stage IIIC or IV EOC Wright et al. J Clin Oncol 2016

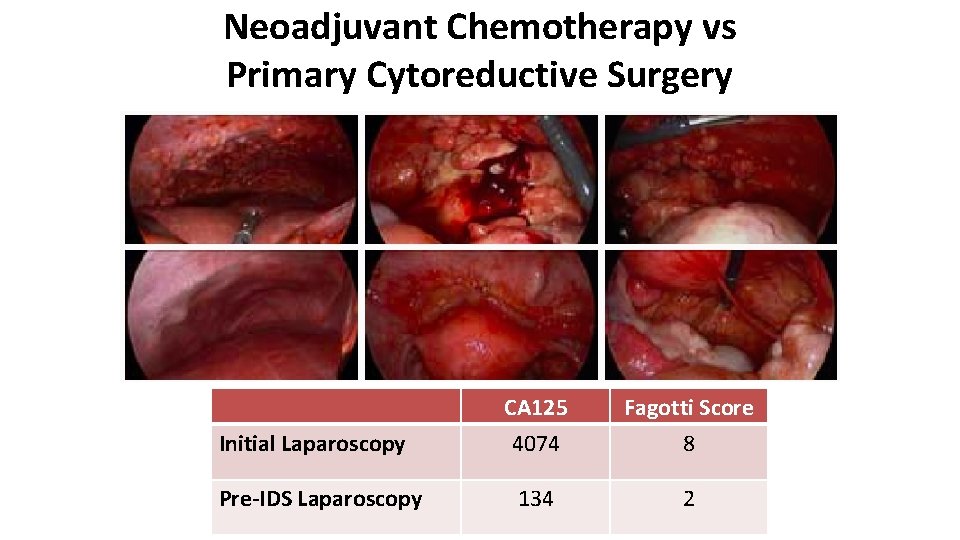
Neoadjuvant Chemotherapy vs Primary Cytoreductive Surgery Initial Laparoscopy Pre-IDS Laparoscopy CA 125 4074 Fagotti Score 8 134 2

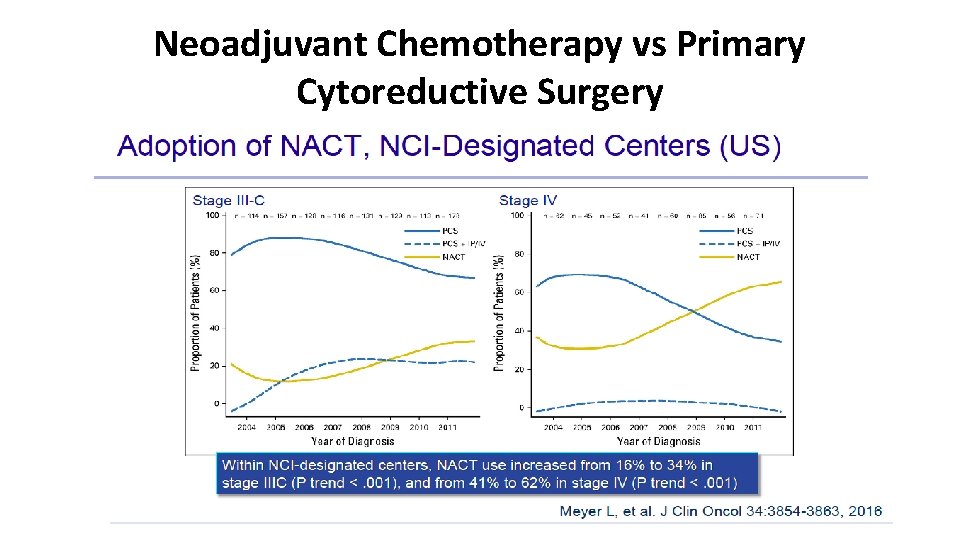
Neoadjuvant Chemotherapy vs Primary Cytoreductive Surgery

