PHYS 172 Modern Mechanics Lecture 14 Energy Quantization







- Slides: 23

PHYS 172: Modern Mechanics Lecture 14 – Energy Quantization Summer 2012 Read 8. 1 -8. 8

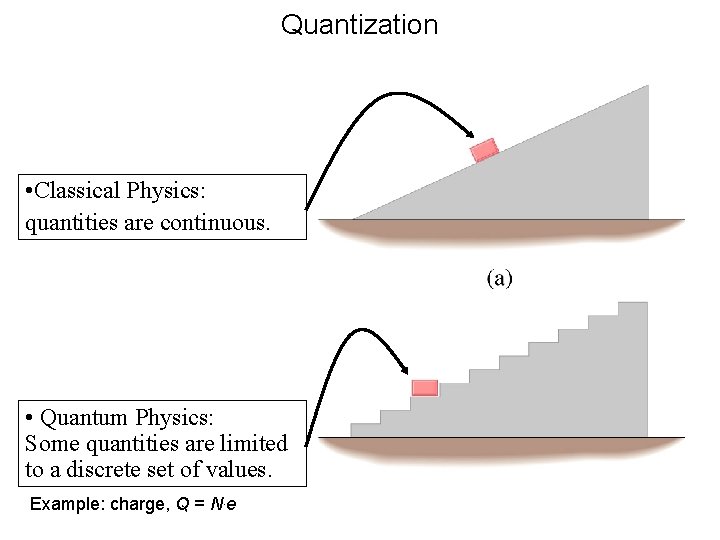
Quantization • Classical Physics: quantities are continuous. • Quantum Physics: Some quantities are limited to a discrete set of values. Example: charge, Q = N. e

Quantum means quantized Answers come in whole numbers Example: The number of unopened Coke cans in your refrigerator is quantized.

Quantum Waves are Quantized There are discrete vibrational modes (normal modes) 1 D: One Dimension Violin string, jumprope 2 D: Two Dimensions Modes of a drumhead, coffee sloshing in your mug http: //demonstrations. wolfram. com/Normal. Modes. Of. ACircular. Drum. Head/ 3 D: Three Dimensions Electron Waves around Atomic Nuclei! http: //www. daugerresearch. com/orbitals/index. shtml Higher Frequency = Higher Energy

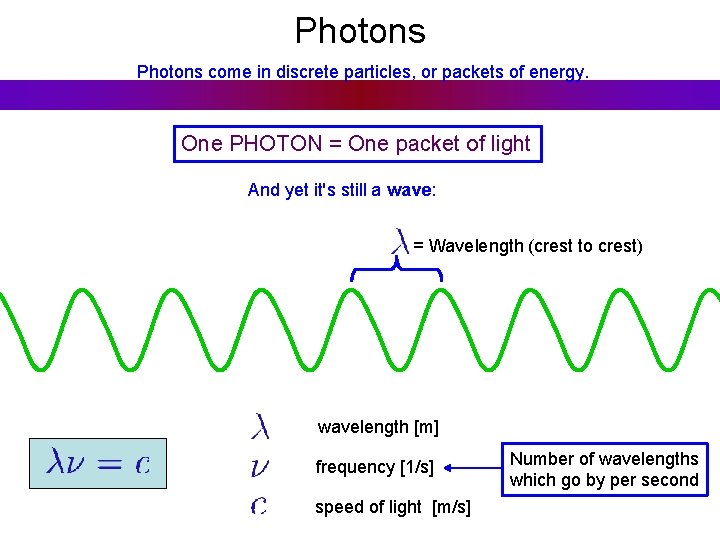
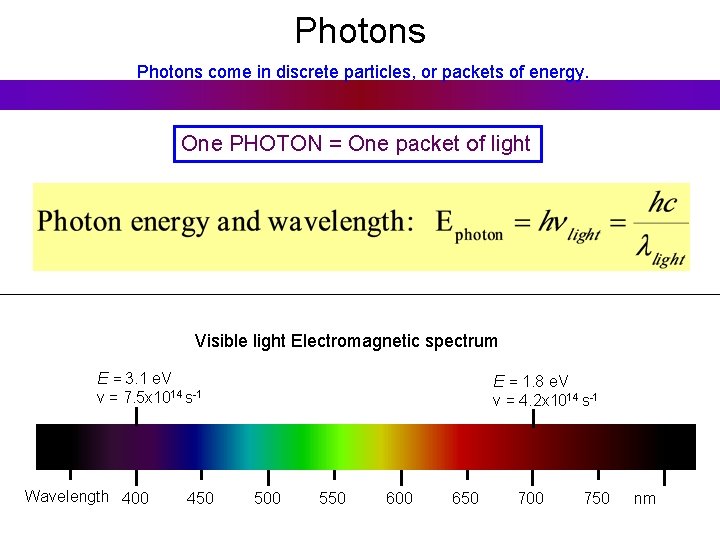
Photons come in discrete particles, or packets of energy. One PHOTON = One packet of light And yet it's still a wave: = Wavelength (crest to crest) wavelength [m] frequency [1/s] speed of light [m/s] Number of wavelengths which go by per second

