Nitric OxideEDRF Signaling Erectile dysfunction and other diseases










- Slides: 34

Nitric Oxide/EDRF Signaling: Erectile dysfunction and other diseases Molecular mechanisms of c. GMP action on vascular and cardiac muscle

NO/c. GMP and Nobel Prize '98 Robert F Furchgott, 1916 -2009 Dept. of Pharmacology, SUNY Health Science Center New York Louis J Ignarro, born 1941 Dept. of Molecular and Medical Pharmacology UCLA School of Medicine Los Angeles Ferid Murad, born 1936 Dept. of Integrative Biology Pharmacology and Physiology University of Texas Medical School, Houston The Nobel Assembly at the Karolinska Institute in Stockholm, Sweden, awarded the Nobel Prize in Physiology or Medicine to Robert F. Furchgott, Louis J. Ignarro and Ferid Murad for their discoveries concerning " nitric oxide as a signaling molecule in the cardiovascular system".

What is Nitric Oxide & What does it do? Nitric oxide is a gas (lipid soluble) Nitric oxide is a moderately reactive - free radical: contains an unpaired - electron; • N=O Nitric oxide is a toxic chemical NO will chemically nitrosylate proteins - macrophages use reactivity to kill pathogens Nitric oxide is a neurotransmitter (NANC) Nitric oxide is a hormone ( EDRF ) Nitric oxide is an activator of guanylyl cyclase

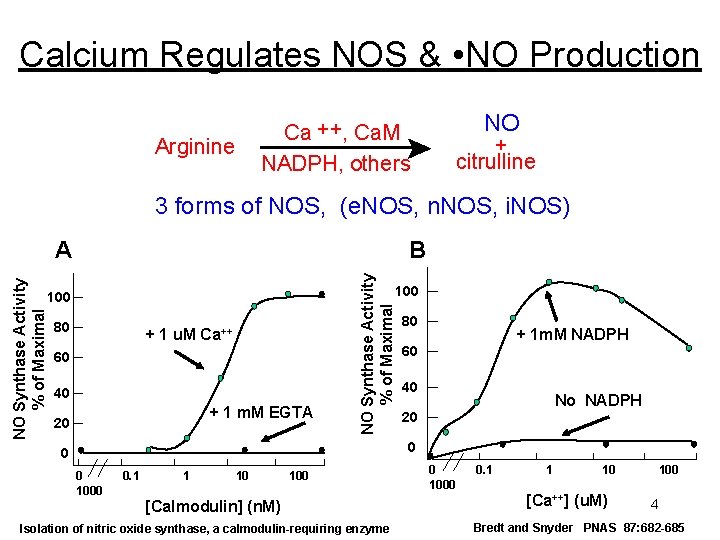
Calcium Regulates NOS & • NO Production Ca ++, Ca. M NADPH, others Arginine NO + citrulline 3 forms of NOS, (e. NOS, n. NOS, i. NOS) B NO Synthase Activity % of Maximal A 100 80 + 1 u. M Ca++ 60 40 + 1 m. M EGTA 20 80 + 1 m. M NADPH 60 40 No NADPH 20 0 1000 0. 1 1 10 100 [Calmodulin] (n. M) Isolation of nitric oxide synthase, a calmodulin-requiring enzyme 0 1000 0. 1 1 10 [Ca++] (u. M) 100 4 Bredt and Snyder PNAS 87: 682 -685

++ What Regulates the Ca & Where? Acetylcholine Histamine Thrombin ATP & ADP Serotonin Vasopressin Bradykinin CGRP Leukotriene Many of these are known to contract muscle. How so? ? 5

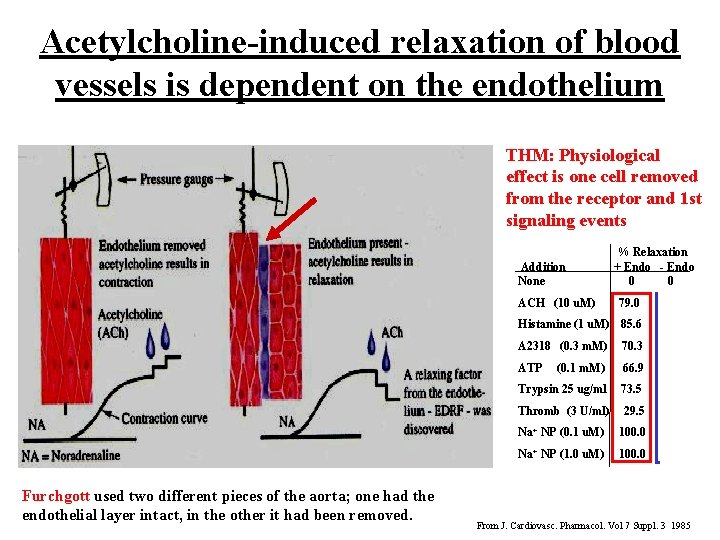
Acetylcholine-induced relaxation of blood vessels is dependent on the endothelium THM: Physiological effect is one cell removed from the receptor and 1 st signaling events Addition None Furchgott used two different pieces of the aorta; one had the endothelial layer intact, in the other it had been removed. % Relaxation + Endo - Endo 0 0 ACH (10 u. M) 79. 0 31. 6 0 Histamine (1 u. M) 85. 6 21. 1 0 A 2318 (0. 3 m. M) 70. 3 28. 3 0 ATP (0. 1 m. M) 66. 9 24. 8 0 Trypsin 25 ug/ml 73. 5 52. 5 0 Thromb (3 U/ml) 29. 5 4. 2 0 Na+ NP (0. 1 u. M) 100. 0 2. 6 100 Na+ NP (1. 0 u. M) 100. 0 30. 2 100 From J. Cardiovasc. Pharmacol. Vol 7 Suppl. 3 1985

