PHYS 342 Modern Physics 2 Course Elements 1

















































- Slides: 76

PHYS 342 Modern Physics 2

Course Elements 1. 2. 3. 4. 5. 6. 7. The Schrödinger Equation The Rutherford-Bohr Model of the Atom The Hydrogen Atom in Wave Mechanics Many-Electron Atoms Molecular Structure Statistical Physics Nuclear Structure and Radioactivity

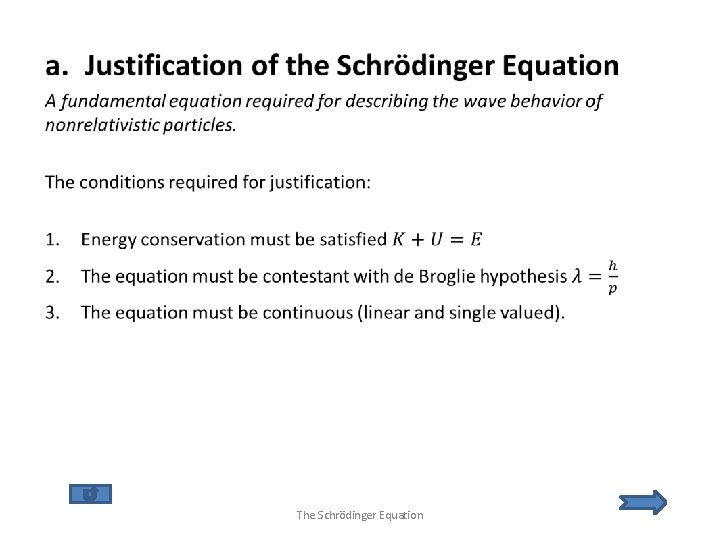
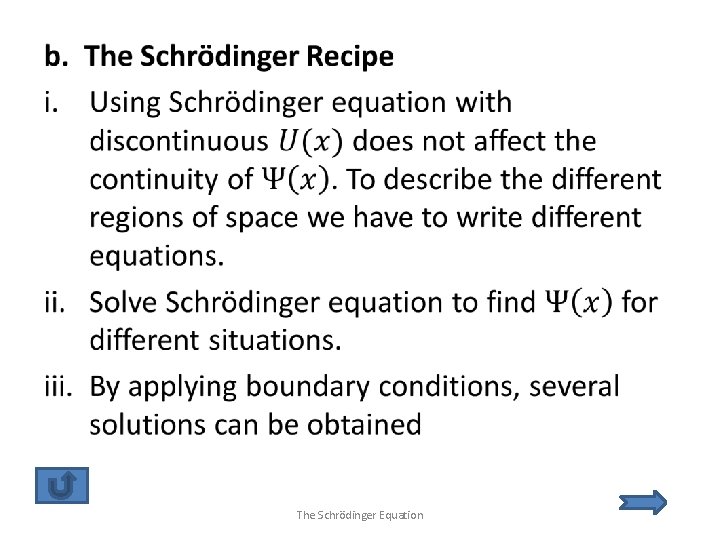
1. The Schrödinger Equation a. b. c. d. e. f. g. Justification of the Schrödinger Equation The Schrödinger Recipe Probabilities and Normalization Applications The Simple Harmonic Oscillator Time Dependence Steps and Barriers

