Physical Properties Physical Property a characteristic of a





- Slides: 18


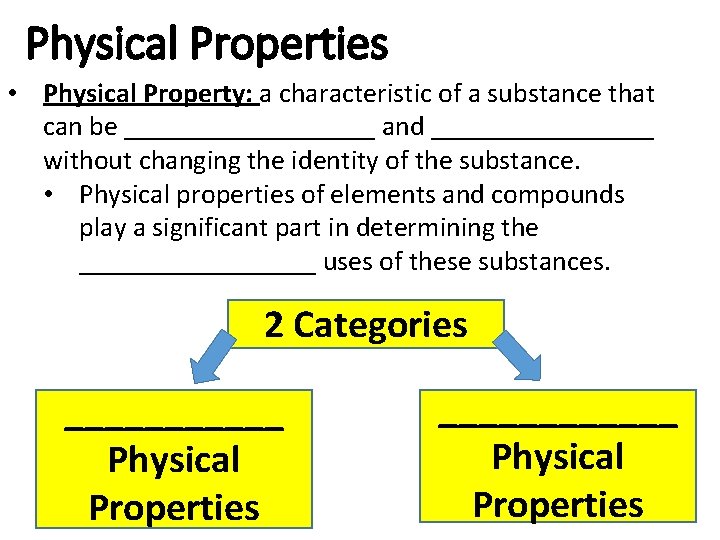
Physical Properties • Physical Property: a characteristic of a substance that can be _________ and ________ without changing the identity of the substance. • Physical properties of elements and compounds play a significant part in determining the _________ uses of these substances. 2 Categories ______ Physical Properties

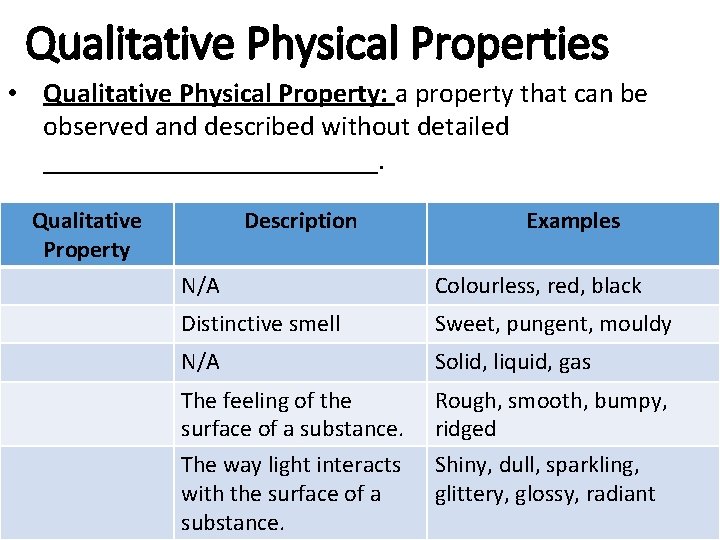
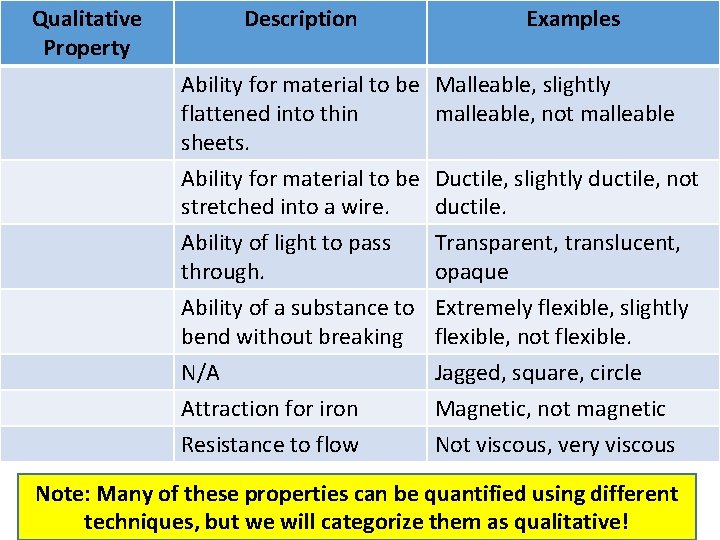
Qualitative Physical Properties • Qualitative Physical Property: a property that can be observed and described without detailed ____________. Qualitative Property Description Examples N/A Colourless, red, black Distinctive smell Sweet, pungent, mouldy N/A Solid, liquid, gas The feeling of the surface of a substance. The way light interacts with the surface of a substance. Rough, smooth, bumpy, ridged Shiny, dull, sparkling, glittery, glossy, radiant