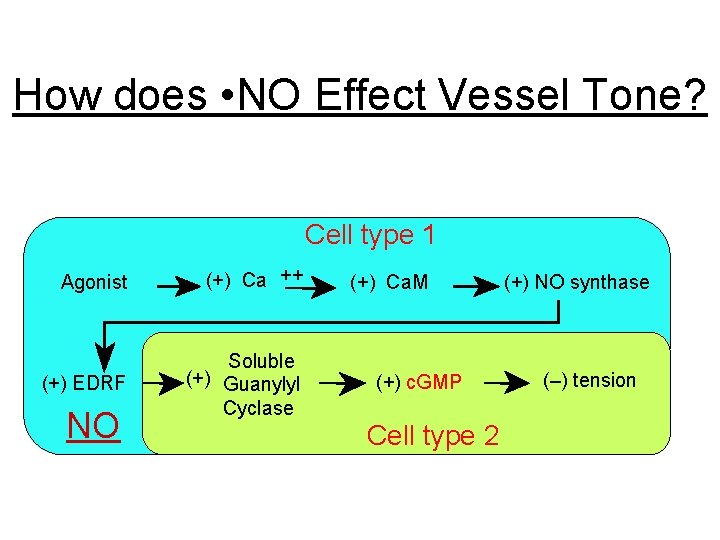
How does • NO Effect Vessel Tone? Cell type 1 Agonist (+) Ca ++ (+) EDRF Soluble (+) Guanylyl Cyclase NO (+) Ca. M (+) c. GMP Cell type 2 (+) NO synthase (–) tension

How does • NO stimulate the production of c. GMP? What is the molecular target of • NO ?

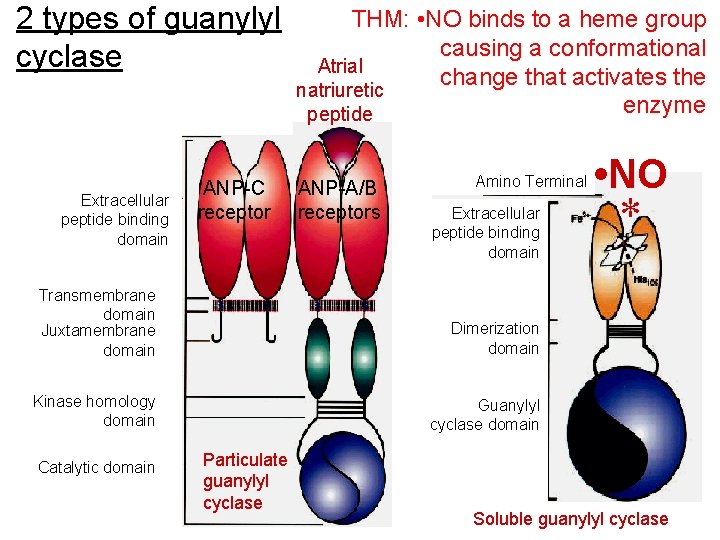
2 types of guanylyl cyclase Extracellular peptide binding domain ANP-C receptor THM: • NO binds to a heme group causing a conformational Atrial change that activates the natriuretic enzyme peptide ANP-A/B receptors Amino Terminal Extracellular peptide binding domain Transmembrane domain Juxtamembrane domain Dimerization domain Kinase homology domain Guanylyl cyclase domain Catalytic domain Particulate guanylyl cyclase • NO * Soluble guanylyl cyclase

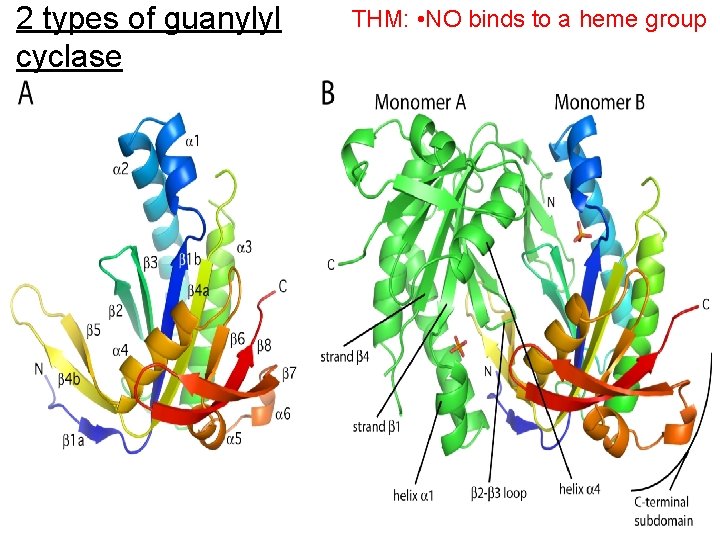
2 types of guanylyl cyclase Extracellular peptide binding domain ANP-C receptor THM: • NO binds to a heme group causing a conformational Atrial change that activates the natriuretic enzyme peptide ANP-A/B receptors • NO * Transmembrane domain Juxtamembrane domain Kinase homology domain Catalytic domain Particulate guanylyl cyclase 10 Soluble guanylyl cyclase