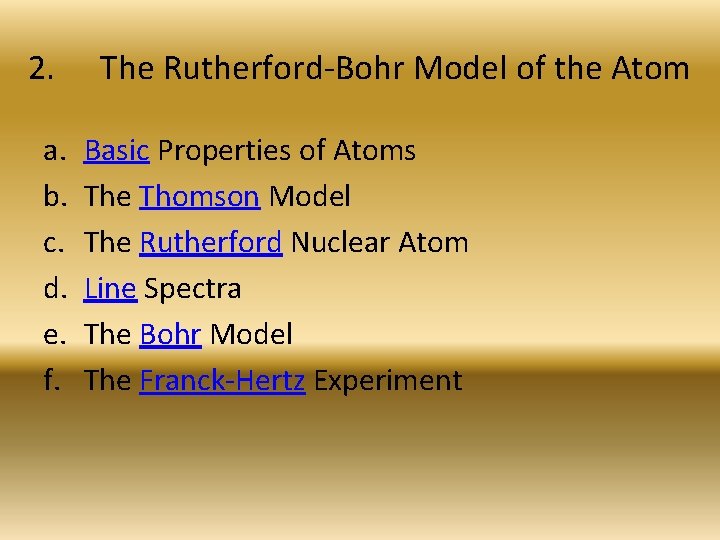
2. a. b. c. d. e. f. The Rutherford-Bohr Model of the Atom Basic Properties of Atoms The Thomson Model The Rutherford Nuclear Atom Line Spectra The Bohr Model The Franck-Hertz Experiment

3. The Hydrogen Atom in Wave Mechanics a. The Schrödinger Equation in Spherical Coordinates b. The Hydrogen Atom Wave Functions c. Radial Probability Densities d. Angular Momentum and Probability Densities e. Intrinsic Spin f. Energy Levels and Spectroscopic Notation g. The Zeeman Effect

4. Many-Electron Atoms a. b. c. d. e. f. g. The Pauli Exclusion Principle Electronic States in Many-Electron Atoms The Periodic Table Properties of the Elements X-Rays Optical Spectra Lasers

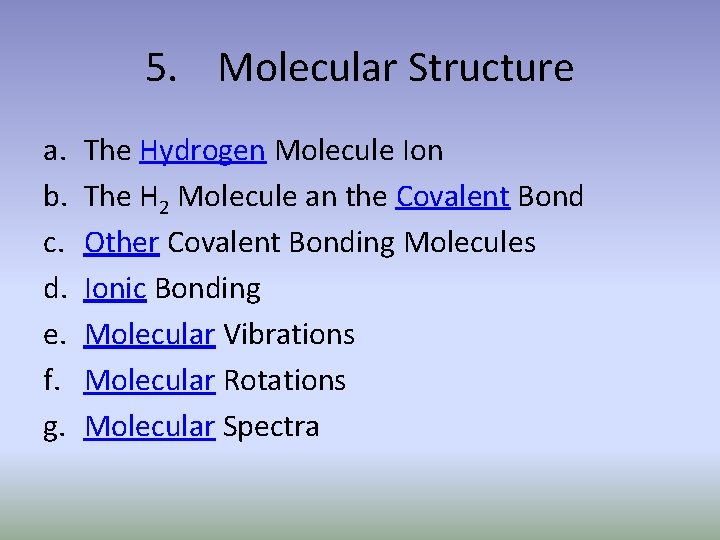
5. Molecular Structure a. b. c. d. e. f. g. The Hydrogen Molecule Ion The H 2 Molecule an the Covalent Bond Other Covalent Bonding Molecules Ionic Bonding Molecular Vibrations Molecular Rotations Molecular Spectra

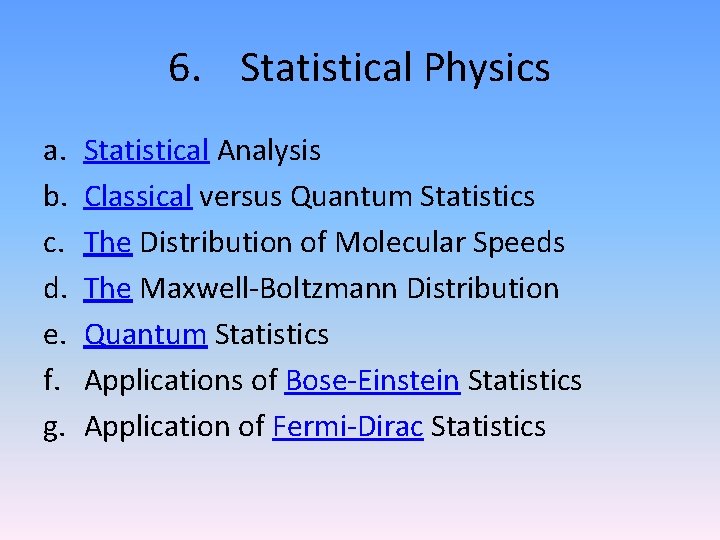
6. Statistical Physics a. b. c. d. e. f. g. Statistical Analysis Classical versus Quantum Statistics The Distribution of Molecular Speeds The Maxwell-Boltzmann Distribution Quantum Statistics Applications of Bose-Einstein Statistics Application of Fermi-Dirac Statistics

