ATAXIA Paraneoplastic causes Loulwah Mukharesh Intern Neurology Rotation









- Slides: 20

ATAXIA - Paraneoplastic causes Loulwah Mukharesh, Intern Neurology Rotation (April 2012)

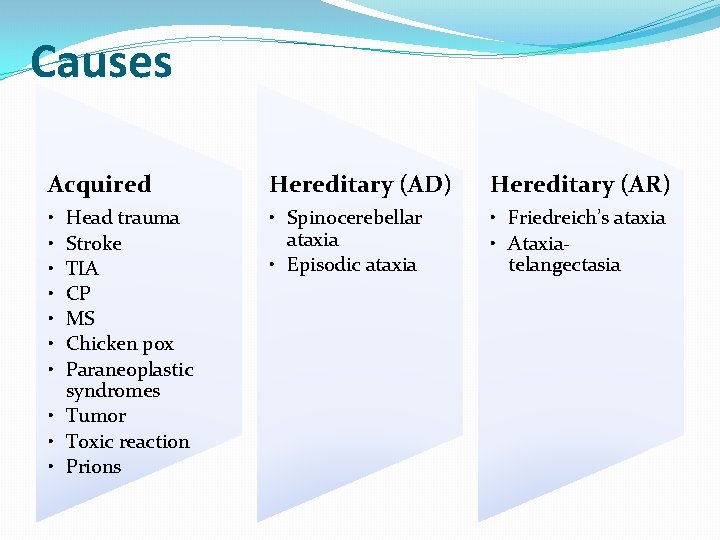
Causes Acquired Hereditary (AD) Hereditary (AR) • • Spinocerebellar ataxia • Episodic ataxia • Friedreich’s ataxia • Ataxiatelangectasia Head trauma Stroke TIA CP MS Chicken pox Paraneoplastic syndromes • Tumor • Toxic reaction • Prions

Paraneoplastic syndrome � A heterogeneous group of neurologic disorders associated with systemic cancer and caused by mechanisms other than metastases, metabolic and nutritional deficits, infections, coagulopathy, or side effects of cancer treatment. �Paraneoplastic cerebellar degeneration is an uncommon disorder that can be associated with any cancer; the most commonly associated are lung cancer (particularly small cell lung cancer (SCLC)), gynecologic and breast cancer, and lymphoma (particularly Hodgkin disease). The neurologic symptoms frequently precede the diagnosis of cancer, sometimes by an interval of years

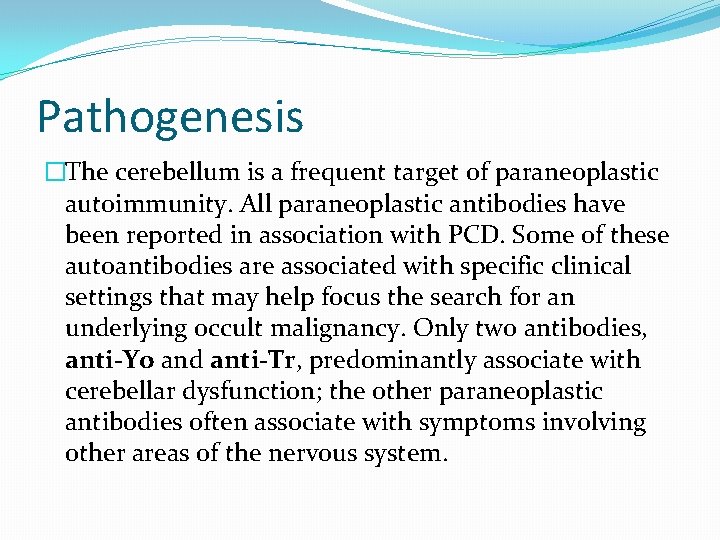
Pathogenesis �The cerebellum is a frequent target of paraneoplastic autoimmunity. All paraneoplastic antibodies have been reported in association with PCD. Some of these autoantibodies are associated with specific clinical settings that may help focus the search for an underlying occult malignancy. Only two antibodies, anti-Yo and anti-Tr, predominantly associate with cerebellar dysfunction; the other paraneoplastic antibodies often associate with symptoms involving other areas of the nervous system.