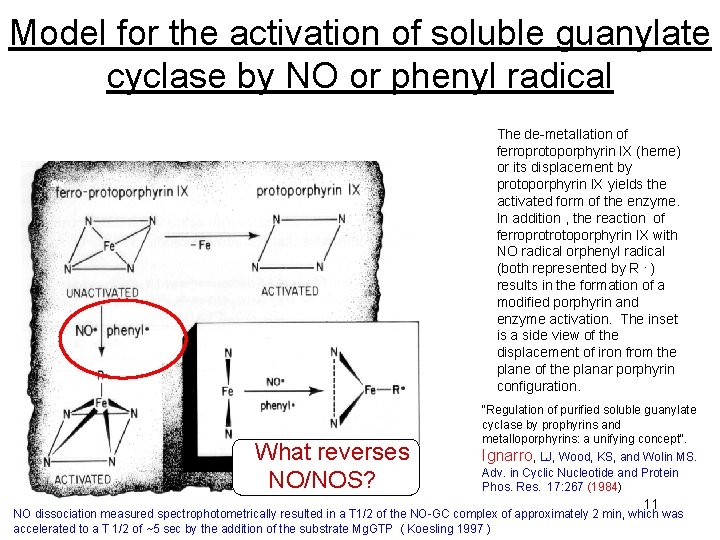
Model for the activation of soluble guanylate cyclase by NO or phenyl radical At the time itit was “heretical” that aa free radical could be be aa At The de-metallation of second messenger second ferroprotoporphyrin IX (heme) or its displacement by protoporphyrin IX yields the activated form of the enzyme. In addition , the reaction of ferroprotrotoporphyrin IX with NO radical orphenyl radical (both represented by R. ) results in the formation of a modified porphyrin and enzyme activation. The inset is a side view of the displacement of iron from the plane of the planar porphyrin configuration. What reverses NO/NOS? "Regulation of purified soluble guanylate cyclase by prophyrins and metalloporphyrins: a unifying concept". Ignarro, LJ, Wood, KS, and Wolin MS. Adv. in Cyclic Nucleotide and Protein Phos. Res. 17: 267 (1984) 11 NO dissociation measured spectrophotometrically resulted in a T 1/2 of the NO-GC complex of approximately 2 min, which was accelerated to a T 1/2 of ~5 sec by the addition of the substrate Mg. GTP ( Koesling 1997 )

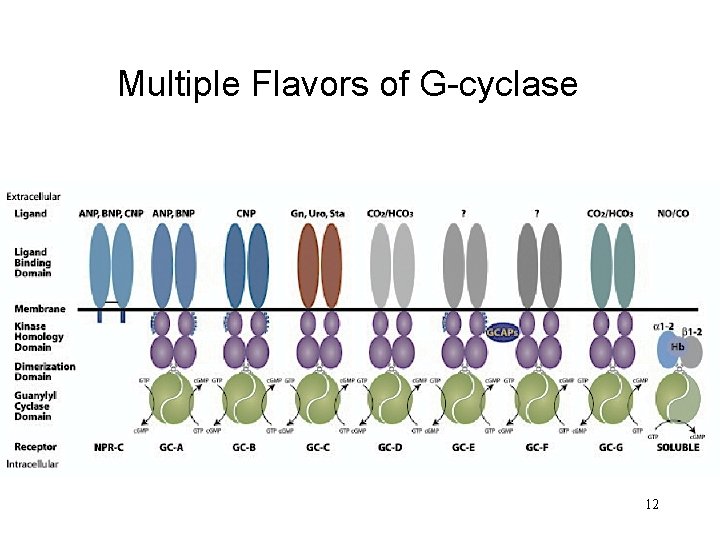
Multiple Flavors of G-cyclase 12

What are receptors for c. GMP? How does it work mechanistically?

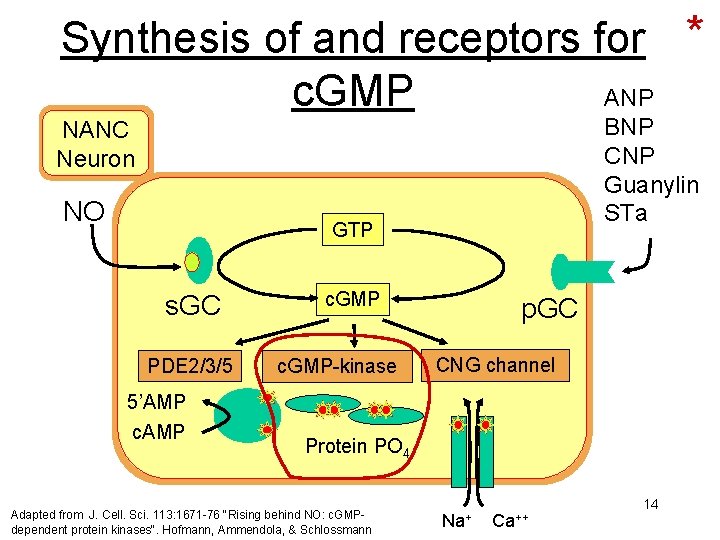
Synthesis of and receptors for * c. GMP ANP BNP CNP Guanylin STa NANC Neuron NO GTP s. GC PDE 2/3/5 c. GMP-kinase p. GC CNG channel 5’AMP c. AMP Protein PO 4 Adapted from J. Cell. Sci. 113: 1671 -76 "Rising behind NO: c. GMPdependent protein kinases". Hofmann, Ammendola, & Schlossmann Na+ Ca++ 14