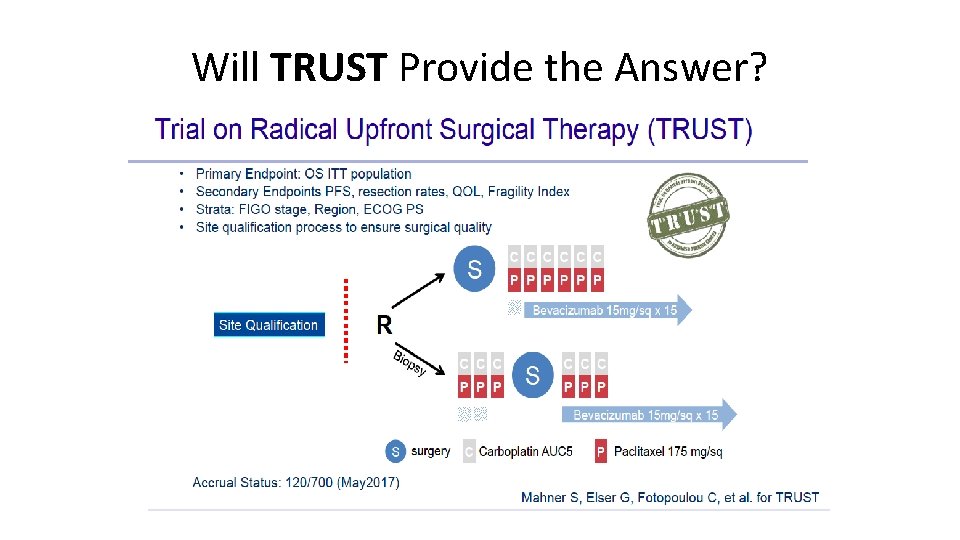
Will TRUST Provide the Answer?

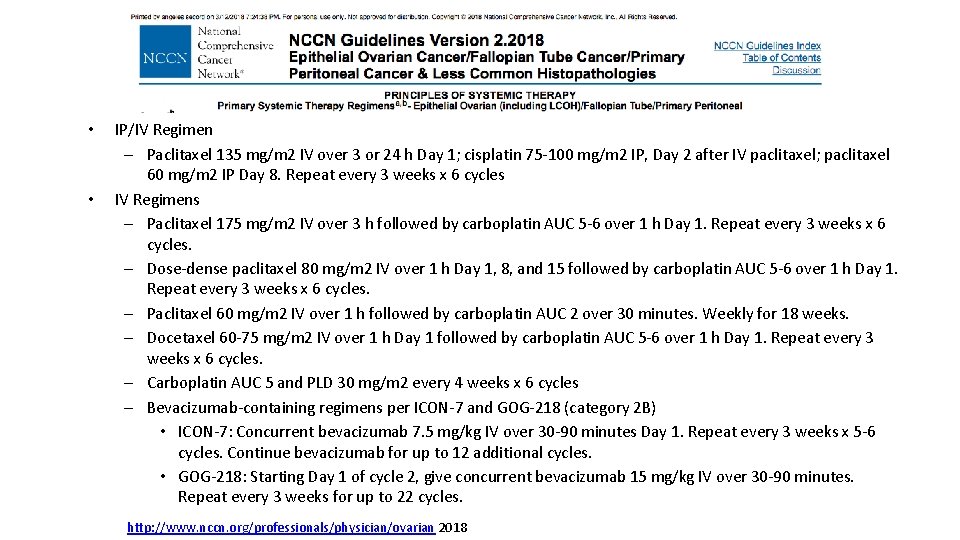
• • IP/IV Regimen – Paclitaxel 135 mg/m 2 IV over 3 or 24 h Day 1; cisplatin 75 -100 mg/m 2 IP, Day 2 after IV paclitaxel; paclitaxel 60 mg/m 2 IP Day 8. Repeat every 3 weeks x 6 cycles IV Regimens – Paclitaxel 175 mg/m 2 IV over 3 h followed by carboplatin AUC 5 -6 over 1 h Day 1. Repeat every 3 weeks x 6 cycles. – Dose-dense paclitaxel 80 mg/m 2 IV over 1 h Day 1, 8, and 15 followed by carboplatin AUC 5 -6 over 1 h Day 1. Repeat every 3 weeks x 6 cycles. – Paclitaxel 60 mg/m 2 IV over 1 h followed by carboplatin AUC 2 over 30 minutes. Weekly for 18 weeks. – Docetaxel 60 -75 mg/m 2 IV over 1 h Day 1 followed by carboplatin AUC 5 -6 over 1 h Day 1. Repeat every 3 weeks x 6 cycles. – Carboplatin AUC 5 and PLD 30 mg/m 2 every 4 weeks x 6 cycles – Bevacizumab-containing regimens per ICON-7 and GOG-218 (category 2 B) • ICON-7: Concurrent bevacizumab 7. 5 mg/kg IV over 30 -90 minutes Day 1. Repeat every 3 weeks x 5 -6 cycles. Continue bevacizumab for up to 12 additional cycles. • GOG-218: Starting Day 1 of cycle 2, give concurrent bevacizumab 15 mg/kg IV over 30 -90 minutes. Repeat every 3 weeks for up to 22 cycles. http: //www. nccn. org/professionals/physician/ovarian 2018