7. Nuclear Structure and Radioactivity a. b. c. d. e. f. g. h. i. Nuclear constituents Nuclear Sizes and Shapes Nuclear Masses and Binding Energies The Nuclear Force Radioactive Decay Conservation Laws in Radioactive Decay Alpha Decay Gamma Decay Natural Radioactivity

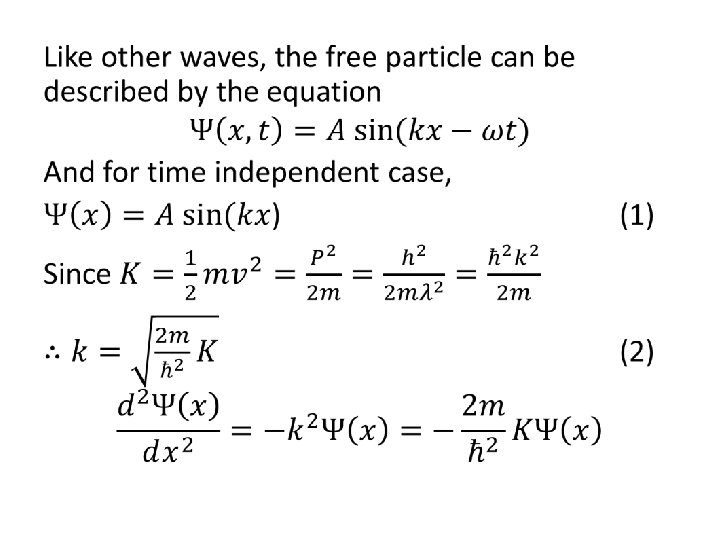
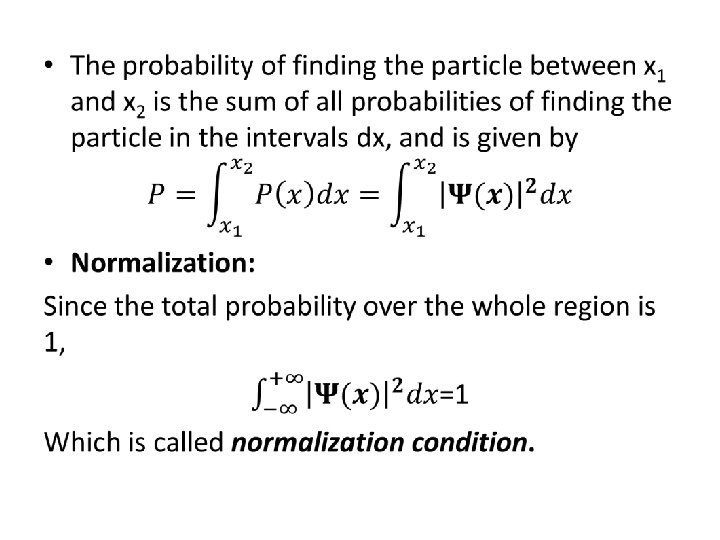
• The Schrödinger Equation