How does c. GK work? What is/are the substrate(s)? 15

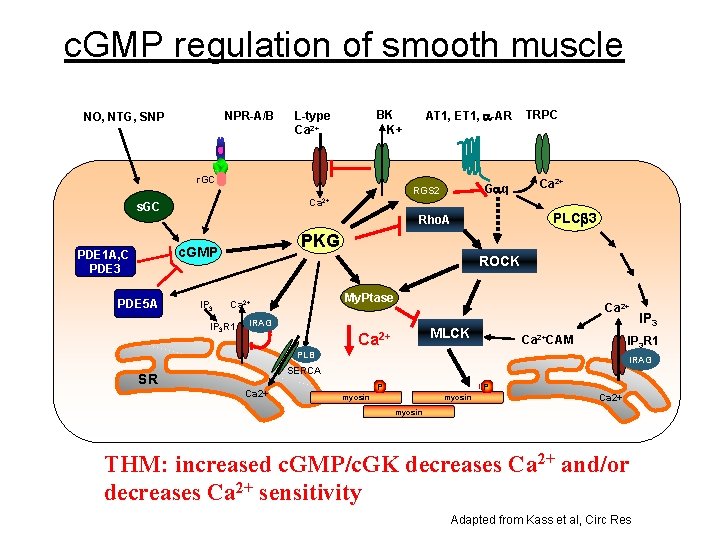
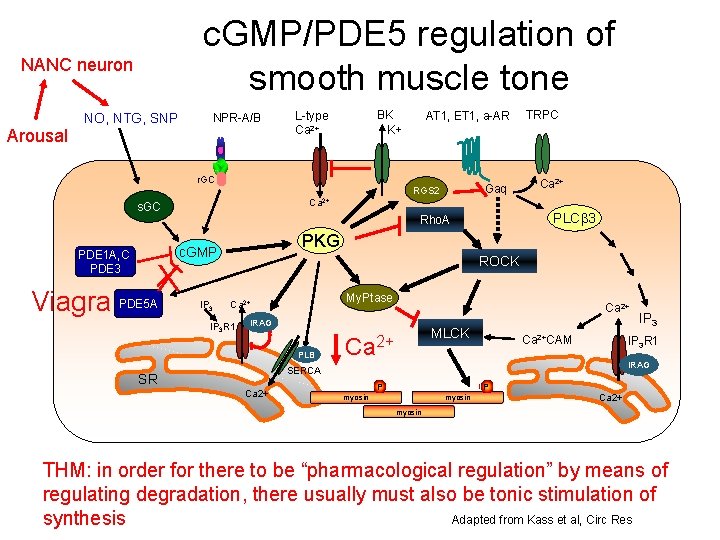
c. GMP regulation of smooth muscle NPR-A/B NO, NTG, SNP r. GC TRPC Ca 2+ PLC 3 Rho. A PKG c. GMP PDE 5 A Gaq RGS 2 s. GC PDE 1 A, C PDE 3 AT 1, ET 1, a-AR BK K+ L-type Ca 2+ IP 3 ROCK My. Ptase Ca 2+ IP 3 R 1 Ca 2+ IRAG MLCK Ca 2+CAM IP 3 R 1 PLB IRAG SERCA SR Ca 2+ IP 3 P P myosin Ca 2+ myosin THM: increased c. GMP/c. GK decreases Ca 2+ and/or decreases Ca 2+ sensitivity Adapted from Kass et al, Circ Res

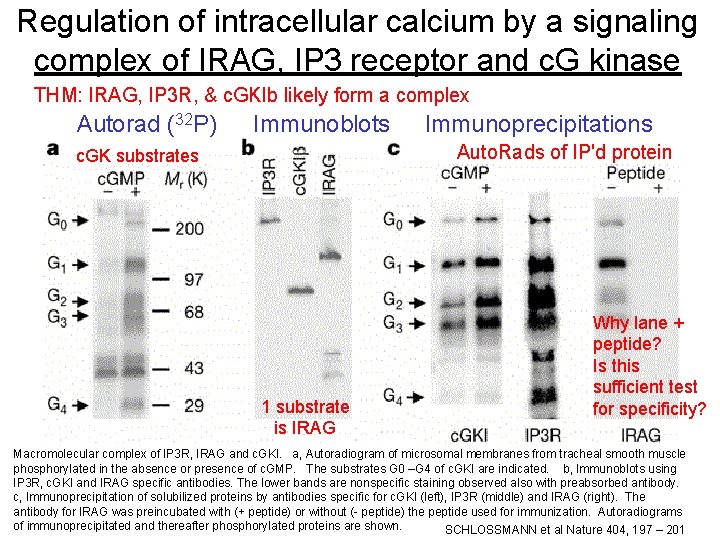
Regulation of intracellular calcium by a signaling complex of IRAG, IP 3 receptor and c. G kinase THM: IRAG, IP 3 R, & c. GKIb likely form a complex Autorad (32 P) Immunoblots Immunoprecipitations Auto. Rads of IP'd protein c. GK substrates 1 substrate is IRAG Why lane + peptide? Is this sufficient test for specificity? Macromolecular complex of IP 3 R, IRAG and c. GKI. a, Autoradiogram of microsomal membranes from tracheal smooth muscle phosphorylated in the absence or presence of c. GMP. The substrates G 0 –G 4 of c. GKI are indicated. b, Immunoblots using IP 3 R, c. GKI and IRAG specific antibodies. The lower bands are nonspecific staining observed also with preabsorbed antibody. c, Immunoprecipitation of solubilized proteins by antibodies specific for c. GKI (left), IP 3 R (middle) and IRAG (right). The 17 antibody for IRAG was preincubated with (+ peptide) or without (- peptide) the peptide used for immunization. Autoradiograms of immunoprecipitated and thereafter phosphorylated proteins are shown. SCHLOSSMANN et al Nature 404, 197 – 201