Qualitative Property Description Examples Ability for material to be Malleable, slightly flattened into thin malleable, not malleable sheets. Ability for material to be stretched into a wire. Ability of light to pass through. Ductile, slightly ductile, not ductile. Transparent, translucent, opaque Ability of a substance to bend without breaking N/A Attraction for iron Extremely flexible, slightly flexible, not flexible. Jagged, square, circle Magnetic, not magnetic Resistance to flow Not viscous, very viscous Note: Many of these properties can be quantified using different techniques, but we will categorize them as qualitative!

Qualitative Properties of Paper Clips 1) Describe six qualitative physical properties of your paper clip. ________________________ ________________________ 2) What provides your paper clip with many of the properties that you observed? ____________________________________________________ 3) Think of what a paper clip is used for. How are the properties that you listed related to the function of the paper clip? ____________________________________________________

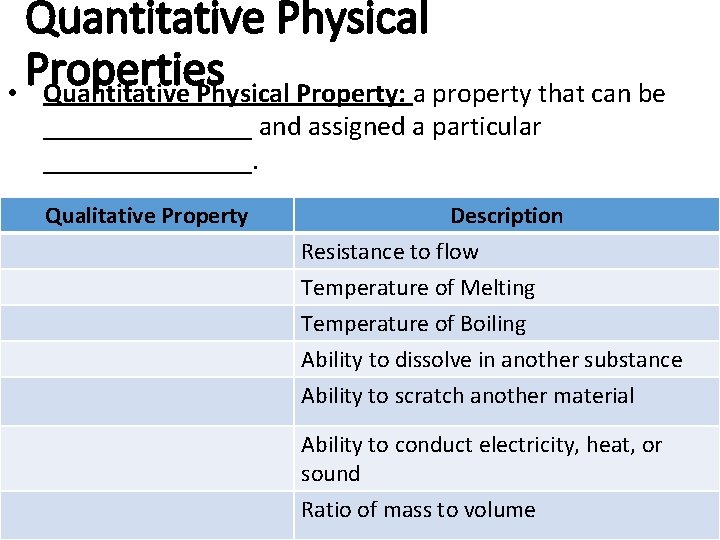
Quantitative Physical Properties • Quantitative Physical Property: a property that can be ________ and assigned a particular ________. Qualitative Property Description Resistance to flow Temperature of Melting Temperature of Boiling Ability to dissolve in another substance Ability to scratch another material Ability to conduct electricity, heat, or sound Ratio of mass to volume

Slow as Molasses – Quick Activity Materials: • 2 beakers of the same size • 2 scoopulas • Water • • • Molasses Medicine droppers Stopwatch Procedure: 1. You and your partner should each place your scoopula into your beaker. Position the scoopula so the wider end is at the top and so it rests against the side of the beaker. 2. Get the stopwatch ready! Add a drop of water to the wide end of one scoopula. Time and record how long it takes for the water to hit the side of the beaker. _____ 3. Repeat step 2 with the molasses. _____ Discussion: 1. What physical property of molasses does the expression “slow as molasses” refer to? __________ 2. What is another way you could measure the difference between the viscosities of water and molasses? _____________________________________________________

Let’s take a closer look at the following physical properties: 1) States of Matter 2) Solubility 3) Hardness 4) Conductivity 5) Density

1) States of Matter • Different substances change states at different temperatures. For example, water boils at ______ whereas helium boils at -269°C. • Substance that sublimates: Solid Carbon Dioxide (Dry Ice) to “fog. ” • Substance that undergoes deposition: water vapour to ice in cloud.