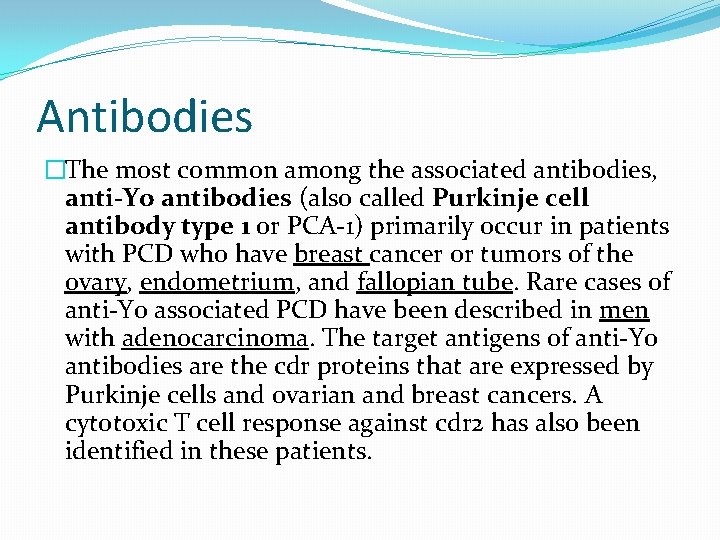
Antibodies �The most common among the associated antibodies, anti-Yo antibodies (also called Purkinje cell antibody type 1 or PCA-1) primarily occur in patients with PCD who have breast cancer or tumors of the ovary, endometrium, and fallopian tube. Rare cases of anti-Yo associated PCD have been described in men with adenocarcinoma. The target antigens of anti-Yo antibodies are the cdr proteins that are expressed by Purkinje cells and ovarian and breast cancers. A cytotoxic T cell response against cdr 2 has also been identified in these patients.

Antibodies �Anti-Hu antibodies (also called antineuronal nuclear antibody, type 1 or ANNA-1) are prevalent in patients with small cell lung cancer (SCLC) and PCD. In one report of 57 such patients, 44 percent had a high titer of anti-Hu antibodies. These patients are more likely to have multifocal neurologic disease and severe disability than other patient groups.

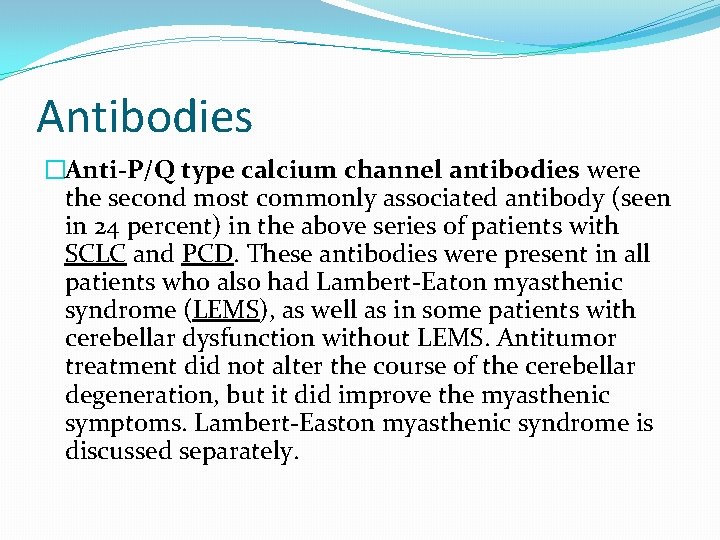
Antibodies �Anti-P/Q type calcium channel antibodies were the second most commonly associated antibody (seen in 24 percent) in the above series of patients with SCLC and PCD. These antibodies were present in all patients who also had Lambert-Eaton myasthenic syndrome (LEMS), as well as in some patients with cerebellar dysfunction without LEMS. Antitumor treatment did not alter the course of the cerebellar degeneration, but it did improve the myasthenic symptoms. Lambert-Easton myasthenic syndrome is discussed separately.