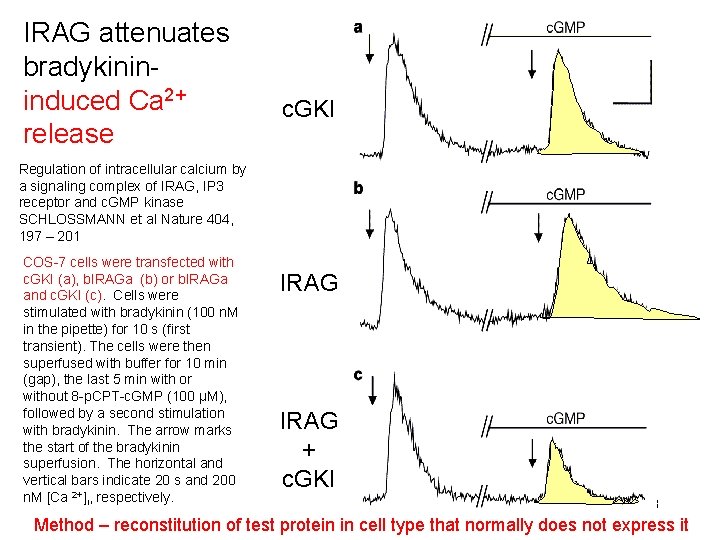
IRAG attenuates bradykinininduced Ca 2+ release c. GKI Regulation of intracellular calcium by a signaling complex of IRAG, IP 3 receptor and c. GMP kinase SCHLOSSMANN et al Nature 404, 197 – 201 COS-7 cells were transfected with c. GKI (a), b. IRAGa (b) or b. IRAGa and c. GKI (c). Cells were stimulated with bradykinin (100 n. M in the pipette) for 10 s (first transient). The cells were then superfused with buffer for 10 min (gap), the last 5 min with or without 8 -p. CPT-c. GMP (100 µM), followed by a second stimulation with bradykinin. The arrow marks the start of the bradykinin superfusion. The horizontal and vertical bars indicate 20 s and 200 n. M [Ca 2+]i, respectively. IRAG + c. GKI 18 Method – reconstitution of test protein in cell type that normally does not express it

Is c. GMP-dependent Protein Kinase Required? If so which isozyme - c. GKI or c. GKII Approaches - Drugs, KOs

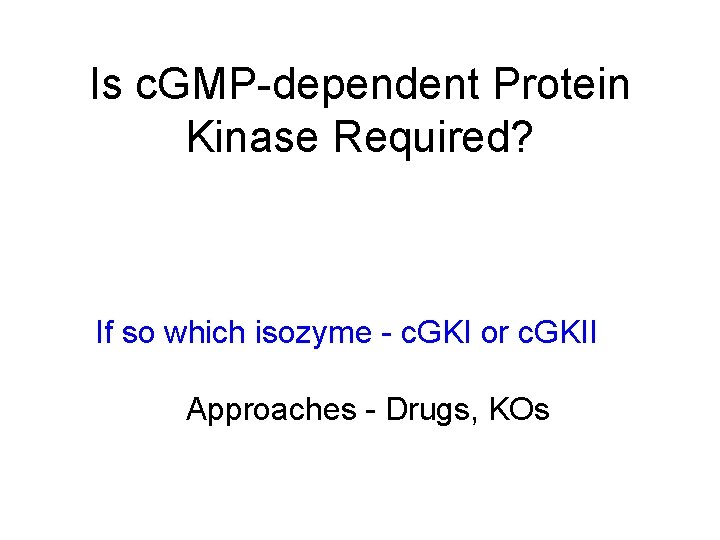
I Effects of ACh, adenosine, c. GMP and c. AMP on isolated aortic rings +/+ -/- 8 -Br c. G(n ) & ACh (n ) & A 2 (l) c. BIMPS (O ) -/+/+ (A) Representative registration of aortic segments from c. GKI+/+ (top) and c. GKI-/(bottom) mice. Aortas were pre-contracted with 100 n. M NE. After reaching steady state, 3 n. M to 10 µM ACh was added to the bath. Numbers above the arrows indicate the log of concentration in mol/l. (B and C) Concentration-response curves to (B) ACh (o, n ) and the adenosine receptor A 2 agonist CGS-21680 (l, O ) and (C) 8 -Brc. GMP (n, o ) and c. BIMPS (l, O). Aortic rings developed the same tension in the presence of 0. 1 µM NE: 7. 5 ± 0. 5 (c. GKI+/+) and 6. 7 ± 0. 5 m. M (c. GKI -/- ). Values are the means ± SEM for 12 aortic strips from 3 -5 different animals of each genotype. Curves are fits using a logarithmic function. Isolated "cells" a good use of genetic KOs 20 in Pfeifer A, et al, Defective smooth muscle regulation c. GMP kinase I-deficient mice. EMBO J 17: 3045 -51 .