Photons come in discrete particles, or packets of energy. One PHOTON = One packet of light Visible light Electromagnetic spectrum E = 3. 1 e. V ν = 7. 5 x 1014 s-1 Wavelength 400 450 E = 1. 8 e. V ν = 4. 2 x 1014 s-1 500 550 600 650 700 750 nm

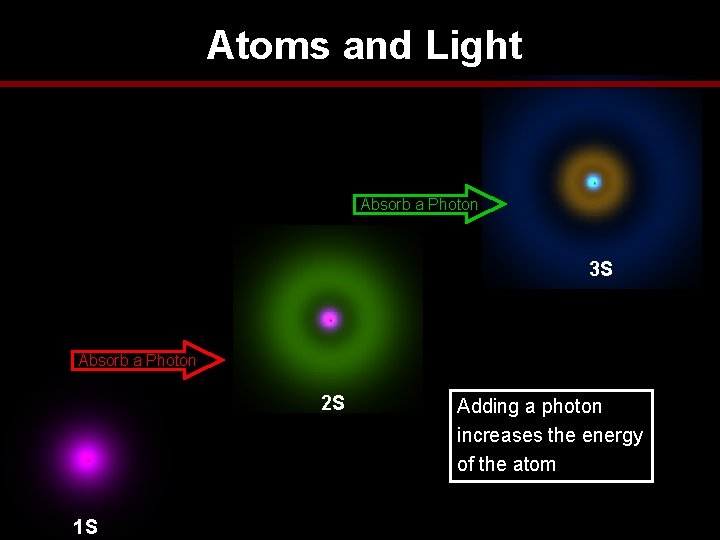
Atoms and Light Absorb a Photon 3 S Absorb a Photon 2 S 1 S Adding a photon increases the energy of the atom

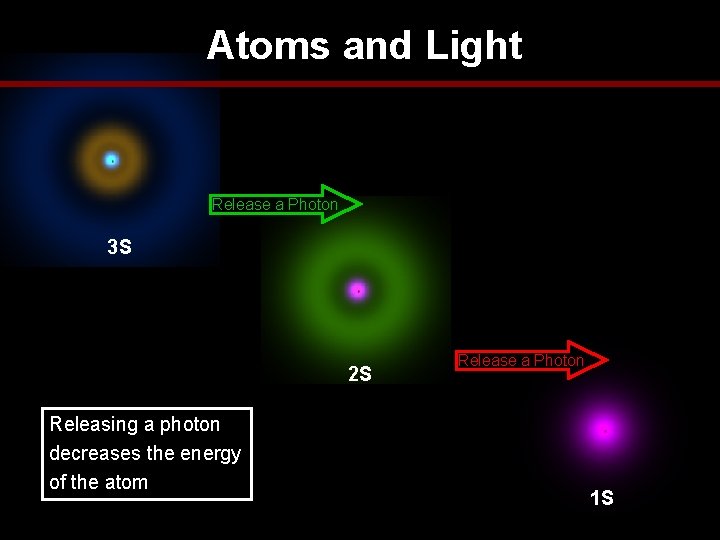
Atoms and Light Release a Photon 3 S 2 S Releasing a photon decreases the energy of the atom Release a Photon 1 S

