Modern Physics The Nucleus Modern Physics Three main














- Slides: 36

Modern Physics The Nucleus

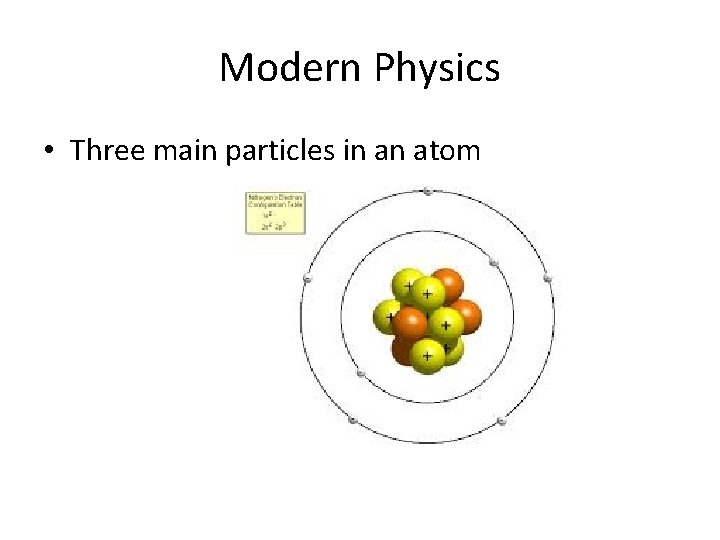
Modern Physics • Three main particles in an atom

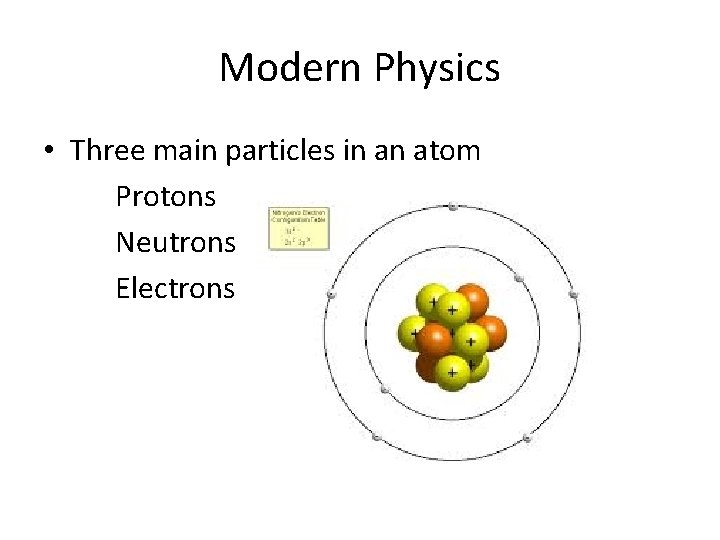
Modern Physics • Three main particles in an atom Protons Neutrons Electrons

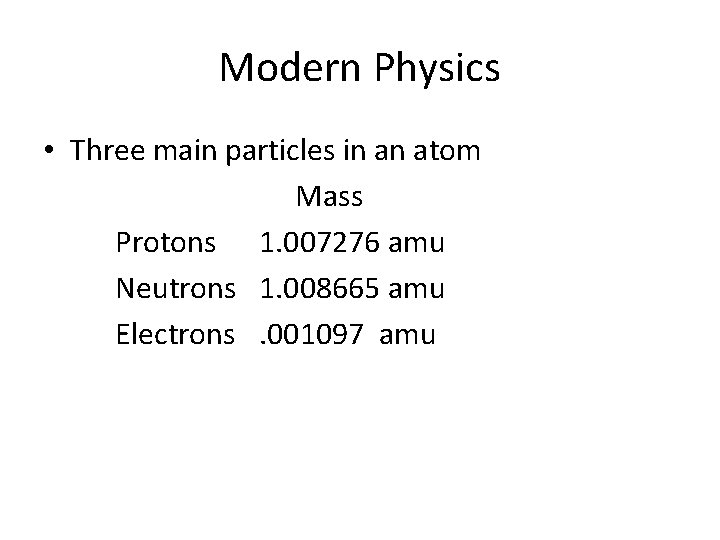
Modern Physics • Three main particles in an atom Mass Protons 1. 007276 amu Neutrons 1. 008665 amu Electrons. 001097 amu