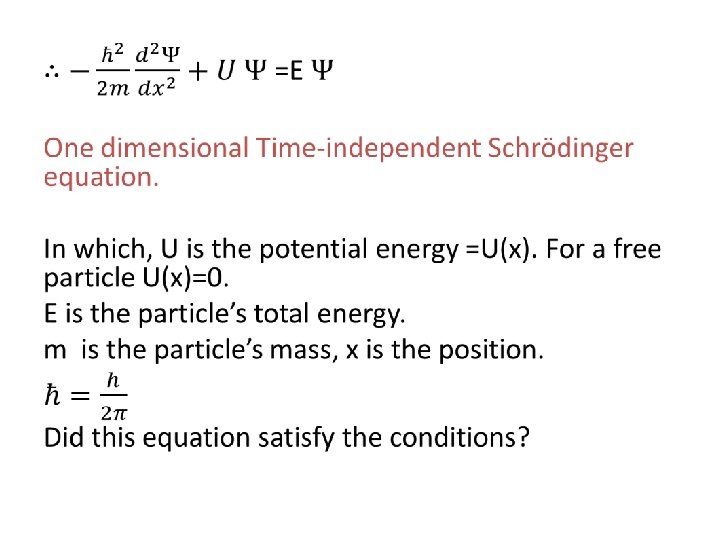
• The Schrödinger Equation

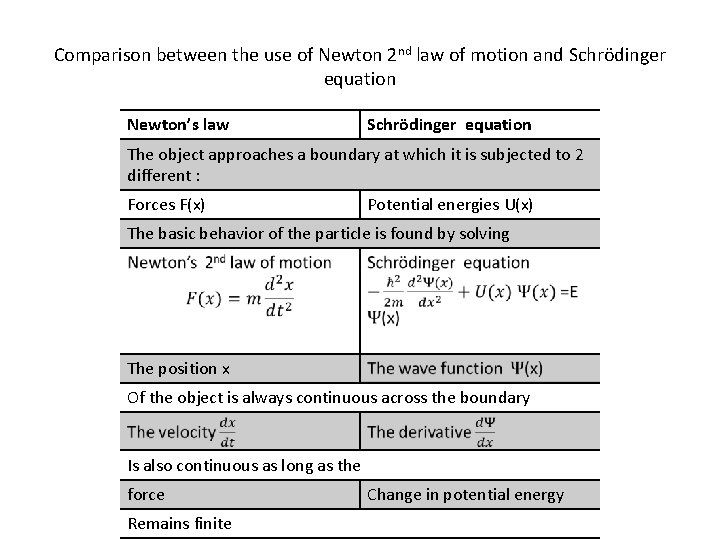
Comparison between the use of Newton 2 nd law of motion and Schrödinger equation Newton’s law Schrödinger equation The object approaches a boundary at which it is subjected to 2 different : Forces F(x) Potential energies U(x) The basic behavior of the particle is found by solving The position x Of the object is always continuous across the boundary Is also continuous as long as the force Remains finite Change in potential energy

• The Schrödinger Equation





• The Schrödinger Equation






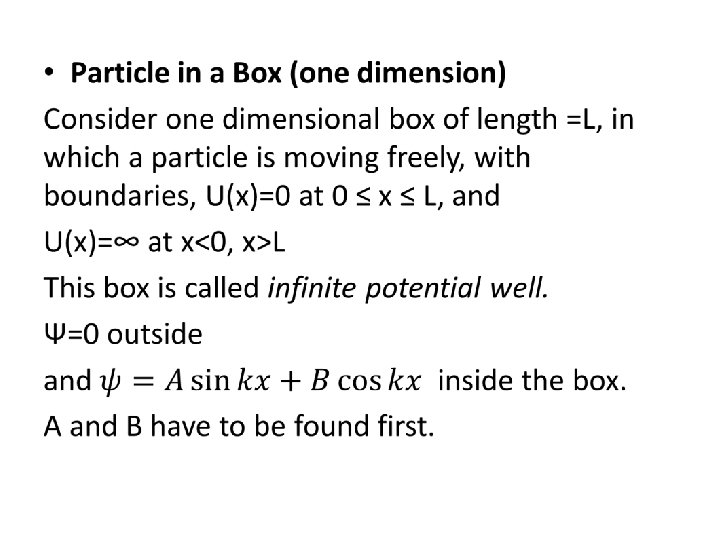
Example 5. 2 An electron is trapped in a one-dimensional region of length 1 X 10 -10 m. a. how much energy must be supplied to excite the electron from the ground state to the first excited state? (111 e. V) b. In the ground state, what is the probability of finding the electron in the region from x=0. 09 X 10 -10 m to 0. 11 X 10 -10 m. (0. 38%) c. in the first excited state, find the probability of finding the electron between x=0 and x=0. 25 X 10 -10 m. (0. 25) Example 5. 3