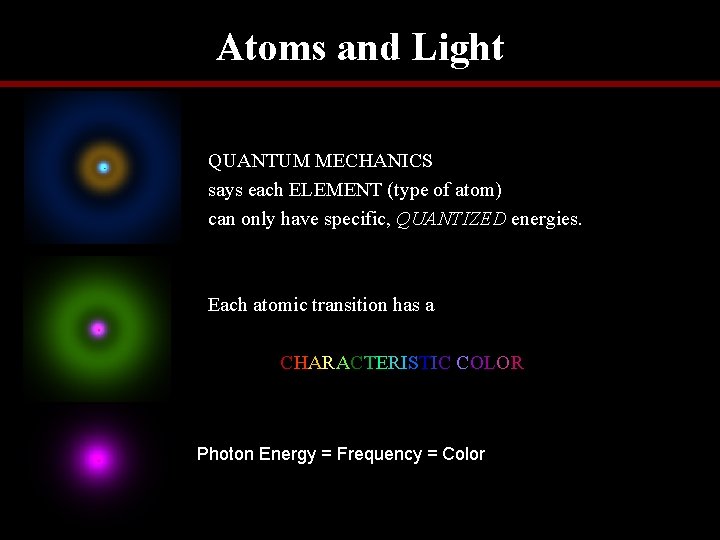
Atoms and Light QUANTUM MECHANICS says each ELEMENT (type of atom) can only have specific, QUANTIZED energies. Each atomic transition has a CHARACTERISTIC COLOR Photon Energy = Frequency = Color

The Sun Dark lines correspond to specific atomic transitions, such as “ 1 s to 2 s in Hydrogen”, or “ 1 s to 2 p in Helium”.

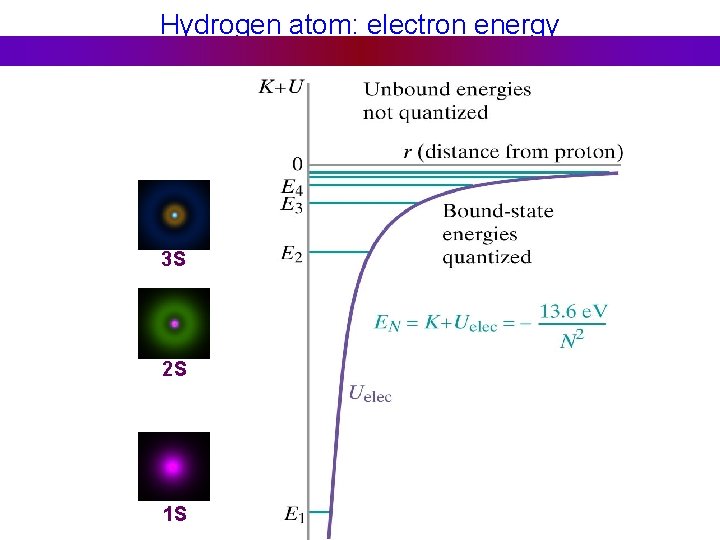
Hydrogen atom: electron energy 3 S 2 S 1 S

Emission spectra Hydrogen atom: Energy of emitted photon: 3 S 2 S Line spectrum – light is emitted at fixed frequencies 1 S

Absorption spectra Hydrogen atom: Energy of absorbed photon: 3 S Line spectrum – absorption at fixed frequencies 2 S Different atoms – different energies Atomic spectra – signature of element Example: He was discovered on Sun first 1 S