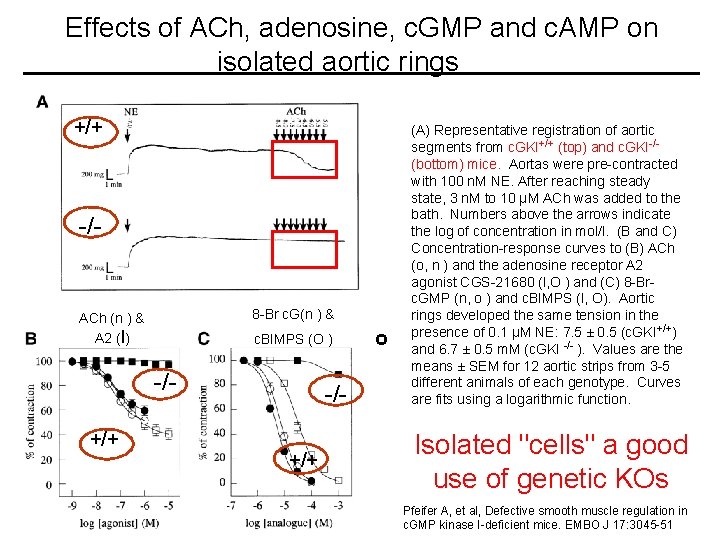
Mean arterial blood pressure (MAP) of c. GKI+/+ (O) and c. GKI -/- (l) mice L-NNA DEA-NO Phenylephrine MAP was measured continuously starting during inhalational anaesthesia KO After recovery (a), and after 60 min recovery (break) in conscious, unrestrained mice (b-e). WT During anesthesia THM: c. GKI is required for effect of c. GMP on smooth muscle relaxation (c), (d) and (e) indicate the administration of L-NNA (12 µg/g i. p. ), DEA-NO (75 ng/g i. v. ) and phenylephrine (200 ng/g i. v. ), respectively. Pfeifer A, et al, Defective smooth muscle regulation in c. GMP kinase I-deficient mice. 2117: 3045 EMBO J 1998 Jun 1; 51

How does it work physiologically? Example of Viagra?

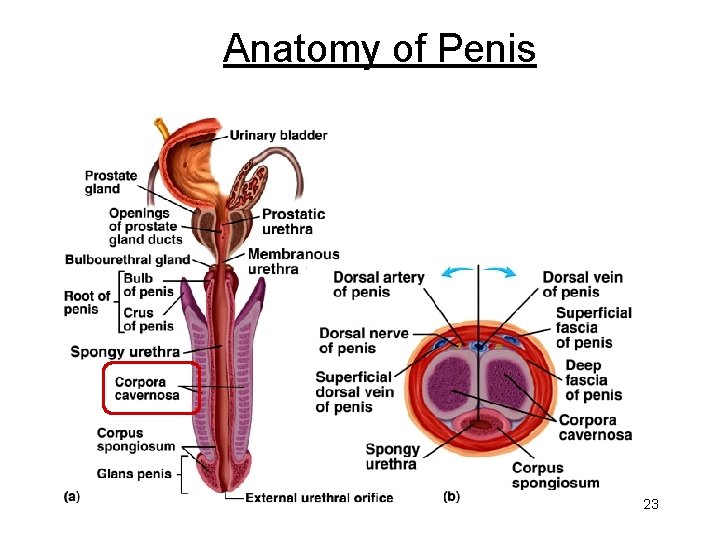
Anatomy of Penis 23

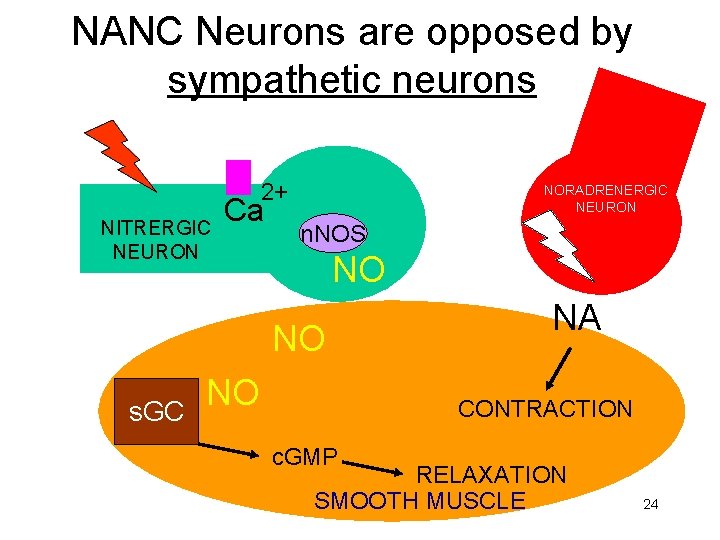
NANC Neurons are opposed by sympathetic neurons 2+ NITRERGIC NEURON Ca NORADRENERGIC NEURON n. NOS NO NO s. GC NO NA CONTRACTION c. GMP RELAXATION SMOOTH MUSCLE 24