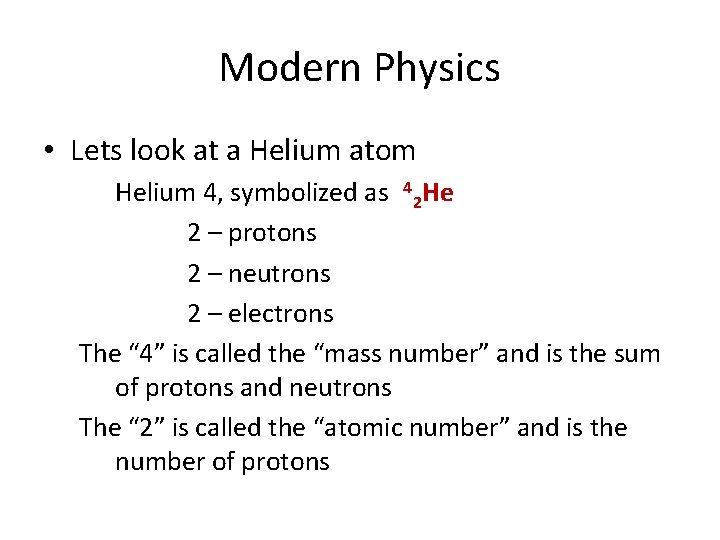
Modern Physics • Lets look at a Helium atom Helium 4, symbolized as 42 He 2 – protons 2 – neutrons 2 – electrons The “ 4” is called the “mass number” and is the sum of protons and neutrons The “ 2” is called the “atomic number” and is the number of protons

Modern Physics • Protons have a positive charge + • Neutrons have NO charge • Electrons have a negative charge -

Modern Physics • Protons are in the nucleus • Neutrons are in the nucleus • Electrons are in clouds at different energy levels away from the nucleus

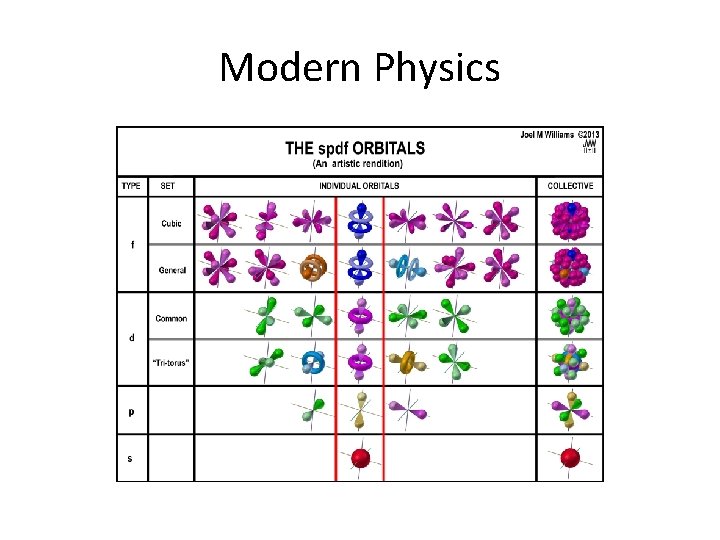
Modern Physics

Modern Physics • Wait, some elements come in different varieties, like Carbon-12 and Carbon 14

Modern Physics • Wait, some elements come in different varieties, like Carbon-12 and Carbon 14 • These are called ISOTOPES Different in the number of neutrons • We identify elements by the number of protons • IF an atom has 6 protons, it is called Carbon

Modern Physics • Carbon comes in two forms • Carbon -12 – 6 protons and 6 neutron • Carbon -14 – 6 protons and 8 neutrons • Carbon -12 is the most common • Carbon -14 is less common and is called an ISOTOPE of Carbon

Modern Physics • Lets go back to the main particles

Modern Physics • • • Protons have a positive charge + Neutrons have NO charge Electrons have a negative charge – Remember like charges repel SO If the nucleus is made up of Positively charged protons and neutral neutrons – why does it stay together? ? Shouldn’t it fly apart?