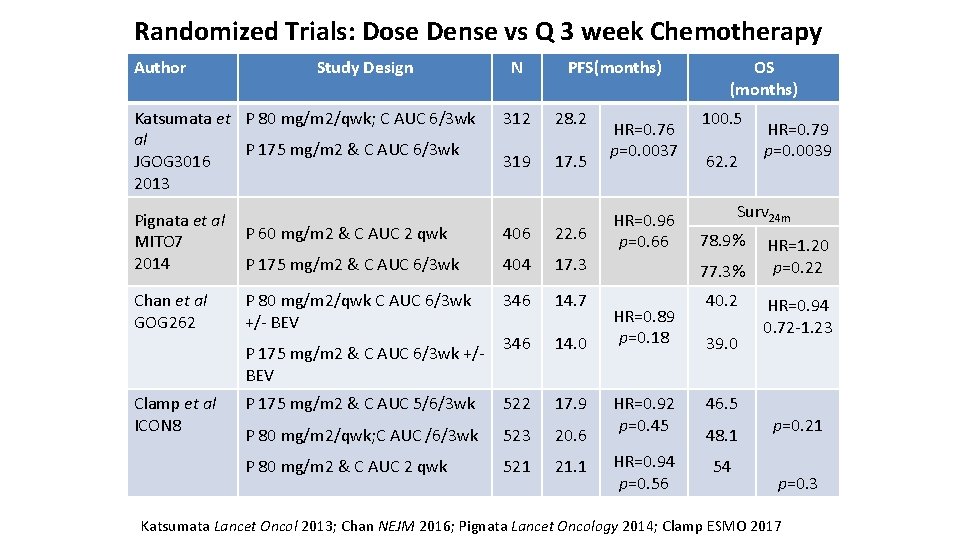
Randomized Trials: Dose Dense vs Q 3 week Chemotherapy Author Study Design Katsumata et P 80 mg/m 2/qwk; C AUC 6/3 wk al P 175 mg/m 2 & C AUC 6/3 wk JGOG 3016 2013 Pignata et al · P 60 mg/m 2 & C AUC 2 qwk MITO 7 ·P 175 mg/m 2 & C AUC 6/3 wk 2014 Chan et al GOG 262 PFS(months) 312 28. 2 319 17. 5 HR=0. 76 p=0. 0037 HR=0. 96 p=0. 66 OS (months) 100. 5 62. 2 22. 6 404 17. 3 77. 3% 346 14. 7 40. 2 346 14. 0 P 175 mg/m 2 & C AUC 5/6/3 wk 522 17. 9 P 80 mg/m 2/qwk; C AUC /6/3 wk 523 20. 6 P 80 mg/m 2 & C AUC 2 qwk 521 21. 1 P 80 mg/m 2/qwk C AUC 6/3 wk +/- BEV HR=0. 89 p=0. 18 HR=0. 79 p=0. 0039 Surv 24 m 406 P 175 mg/m 2 & C AUC 6/3 wk +/BEV Clamp et al ICON 8 N 78. 9% 39. 0 HR=0. 92 p=0. 45 46. 5 HR=0. 94 p=0. 56 54 48. 1 HR=1. 20 p=0. 22 HR=0. 94 0. 72 -1. 23 p=0. 21 p=0. 3 Katsumata Lancet Oncol 2013; Chan NEJM 2016; Pignata Lancet Oncology 2014; Clamp ESMO 2017