• Solution

• An electron is trapped in an infinitely deep potential well of width L = 106 fm. Calculate the wavelength of photon emitted from the transition E 4 → E 3. (472 nm)

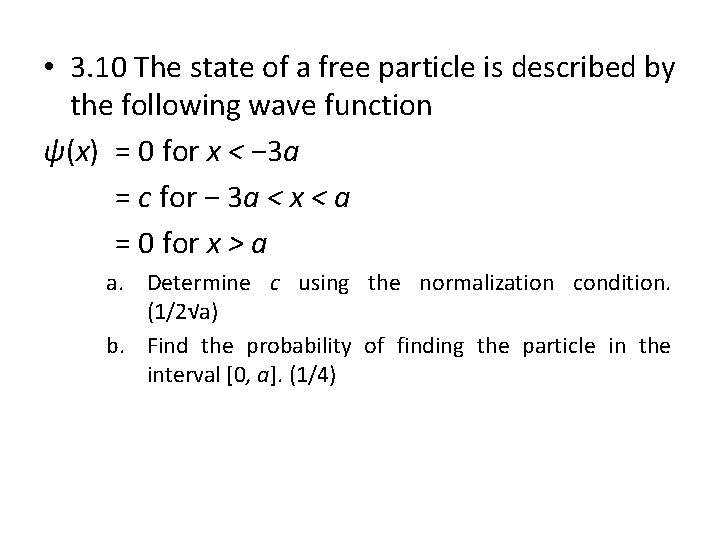
• 3. 10 The state of a free particle is described by the following wave function ψ(x) = 0 for x < − 3 a = c for − 3 a < x < a = 0 for x > a a. Determine c using the normalization condition. (1/2√a) b. Find the probability of finding the particle in the interval [0, a]. (1/4)

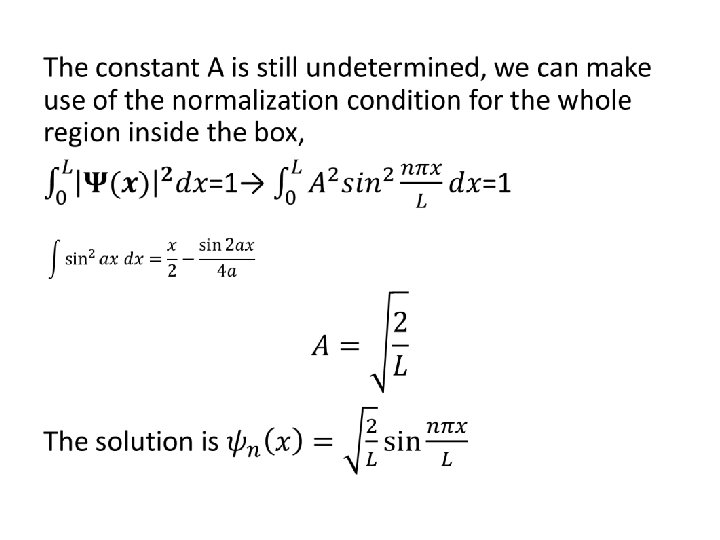
Example 5. 3 • Show that the average value of x is L/2, independent of the quantum state.

