Newer Antihypertensive Drugs Vinod Sharma National Heart Institute




















- Slides: 38

Newer Antihypertensive Drugs Vinod Sharma National Heart Institute New Delhi 1

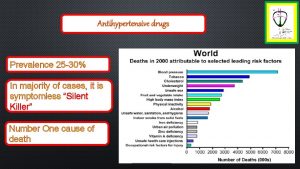
Newer Antihypertensive Drugs Arterial Hypertension, a widespread but controllable disease affecting as much as 30 -45% of general population. Despite the Broad spectrum of already available pharmacological (as well as non-pharmacological) means of BP control, there is no evidence for a change in average blood pressure value over the past decade. Eur Heart J 2013 Rate of stroke (as an indirect indicator of blood pressure levels in population) tends to increase in Eastern European Countries & Central Asia. - Obvious medical demand for novel approach / drugs to treat high blood pressure. Eur Heart J 2011 2

Newer Antihypertensive Drugs Current “Gold Standard” Therapy Ø ACE Inhibitors Ø 16 ACE Inhibitors CAPP Imidapril STOP – 2 Cilazapril HOPE Ø AT 1 R Blockers LIFE Ø 7 AT 1 R Blockers Azilsartan VALUE ONTARGET 3

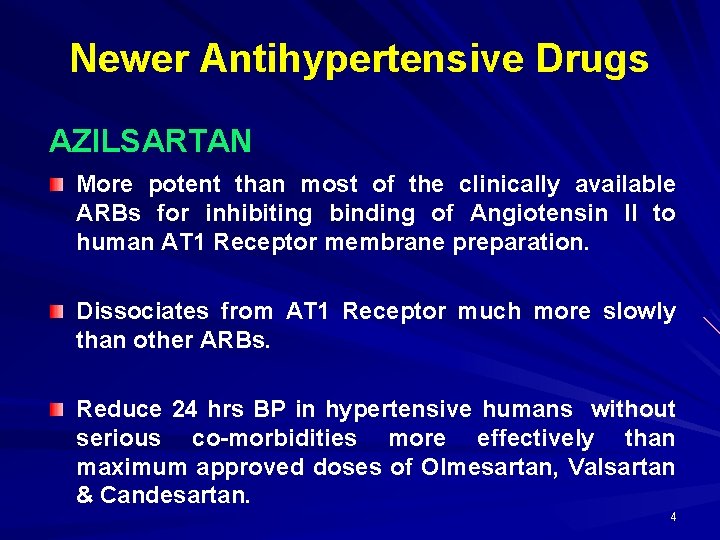
Newer Antihypertensive Drugs AZILSARTAN More potent than most of the clinically available ARBs for inhibiting binding of Angiotensin II to human AT 1 Receptor membrane preparation. Dissociates from AT 1 Receptor much more slowly than other ARBs. Reduce 24 hrs BP in hypertensive humans without serious co-morbidities more effectively than maximum approved doses of Olmesartan, Valsartan & Candesartan. 4

Effects of the Angiotensin Receptor Blocker Azilsartan Medoxomil Versus Olmesartan & Valsartan on Ambulatory and Clinic Blood Pressure in Patients with Stages 1 and 2 Hypertension William B White, Michael A Weber, Domenic Sica, George L Bakris, Alfonso Perez, Charlie Cao, Stuart Kupfer Azilsartan Medoxomil at its maximal dose has superior efficacy to both Olmesartan and Valsartan at their maximal, approved doses without increasing adverse events. Azilsartan Medoxomil could provide higher rates of hypertension control within the ARB class. Hypertension 2011; 57: 413 -4205