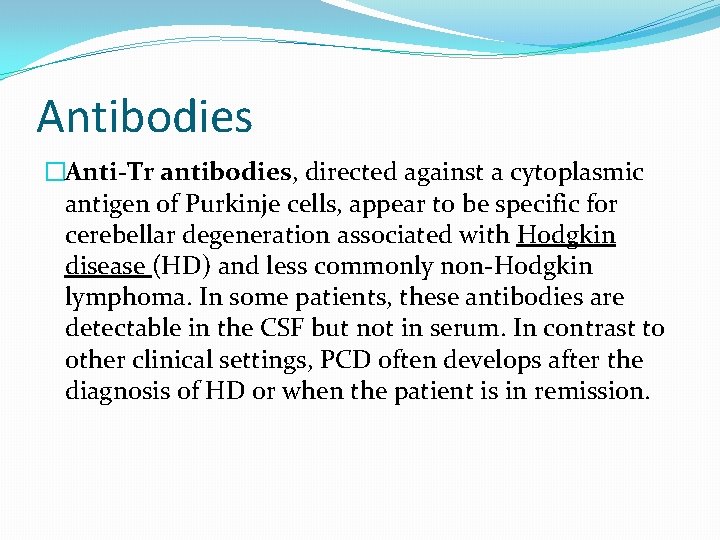
Antibodies �Anti-Tr antibodies, directed against a cytoplasmic antigen of Purkinje cells, appear to be specific for cerebellar degeneration associated with Hodgkin disease (HD) and less commonly non-Hodgkin lymphoma. In some patients, these antibodies are detectable in the CSF but not in serum. In contrast to other clinical settings, PCD often develops after the diagnosis of HD or when the patient is in remission.

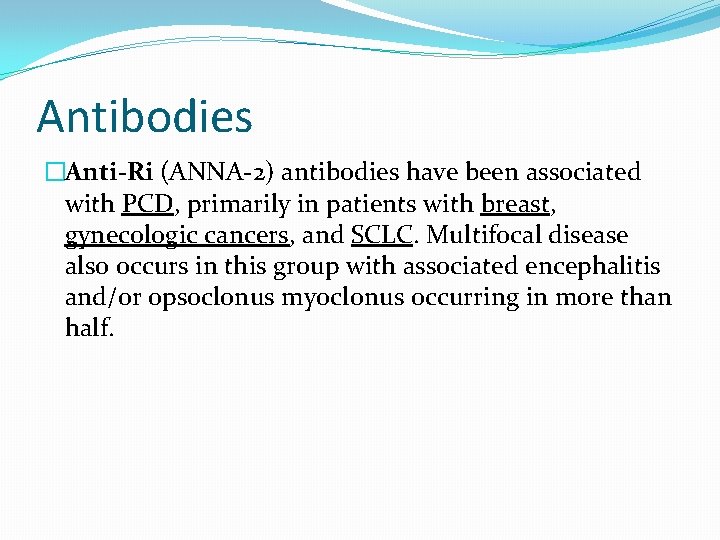
Antibodies �Anti-Ri (ANNA-2) antibodies have been associated with PCD, primarily in patients with breast, gynecologic cancers, and SCLC. Multifocal disease also occurs in this group with associated encephalitis and/or opsoclonus myoclonus occurring in more than half.

Antibodies �Anti-CV 2 antibodies, directed against a cytoplasmic antigen in some glial cells, and against peripheral nerve antigens, have been associated with several syndromes, including PCD as well as limbic encephalitis, encephalomyelitis, peripheral neuropathy, and optic neuritis. The most common tumors are SCLC and thymoma. �Antibodies to Ma proteins, which are selectively expressed in brain, testis, and some tumors can occur in patients with paraneoplastic brainstem and cerebellar dysfunction associated with a variety of tumors arising in the testes (germ-cell tumors), breast, colon, and parotid gland, among others.

Antibodies �Antibodies to the Zic 4 have been described in patients with neurologic disorders including cerebellar dysfunction and SCLC. These patients often have concurrent anti-Hu or CRMP 5 antibodies. Patients with isolated Zic 4 antibodies are more likely to develop cerebellar dysfunction than those with concurrent immunities.

Antibodies �Other, less well characterized antibodies have been reported in patients with PCD. PCA-2 and ANNA-3 are not syndrome specific and have unknown autoantigens. Antibodies to a glutamate receptor in the brain, designated anti-m. Glu. R 1, have only been identified in two patients with PCD and Hodgkin lymphoma as well as one patient without cancer. Antibodies against the carbonic anhydrase related protein VIII (CARP VIII) were identified in a patient with PCD and melanoma. In one patient with non. SCLC and PCD, immunoreactivity was observed to protein kinase C, a protein highly expressed in the Purkinje cells of the cerebellum.