2) Solubility • Measuring Solubility: the maximum quantity of a substance that can ______ in a given amount of solvent at a particular temperature and pressure. • Expressed as a ________: • Mass of Solute / Mass of Solvent • Mass of Solute / Volume of Solvent • Example: salt (sodium chloride) has a solubility of 39. 5 g/ 100 m. L at 25°C and atmospheric pressure. Aqueous Solution: a solution where water is the solvent A substance with very low solubility is described as ____________.

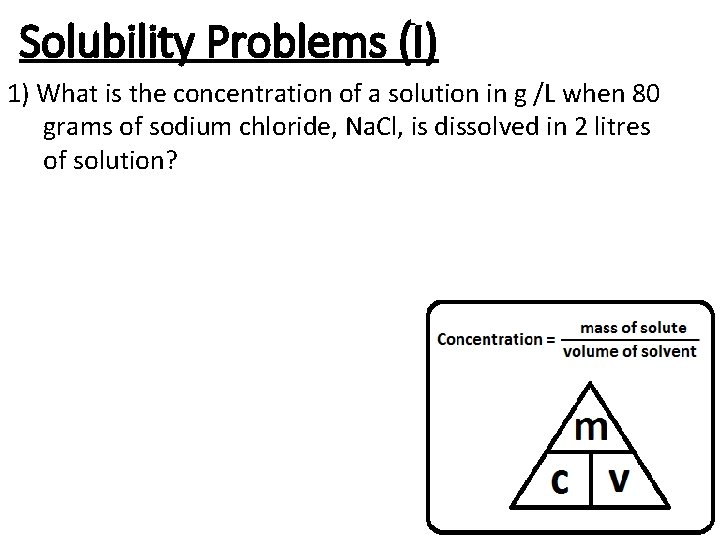
Solubility Problems (I) 1) What is the concentration of a solution in g /L when 80 grams of sodium chloride, Na. Cl, is dissolved in 2 litres of solution?

Solubility Problems (II) 2) At 20°C and atmospheric pressure, the maximum amount of table sugar that can dissolve into 100 m. L of water is 179 grams. What is the solubility of table sugar?

Solubility Problems (III) 3) The concentration of an aqueous solution is 6. 2 g /L. The volume of the solvent was 3. 5 L. How much solute was dissolved into the solvent?

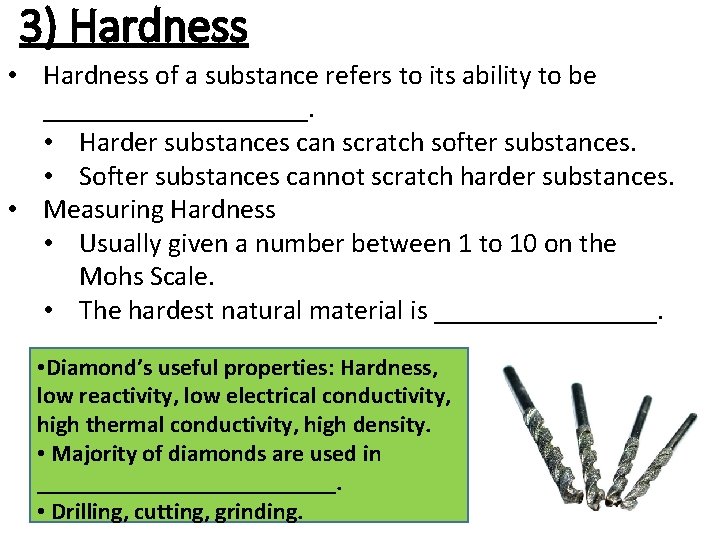
3) Hardness • Hardness of a substance refers to its ability to be __________. • Harder substances can scratch softer substances. • Softer substances cannot scratch harder substances. • Measuring Hardness • Usually given a number between 1 to 10 on the Mohs Scale. • The hardest natural material is ________. • Diamond’s useful properties: Hardness, low reactivity, low electrical conductivity, high thermal conductivity, high density. • Majority of diamonds are used in _____________. • Drilling, cutting, grinding.