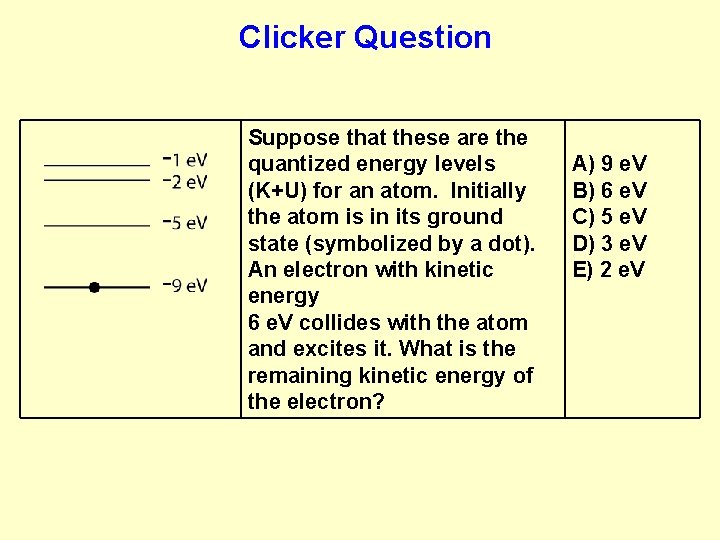
Clicker Question Suppose that these are the quantized energy levels (K+U) for an atom. Initially the atom is in its ground state (symbolized by a dot). An electron with kinetic energy 6 e. V collides with the atom and excites it. What is the remaining kinetic energy of the electron? A) 9 e. V B) 6 e. V C) 5 e. V D) 3 e. V E) 2 e. V

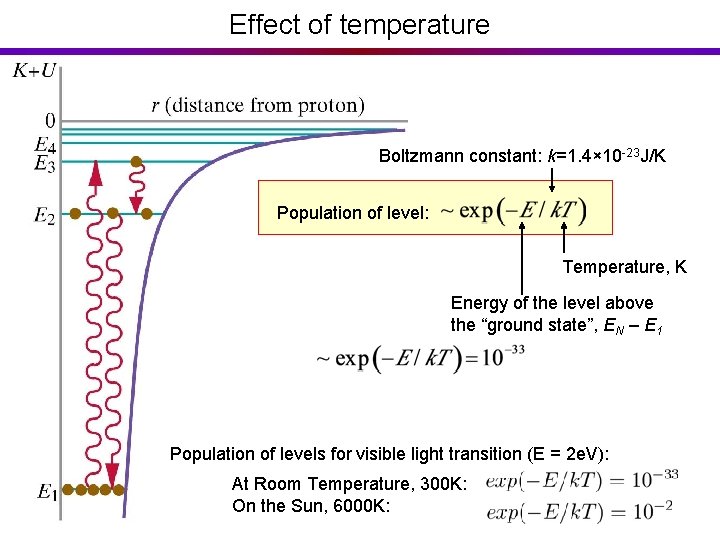
Effect of temperature Boltzmann constant: k=1. 4× 10 -23 J/K Population of level: Temperature, K Energy of the level above the “ground state”, EN – E 1 Population of levels for visible light transition (E = 2 e. V): At Room Temperature, 300 K: On the Sun, 6000 K:

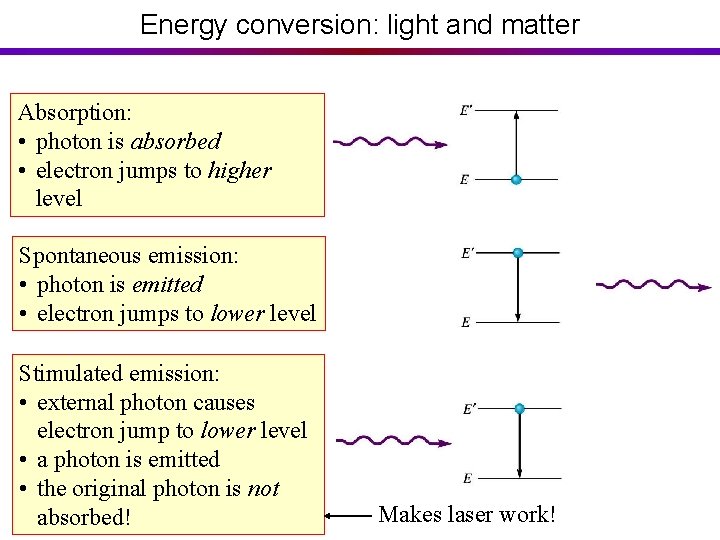
Energy conversion: light and matter Absorption: • photon is absorbed • electron jumps to higher level Spontaneous emission: • photon is emitted • electron jumps to lower level Stimulated emission: • external photon causes electron jump to lower level • a photon is emitted • the original photon is not absorbed! Makes laser work!

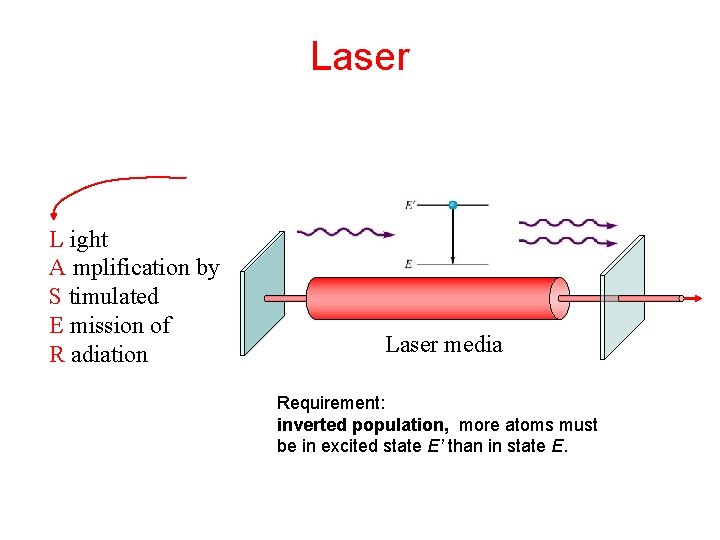
Laser L ight A mplification by S timulated E mission of R adiation Laser media Requirement: inverted population, more atoms must be in excited state E’ than in state E.

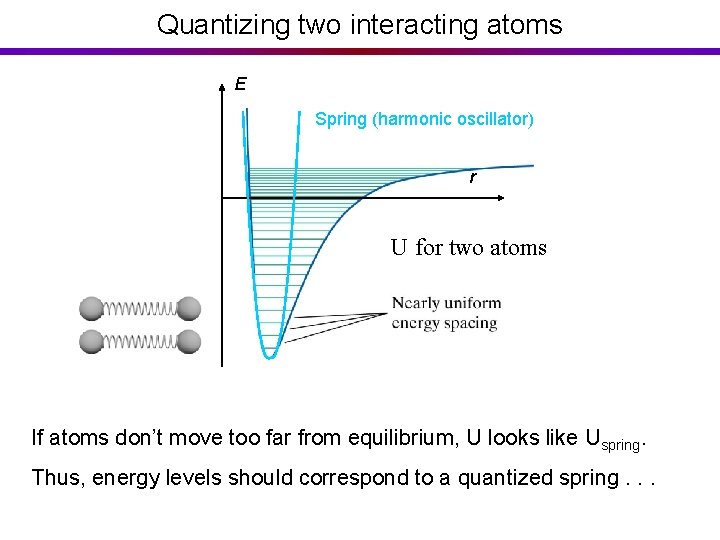
Quantizing two interacting atoms E Spring (harmonic oscillator) r U for two atoms If atoms don’t move too far from equilibrium, U looks like Uspring. Thus, energy levels should correspond to a quantized spring. . .