Clinical features � Dizziness, nausea, and vomiting (acute) gait instability, oscillopsia, diplopia, both truncal and appendicular ataxia, dysarthria, and dysphagia. Worsen for weeks to months before stabilizing. Severe disability with inability to walk or even sit unsupported, inability to write or feed oneself, and limited communication secondary to dysarthria are common, with some patients having a somewhat better outcome. � Many of these patients with paraneoplastic syndromes develop cerebellar dysfunction along with other neurologic signs. As an example, when cerebellar degeneration occurs with encephalomyelitis, the underlying tumor is SCLC in approximately 80 percent of cases. LEMS can occur with PCD in patients with SCLC who have anti-P/Q type calcium channel antibodies. Even patients with anti-Yo antibodies and relatively isolated PCD have detectable cognitive deficits approximately 20 percent of the time. � Most patients (60 to 70 percent) with PCD do not have a cancer diagnosis at the onset of their neurologic symptoms

Diagnosis �DIAGNOSIS — The diagnostic approach to patients with PCD includes considering and ruling out other diagnoses, confirming and characterizing the paraneoplastic syndrome, and identifying the underlying neoplasm, if this is not previously known. �Differential diagnosis — cerebellar and brainstem metastases, toxic and metabolic causes, and neurodegenerative causes. Alcoholism, vitamin deficiency (thiamine, vitamin E, cobalamin), hypothyroidism, cerebrovascular disease, demyelination, infectious or postinfectious cerebellitis, Miller. Fisher syndrome, anti-glutamic acid decarboxylase (GAD)associated cerebellar ataxia, Creutzfeldt Jakob disease, HIV, and celiac disease should be specifically considered. Medication lists should also be reviewed for possible contributors.

Investigation � MRI is usually not helpful for positive diagnosis of PCD but is important to exclude metastatic and cerebrovascular disease. In rare instances of PCD, contrast enhancement in the cerebellar folia can be found in acute phases of the disease, and PET may reveal hypermetabolism. Diffuse cerebellar atrophy develops subsequently. � Laboratory: TFT, vitamin levels, and other testing (anti-gliadin, anti-GAD antibodies, HIV serology) in the appropriate clinical setting. � CSF examination can show inflammatory changes, at least in the early stages of the illness. A mild pleocytosis (10 -50 lymphocytes) and a mild elevation of protein are characteristic. Some patients with PCD or extensive encephalitis have the 14 -3 -3 protein characteristic of Creutzfeldt-Jakob disease (CJD) in the CSF. This might create some diagnostic confusion if a tumor is not evident because of symptom overlap between paraneoplastic disease and CJD. It is likely that the 14 -3 -3 protein in this setting reflects extensive CNS damage rather than CJD. Immunoblotting reveals the 14 -3 -3 protein as a double band (similar to other false positive results) rather than the single band in CJD. � Paraneoplastic biomarkers. Negative results do not exclude a paraneoplastic or autoimmune disorder. A sample of a patient’s CSF and serum should be sent to a research laboratory for examination in these cases. � Evaluation for occult malignancy. The presence of paraneoplastic biomarkers may help direct further evaluation if initial screening is negative. Tumors may be small; in some cases FDG-PET or exploratory laparotomy was the only means of identifying the underlying lesion. In a minority of patients with PCD, no tumor is identified despite vigorous investigations. In such cases, serial investigations are advised

Treatment � Neurologic outcome is often poor, most patients (75 to 80 percent) become and remain nonambulatory. Treatment of the underlying tumor is considered essential for neurologic stabilization; clinical improvement is less likely but can occur. � Immunotherapy is often used, but does not clearly affect neurologic outcome. In one larger case series, immunotherapy was not significantly associated with recovery. However, there anecdotal reports of neurologic improvement in patients tried on various immunological therapies, including plasma exchange, IVIG, corticosteroids, azathioprine, cyclophosphamide, and rituximab, given individually or in combination. One literature review of 15 cases found that treatment with intravenous immune globulin was more likely to be associated with a good outcome if it was administered between within three months of symptom onset. There also rare reports of spontaneous improvement. Given the overall poor prognosis with substantial neurologic disability, a trial of immunotherapy seems reasonable.