4) Conductivity • Can refer to electricity, heat (thermal energy), or sound. • Electrical: • A substance with high electrical conductivity is ___________. A substance with low electrical conductivity is ________. • Thermal: • A substance with high thermal conductivity is _________ (which is why it is useful for cooking). A substance with low • Sound (at 20°C): • Air has a fairly poor ability to conduct sound (344 m/s) • Water conducts sound better at 1, 462 m/s. • Stone has a great ability to conduct sound (6, 000 m/s)

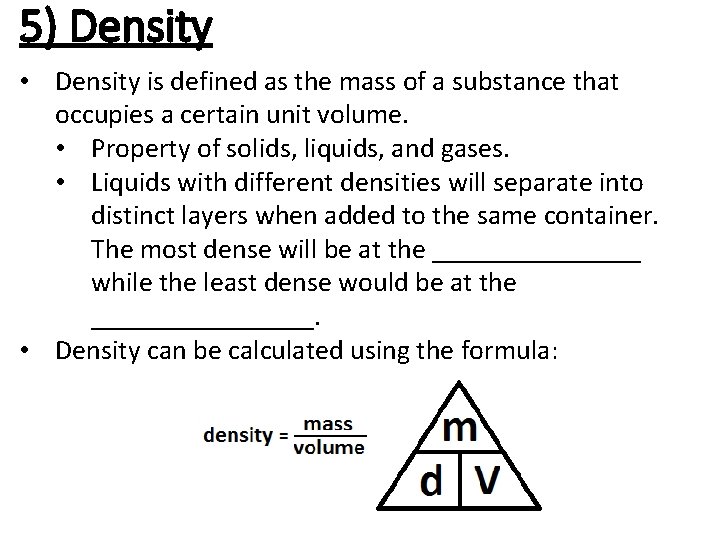
5) Density • Density is defined as the mass of a substance that occupies a certain unit volume. • Property of solids, liquids, and gases. • Liquids with different densities will separate into distinct layers when added to the same container. The most dense will be at the ________ while the least dense would be at the ________. • Density can be calculated using the formula:

Density Problem 4) A sample of silver has a mass of 5. 04 g and a volume of 0. 480 cm 3. What is the density of silver?

Homework 1) See page 158. In your notebook, list the 4 unique properties of water that allow it to support life on Earth. 2) Page 156, #1, 2 (read pg. 154), 3, 4. 3) Page 157, #1 -5. 4) Page 159, #1, 2, 4 – 7.
 Any characteristic of a material that can be observed
Any characteristic of a material that can be observed Characteristic physical property
Characteristic physical property Chemical property definition
Chemical property definition Examples of non characteristic properties
Examples of non characteristic properties What are measurable properties
What are measurable properties Pure substance examples pictures
Pure substance examples pictures Commutative and associative properties
Commutative and associative properties Sapratibandha
Sapratibandha Intensive property and extensive properties
Intensive property and extensive properties What is intensive in chemistry
What is intensive in chemistry Physical properties
Physical properties Physical property of starch
Physical property of starch Blue color physical or chemical
Blue color physical or chemical Physical properties
Physical properties Is making orange juice a physical or chemical change
Is making orange juice a physical or chemical change Whats the difference between a chemical and physical change
Whats the difference between a chemical and physical change Qualitative and quantitative physical properties
Qualitative and quantitative physical properties Which is one physical property that all stars have?
Which is one physical property that all stars have? Physical properties that can be measured
Physical properties that can be measured