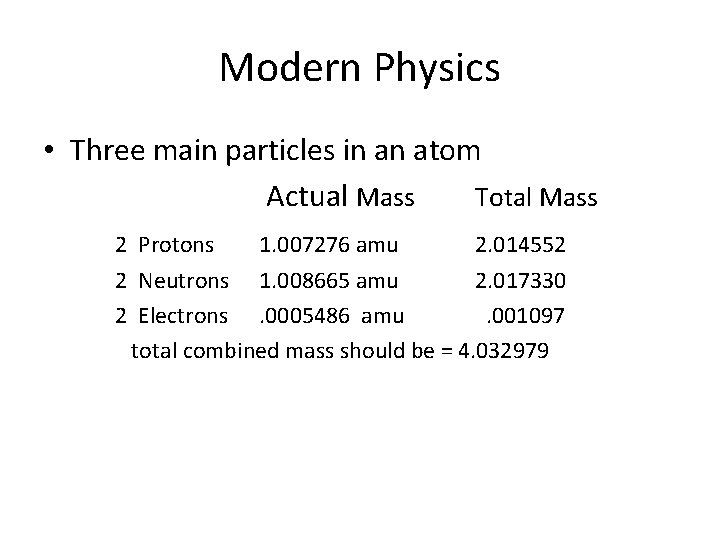
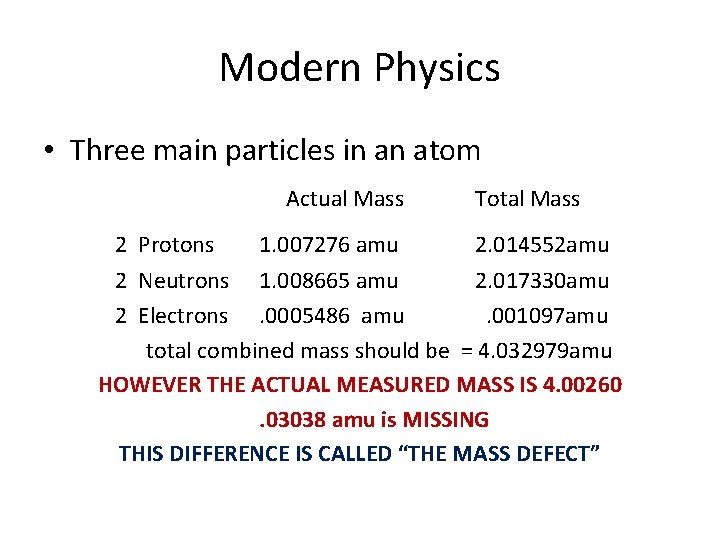
Modern Physics • Three main particles in an atom Actual Mass Total Mass 2 Protons 1. 007276 amu 2. 014552 2 Neutrons 1. 008665 amu 2. 017330 2 Electrons. 0005486 amu. 001097 total combined mass should be = 4. 032979

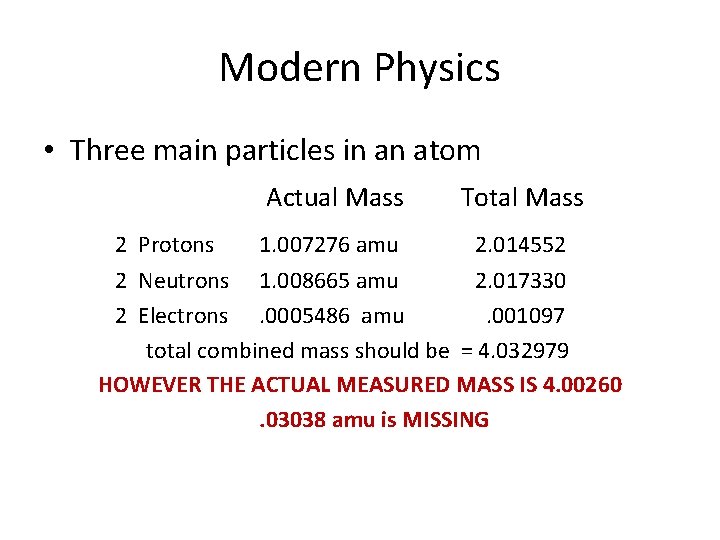
Modern Physics • Three main particles in an atom 2 Protons Actual Mass Total Mass 1. 007276 amu 2. 014552 2 Neutrons 1. 008665 amu 2. 017330 2 Electrons. 0005486 amu. 001097 total combined mass should be = 4. 032979 HOWEVER THE ACTUAL MEASURED MASS IS 4. 00260. 03038 amu is MISSING