Prognosis � The type of antibody appears to affect neurologic prognosis. Patients with PCD associated with anti-Hu or anti-Yo antibodies are less likely to recover, while those with anti-Tr, anti-Ri, or anti-CV 2 antibodies may have a somewhat better chance of neurologic improvement. In some reports, younger age and less severe disability at diagnosis and intervention appear to favorably affect prognosis. � Survival also varies with the type of associated antibody as well as the underlying neoplasm. In one series, survival was worse in patients with anti. Hu antibodies (median seven months) and those with anti-Yo antibodies (median 13 months) compared with patients who had anti-Tr or anti-Ri antibodies (median survival 113 and 69 months, respectively). In another series, median survival was 11 months in patients with anti-Hu antibodies compared with 48 months in those with anti-CV 2 antibodies. Advanced age has a negative impact on survival. � While some reports find that neurologic morbidity contributes most substantively to mortality, others have found that cancer progression was more important

“A patient with breast cancer and paraneoplastic cerebellar syndrome associated with anti-Purkinje cell antibodies: Response to CMF chemotherapy” �M. Faris et al �A 41 -year-old lady underwent a left mastectomy and axillary clearance in 1992, for T 2 N 0 breast cancer. She remained well until December 1995, when she presented with a rapidly progressive cerebellar ataxia. Full investigations for metastatic disease were negative. A clinical diagnosis of paraneoplastic cerebellar degeneration was confirmed by a high titre of anti. Purkinje cell antibodies. She was treated with cyclophosphomide, methotrexate and 5 -fluorouracil. The improvement in neurological symptoms was dramatic and has been maintained by further hormone manipulation (ovarian ablation). The patient now leads a normal life without medication.

“Anti-GAD antibodies in paraneoplastic cerebellar ataxiaassociated with limbic encephalitis and autonomic dysfunction” � C. Carra-Dalliere et al. � A 59 -year-old patient, with history of polymyalgia rheumatica and active smoking, was admitted for subacute cerebellar ataxia and memory dysfunction explained by limbic encephalitis on brain MRI. He also presented with orthostatic hypotension and erectile dysfunction revealing autonomic dysfunction. CSF was inflammatory and antibodies to GAD were positive. Onconeuronal antibodies including GABAB receptor antibodies were negative. Patient's condition quickly improved after intravenous immunoglobulins. A few months later, a small cell lung carcinoma was diagnosed and precociously treated. � Conclusion � This case report underlines the importance of appropriate studies to confirm a primitive neoplasia, when confronted with limbic encephalitis and cerebellar ataxia, even if anti-GAD antibodies rarely defineparaneoplastic syndromes.

“Clinical presentation of immune-mediated cerebellar ataxia” � G. Demarquay et al. � Accumulation of recent clinical evidence indicates that the immune system plays an important role in some central nervous system diseases usually regarded as degenerative. The most striking example is paraneoplastic cerebellar ataxia (PCA), which is characterized by autoimmune cross-reaction between tumoral and nervous system antigens. � In the past 20 years, several antibodies directed against neuronal and tumoral antigens have been described in association with PCA, leading to the description of different subtypes of PCA based on the associated antibodies, the clinical course and the type of tumor. In some subtypes, cerebellar ataxia occurs in isolation, whereas in others, cerebellar ataxia is a syndrome that occurs in conjunction with extensive nervous system disease. Circulating antibodies have also been described in patients with non-paraneoplasticcerebellar ataxia (N-PCA), suggesting that the immune system may be involved in certain cases of sporadic cerebellar ataxia. � Immune-mediated cerebellar ataxia does not seem to be limited to paraneoplastic neurological syndromes. Further studies are however necessary to understand the exact pathophysiology of these disorders and offer effective treatments.
 What causes ataxia
What causes ataxia Loulwah mukharesh
Loulwah mukharesh Difference between cerebellar ataxia and sensory ataxia
Difference between cerebellar ataxia and sensory ataxia Paraneoplastic syndrome symptoms
Paraneoplastic syndrome symptoms How to calculate optical activity
How to calculate optical activity Optic ataxia
Optic ataxia Gait ataxia spokane
Gait ataxia spokane Nihss best language
Nihss best language Limb ataxia
Limb ataxia Ataxia
Ataxia Exercises for ataxia
Exercises for ataxia Ataxia definition
Ataxia definition Gait patterns with walker
Gait patterns with walker What is a waddling gait
What is a waddling gait What causes day and night rotation or revolution
What causes day and night rotation or revolution Seasons
Seasons There are four season all over the world true or false
There are four season all over the world true or false Proximate vs ultimate causation
Proximate vs ultimate causation Animal behavior biology
Animal behavior biology Midwest neurology
Midwest neurology Midwest neurology
Midwest neurology