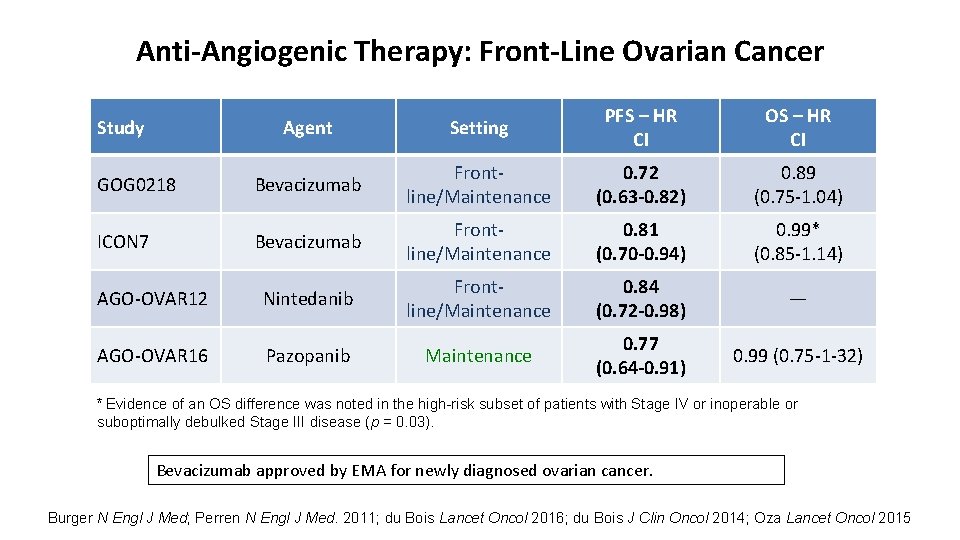
Anti-Angiogenic Therapy: Front-Line Ovarian Cancer Agent Setting PFS – HR CI OS – HR CI GOG 0218 Bevacizumab Frontline/Maintenance 0. 72 (0. 63 -0. 82) 0. 89 (0. 75 -1. 04) ICON 7 Bevacizumab Frontline/Maintenance 0. 81 (0. 70 -0. 94) 0. 99* (0. 85 -1. 14) AGO-OVAR 12 Nintedanib Frontline/Maintenance 0. 84 (0. 72 -0. 98) — AGO-OVAR 16 Pazopanib Maintenance 0. 77 (0. 64 -0. 91) 0. 99 (0. 75 -1 -32) Study * Evidence of an OS difference was noted in the high-risk subset of patients with Stage IV or inoperable or suboptimally debulked Stage III disease (p = 0. 03). Bevacizumab approved by EMA for newly diagnosed ovarian cancer. Burger N Engl J Med; Perren N Engl J Med. 2011; du Bois Lancet Oncol 2016; du Bois J Clin Oncol 2014; Oza Lancet Oncol 2015

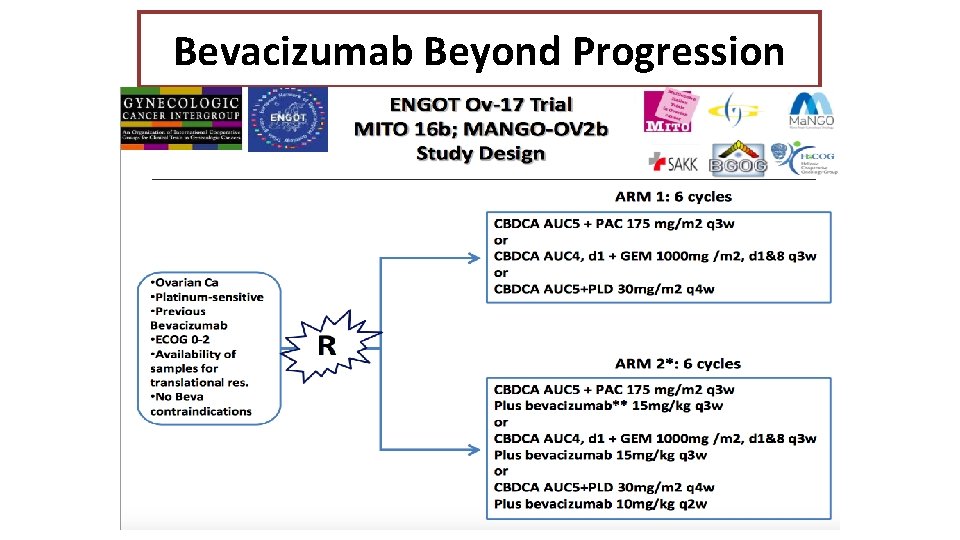
Bevacizumab Beyond Progression

The Future: Current Phase III Front-Line Ovarian Cancer Trials • PARPi – SOLO 1 – Olaparib BRCAm OC – GOG 3005 Veliparib – PRIMA -Niraparib • Immunotherapy – JAVELIN OVARIAN 100 – Chemo +/- Avelumab followed by maintenance • Combination Therapy – PAOLA-1 – Chemo + Bevacizumab followed by maintenance Bevacizumab +/- Olaparib – IMagyn 050 – Chemo + Bevacizumab +/- Atezolizumab followed by maintenance Bevacizumab +/Atezolizumab – FIRST – Chemo +/- Bevacizumab +/- TSR 042 followed by maintenance Placebo +/- Bevacizumab vs Niraparib + TSR 042 +/- Bevacizumab – DUO – anti-VEGF and IO strategy – ATHENA – Rucaparib + Nivolumab maintenance