c. GMP/PDE 5 regulation of smooth muscle tone NANC neuron Arousal NO, NTG, SNP NPR-A/B BK K+ L-type Ca 2+ r. GC TRPC Ca 2+ PLC 3 Rho. A PKG c. GMP ROCK X Viagra PDE 5 A Gaq RGS 2 s. GC PDE 1 A, C PDE 3 AT 1, ET 1, a-AR IP 3 My. Ptase Ca 2+ IP 3 R 1 Ca 2+ IRAG PLB MLCK Ca 2+CAM IP 3 R 1 IRAG SERCA SR Ca 2+ IP 3 P P myosin Ca 2+ myosin THM: in order for there to be “pharmacological regulation” by means of regulating degradation, there usually must also be tonic stimulation of Adapted from Kass et al, Circ Res synthesis

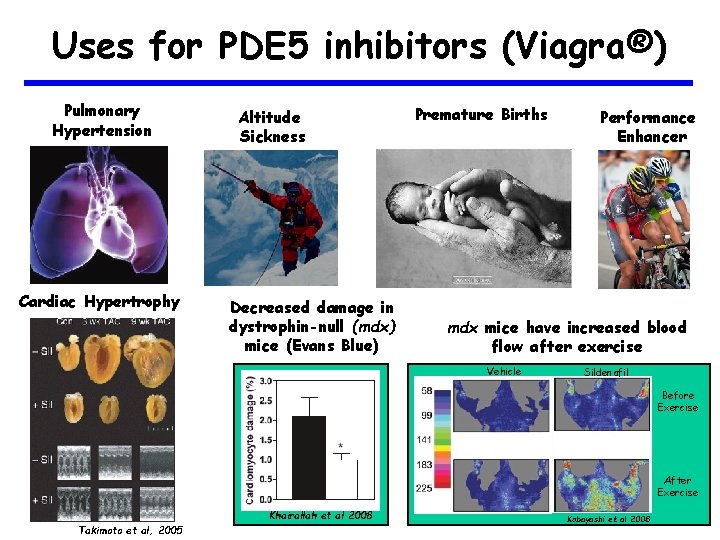
Uses for PDE 5 inhibitors (Viagra®) Pulmonary Hypertension Cardiac Hypertrophy Altitude Sickness Decreased damage in dystrophin-null (mdx) mice (Evans Blue) Premature Births Performance Enhancer mdx mice have increased blood flow after exercise Vehicle Sildenafil Before Exercise After Exercise Khairallah et al 2008 Takimoto et al, 2005 Kobayashi et al 2008

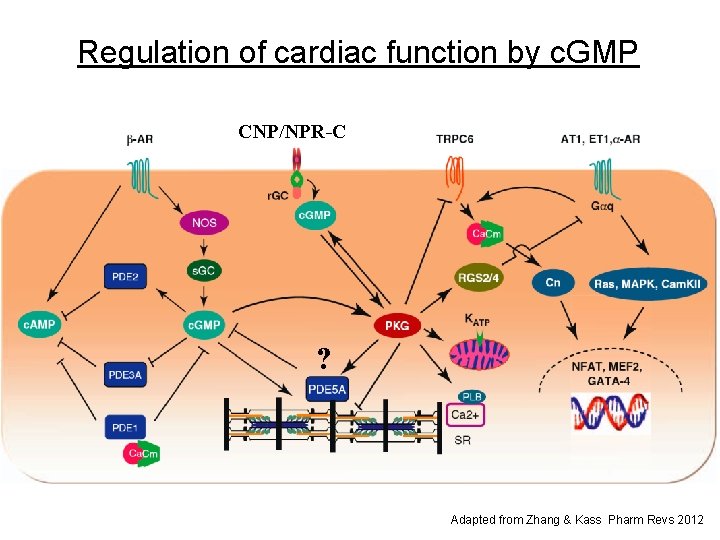
Regulation of cardiac function by c. GMP CNP/NPR-C ? Adapted from Zhang & Kass Pharm Revs 2012

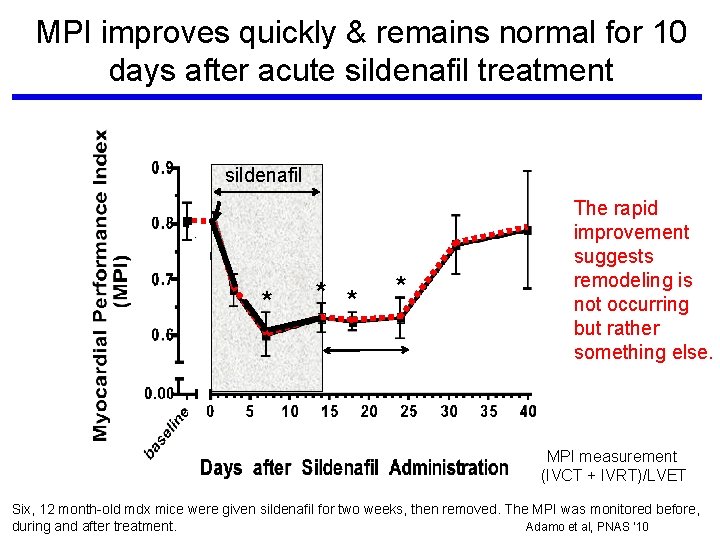
MPI improves quickly & remains normal for 10 days after acute sildenafil treatment sildenafil * * The rapid improvement suggests remodeling is not occurring but rather something else. MPI measurement (IVCT + IVRT)/LVET Six, 12 month-old mdx mice were given sildenafil for two weeks, then removed. The MPI was monitored before, during and after treatment. Adamo et al, PNAS ‘ 10