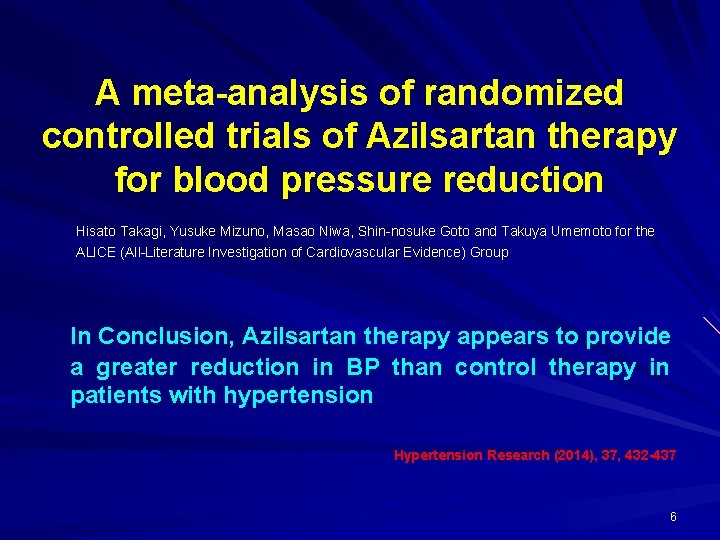
A meta-analysis of randomized controlled trials of Azilsartan therapy for blood pressure reduction Hisato Takagi, Yusuke Mizuno, Masao Niwa, Shin-nosuke Goto and Takuya Umemoto for the ALICE (All-Literature Investigation of Cardiovascular Evidence) Group In Conclusion, Azilsartan therapy appears to provide a greater reduction in BP than control therapy in patients with hypertension Hypertension Research (2014), 37, 432 -437 6

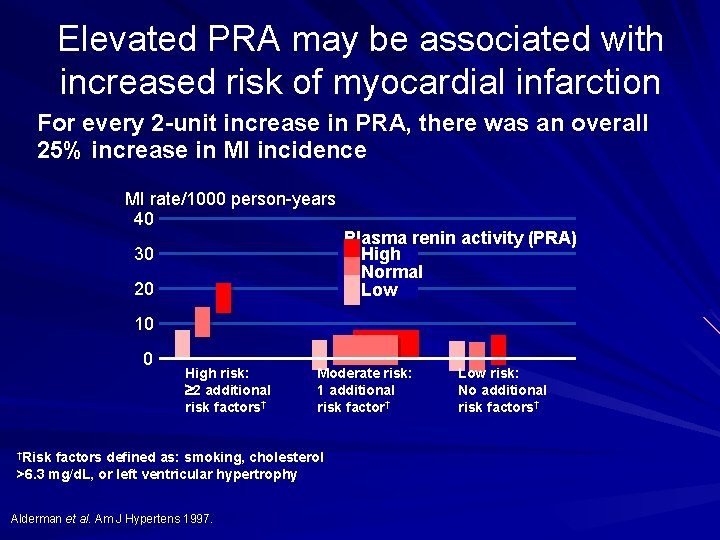
Elevated PRA may be associated with increased risk of myocardial infarction For every 2 -unit increase in PRA, there was an overall 25% increase in MI incidence MI rate/1000 person-years 40 30 20 Plasma renin activity (PRA) High Normal Low 10 0 High risk: 2 additional risk factors† †Risk Moderate risk: 1 additional risk factor† factors defined as: smoking, cholesterol >6. 3 mg/d. L, or left ventricular hypertrophy Alderman et al. Am J Hypertens 1997. Low risk: No additional risk factors†