Modern Physics • Three main particles in an atom Actual Mass 2 Protons 1. 007276 amu Total Mass 2. 014552 amu 2 Neutrons 1. 008665 amu 2. 017330 amu 2 Electrons. 0005486 amu. 001097 amu total combined mass should be = 4. 032979 amu HOWEVER THE ACTUAL MEASURED MASS IS 4. 00260. 03038 amu is MISSING THIS DIFFERENCE IS CALLED “THE MASS DEFECT”

Modern Physics • Where did the mass go? ? ?

Modern Physics • Where did the mass go? ? ? • E=mc 2 • Mass was changed into the BINDING energy to hold together the positively charged protons • This is called the “Nuclear FORCE”

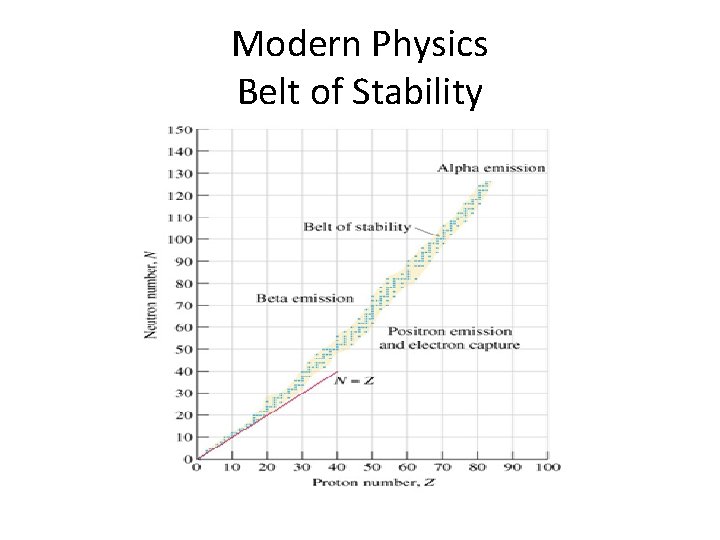
Modern Physics • The Nuclear Force must be strong enough to hold together the positively charged protons • For smaller atoms, an equal number of protons and neutrons generate a sufficient Nuclear Force to hold the atoms together • For larger atoms, more neutrons are needed to generate a sufficient Nuclear Force

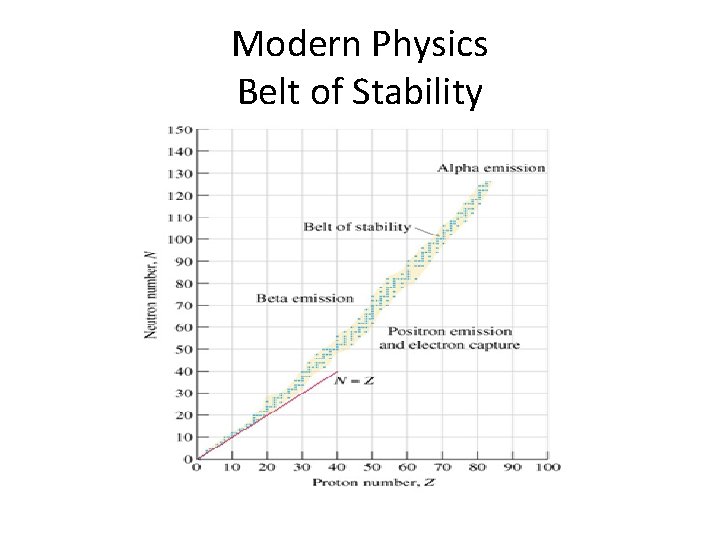
Modern Physics Belt of Stability

Modern Physics • Atoms are stable if they are inside the Belt of Stability • Atoms are unstable if they are outside the Belt of Stability • An unstable atom is said to be RADIOACTIVE

Modern Physics • Are all ISOTOPES radioactive? ?