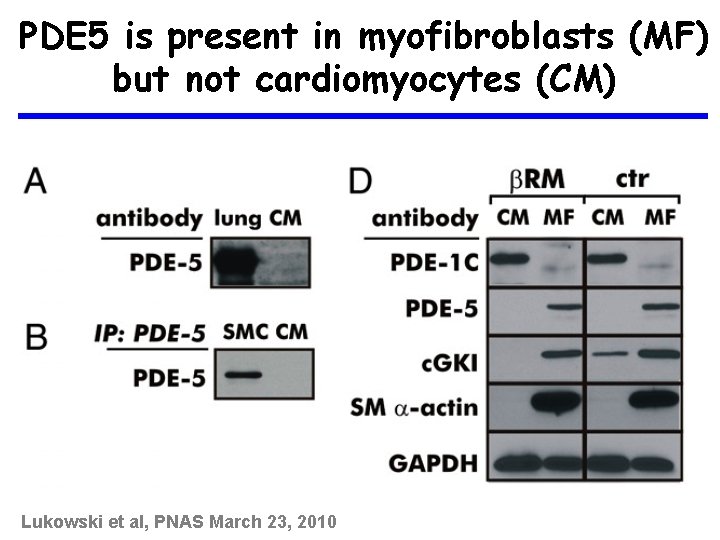
PDE 5 is present in myofibroblasts (MF) but not cardiomyocytes (CM) Lukowski et al, PNAS March 23, 2010

Toby, born 2/16/2004 @ 2#, 7 oz: shown here at 1 week post, (27 wk) 2/24/04 Toby put on Hi-Fi ventillation, pavulon, and nitric oxide http: //www. jendogger. com 30

Later Pictures http: //www. jendogger. com Toby Haloween, 2004 Toby ~ 21 mo 31

Toby, 08 http: //www. jendogger. com Toby, 07 Toby, 09 32

Enough - Ugh, too much!!! http: //av. vimeo. com/58872/195/21670489. mp 4? token=1383606445_56 dd 454 a 55 ef 55028616 d 0 df 92642 c 67 33

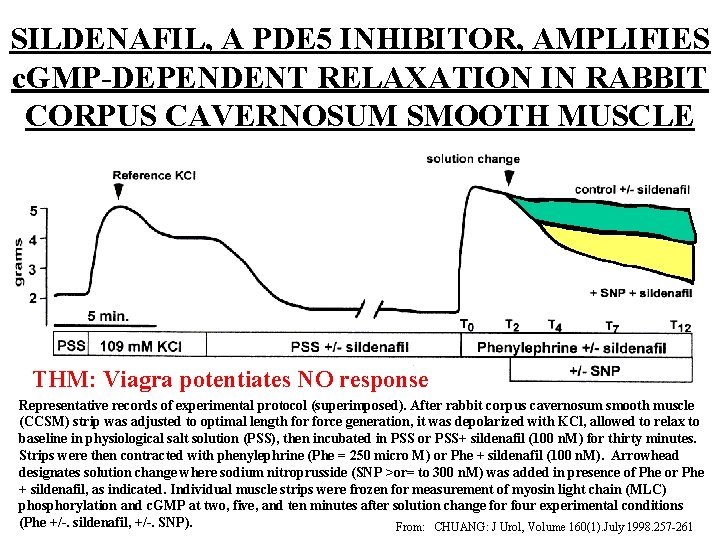
SILDENAFIL, A PDE 5 INHIBITOR, AMPLIFIES c. GMP-DEPENDENT RELAXATION IN RABBIT CORPUS CAVERNOSUM SMOOTH MUSCLE THM: Viagra potentiates NO response Representative records of experimental protocol (superimposed). After rabbit corpus cavernosum smooth muscle (CCSM) strip was adjusted to optimal length force generation, it was depolarized with KCl, allowed to relax to baseline in physiological salt solution (PSS), then incubated in PSS or PSS+ sildenafil (100 n. M) for thirty minutes. Strips were then contracted with phenylephrine (Phe = 250 micro M) or Phe + sildenafil (100 n. M). Arrowhead designates solution change where sodium nitroprusside (SNP >or= to 300 n. M) was added in presence of Phe or Phe + sildenafil, as indicated. Individual muscle strips were frozen for measurement of myosin light chain (MLC) 34 phosphorylation and c. GMP at two, five, and ten minutes after solution change for four experimental conditions (Phe +/-. sildenafil, +/-. SNP). From: CHUANG: J Urol, Volume 160(1). July 1998. 257 -261