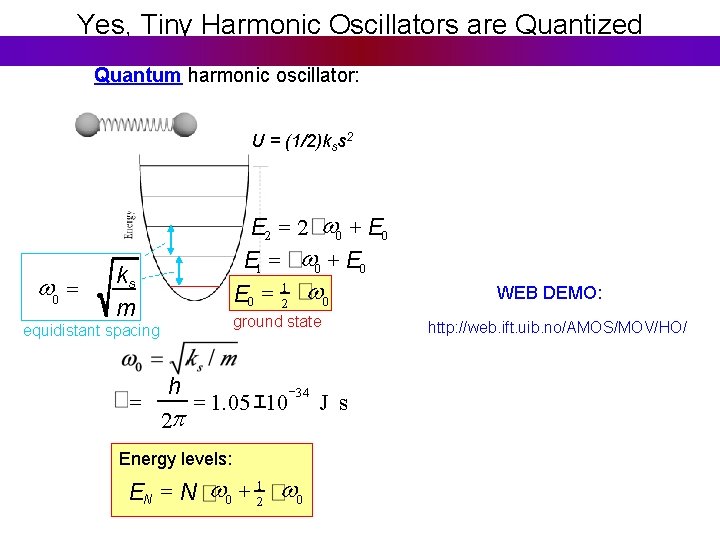
Quantizing two interacting atoms Classical harmonic oscillator: Quantum harmonic oscillator: U = (1/2)kss 2 w 0 = E 2 = 2 w 0 + E 0 E 1 = w 0 + E 0 = 12 w 0 ks m ground state equidistant spacing w 0 = ks / m Any value of A is allowed any E is possible. h = 1. 05 X 10 34 J s 2π Energy levels: EN = N w 0 + 12 w 0

Yes, Tiny Harmonic Oscillators are Quantized Quantum harmonic oscillator: U = (1/2)kss 2 w 0 = E 2 = 2 w 0 + E 0 E 1 = w 0 + E 0 = 12 w 0 ks m ground state equidistant spacing = h = 1. 05 エ 10 34 J s 2 p Energy levels: EN = N w 0 + 12 w 0 WEB DEMO: http: //web. ift. uib. no/AMOS/MOV/HO/

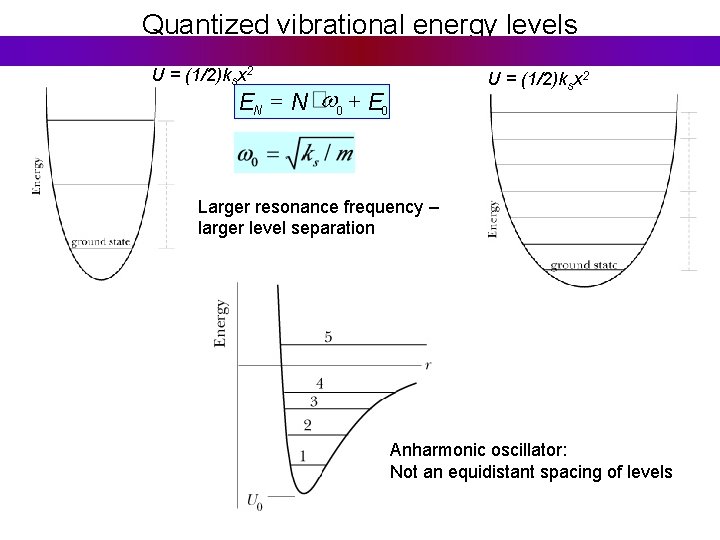
Quantized vibrational energy levels U = (1/2)ksx 2 EN = N w 0 + E 0 Larger resonance frequency – larger level separation Anharmonic oscillator: Not an equidistant spacing of levels

Home study: Rotational energy levels (8. 5, page 338) Nuclear & Hadronic energy levels (8. 6) Comparison of energy level spacing (8. 7)

Laser Ruby: aluminum oxide crystal (sapphire) where some Al were replaced by Cr