Modern Physics • Are all ISOTOPES radioactive? ? • NO, only the ones outside of the belt of stability • Lets take a look at Carbon -12 and Carbon 14

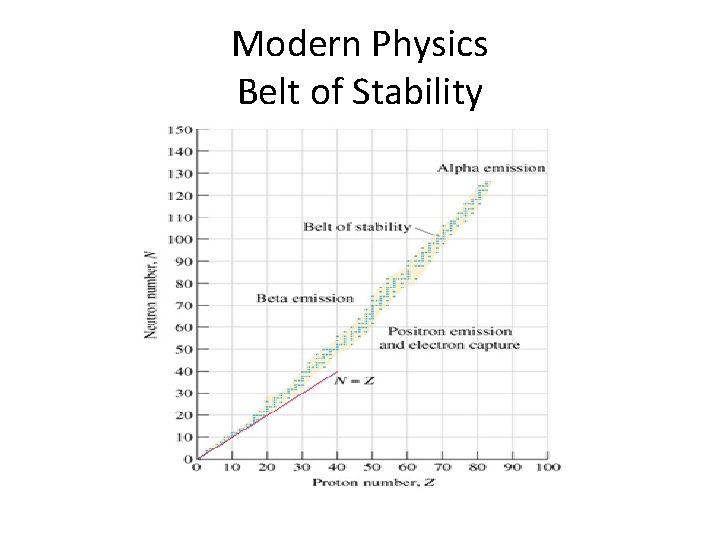
Modern Physics Belt of Stability

Modern Physics • Carbon – 12 is stable with 6 protons and 6 neutrons • Carbon – 14 is unstable with 6 protons and 8 neutrons

Modern Physics • Carbon – 12 is stable with 6 protons and 6 neutrons • Carbon – 14 is unstable with 6 protons and 8 neutrons • Carbon -14 is RADIOACTIVE

Modern Physics • Carbon – 12 is stable with 6 protons and 6 neutrons • Carbon – 14 is unstable with 6 protons and 8 neutrons • Carbon -14 is RADIOACTIVE • Carbon – 14 occurs naturally in our environment – a certain percentage of our bodies contain carbon -14

Modern Physics • Carbon -14 dating

Modern Physics • Carbon -14 dating • All living things have a mixture of Carbon -12 and Carbon -14 atoms incorporated into our living cells • Carbon – 14 is always slowly decaying into Carbon -12 • The ratio of Carbon-12 to Carbon -14 in the cells of living things is representative of the environment

Modern Physics • When an organism is living, the carbon in our bodies is constantly exchanging with carbon in our environment • Therefore the ratio of Carbon-12 and Carbon 14 in our cells reflects that of the environment

Modern Physics • However, when an organism dies, the exchange with the environment stops • The carbon-14 in the dead organism continues to decay and is not replaced with an exchange with the environment • This changes the ratio of Carbon-12 to Carbon -14 in the dead organism’s cells

Modern Physics • We know how fast Carbon -14 decays, therefore by measuring how much is missing we can determine how long the exchange of carbon with the environment has been stopped i. e. when the organism died • This allows us to use Carbon-14 dating to tell how old things are

Modern Physics • Mesa Verde cliff dwellings have been dated using Carbon -14 dating

Modern Physics • By taking samples of the cedar wood beams and measuring the ratios of Carbon -12 to Carbon 14 atoms it was determined that the tree died (was cut down) between 900 and 1300 AD (over 1000 years ago) • Therefore we believe this is the time the cliff dwellings were built

Modern Physics • Carbon dating can only be used on previously living things • Cannot be used to measure the age of rocks • Other similar dating systems exist that use radioactive isotopes and their decay rates

Modern Physics Belt of Stability