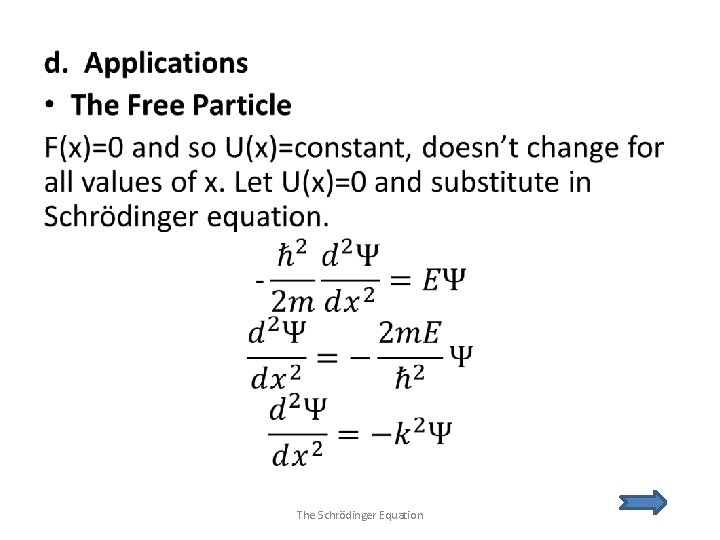
e. The Simple Harmonic Oscillator The Schrödinger Equation

f. Time Dependence The Schrödinger Equation

g. Steps and Barriers The Schrödinger Equation

a. Basic Properties of Atoms The Rutherford-Bohr Model of the Atom

b. The Thomson Model The Rutherford-Bohr Model of the Atom

c. The Rutherford Nuclear Atom The Rutherford-Bohr Model of the Atom

d. Line Spectra The Rutherford-Bohr Model of the Atom

e. The Bohr Model The Rutherford-Bohr Model of the Atom

f. The Franck-Hertz Experiment The Rutherford-Bohr Model of the Atom

a. The Schrödinger Equation in Spherical Coordinates 3. The Hydrogen Atom in Wave Mechanics

b. The Hydrogen Atom Wave Functions 3. The Hydrogen Atom in Wave Mechanics

c. Radial Probability Densities 3. The Hydrogen Atom in Wave Mechanics

d. Angular Momentum and Probability Densities 3. The Hydrogen Atom in Wave Mechanics

e. Intrinsic Spin 3. The Hydrogen Atom in Wave Mechanics

f. Energy Levels and Spectroscopic Notation 3. The Hydrogen Atom in Wave Mechanics

g. The Zeeman Effect 3. The Hydrogen Atom in Wave Mechanics

a. The Pauli Exclusion Principle 4. Many-Electron Atoms

b. Electronic States in Many-Electron Atoms 4. Many-Electron Atoms

c. The Periodic Table 4. Many-Electron Atoms

d. Properties of the Elements 4. Many-Electron Atoms

e. X-Rays 4. Many-Electron Atoms

f. Optical Spectra 4. Many-Electron Atoms

g. Lasers 4. Many-Electron Atoms

a. The Hydrogen Molecule Ion 5. Molecular Structure

b. The H 2 Molecule an the Covalent Bond 4. Many-Electron Atoms

c. Other Covalent Bonding Molecules 4. Many-Electron Atoms

d. Ionic Bonding 4. Many-Electron Atoms

e. Molecular Vibrations 4. Many-Electron Atoms

f. Molecular Rotations 4. Many-Electron Atoms

g. Molecular Spectra 4. Many-Electron Atoms

a. Statistical Analysis 6. Statistical Physics

b. Classical versus Quantum Statistics 6. Statistical Physics

c. The Distribution of Molecular Speeds 6. Statistical Physics

d. The Maxwell-Boltzmann Distribution 6. Statistical Physics

e. Quantum Statistics 6. Statistical Physics

f. Applications of Bose-Einstein Statistics 6. Statistical Physics

g. Application of Fermi-Dirac Statistics 6. Statistical Physics

a. Nuclear constituents 7. Nuclear Structure and Radioactivity

b. Nuclear Sizes and Shapes 7. Nuclear Structure and Radioactivity

c. Nuclear Masses and Binding Energies 7. Nuclear Structure and Radioactivity

d. The Nuclear Force 7. Nuclear Structure and Radioactivity

e. Radioactive Decay 7. Nuclear Structure and Radioactivity

f. Conservation Laws in Radioactive Decay 7. Nuclear Structure and Radioactivity

g. Alpha Decay 7. Nuclear Structure and Radioactivity

h. Gamma Decay 7. Nuclear Structure and Radioactivity

i. Natural Radioactivity 7. Nuclear Structure and Radioactivity