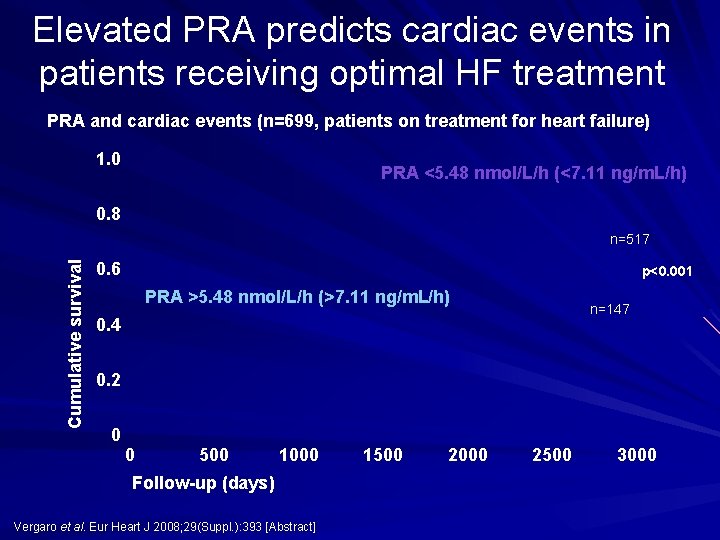
Elevated PRA predicts cardiac events in patients receiving optimal HF treatment PRA and cardiac events (n=699, patients on treatment for heart failure) 1. 0 PRA <5. 48 nmol/L/h (<7. 11 ng/m. L/h) 0. 8 Cumulative survival n=517 0. 6 p<0. 001 PRA >5. 48 nmol/L/h (>7. 11 ng/m. L/h) n=147 0. 4 0. 2 0 0 500 1000 Follow-up (days) Vergaro et al. Eur Heart J 2008; 29(Suppl. ): 393 [Abstract] 1500 2000 2500 3000

Aliskiren 150 -300 mg daily – new generation in antihypertensive treatment First direct renin inhibitor for hypertension Uniquely lowers PRA in monotherapy and combination Effective and sustained monotherapy Additional BP lowering when combined with other antihypertensives Sustained 24 -hour BP control with prolonged effect after withdrawal Placebo-like safety and tolerability profile Potential for improved end-organ protection via optimal suppression of the renin system – BNP reduction in heart failure – LVH regression in hypertensive obesity – Proteinuria reduction in DM nephropathy

“It is said that present is pregnant with future” “Voltaire” 10

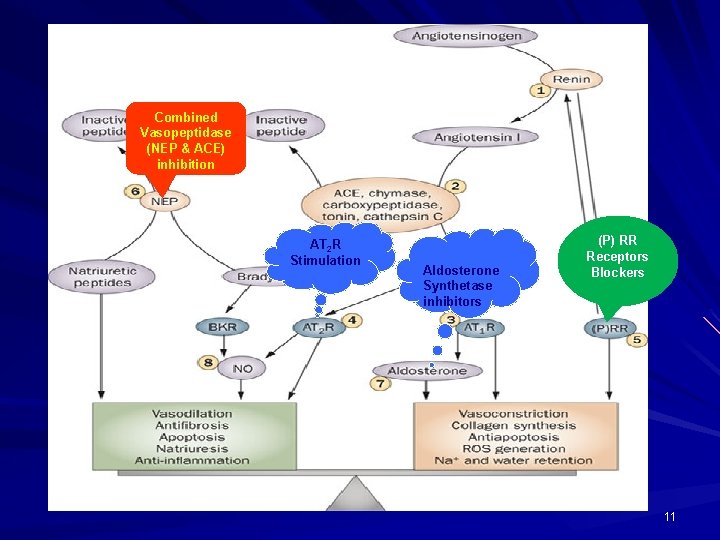
Combined Vasopeptidase (NEP & ACE) inhibition AT 2 R Stimulation Aldosterone Synthetase inhibitors (P) RR Receptors Blockers 11

Newer Antihypertensive Drugs AT 2 R Agonists: Stimulating the RAAS AT 2 R mediates actions opposing AT 1 R stimulation: INHIBITION of cell growth & proapoptotic actions Enhanced NO formation in vascular tissue Attenuation of myointimal hyperplasia after endothelial denudation Reduction of undesired neointimal growth, cardiac remodeling or proliferative retinopathy. AT 2 R stimulation, an exciting & innovative approach to treat hypertension. 12 12

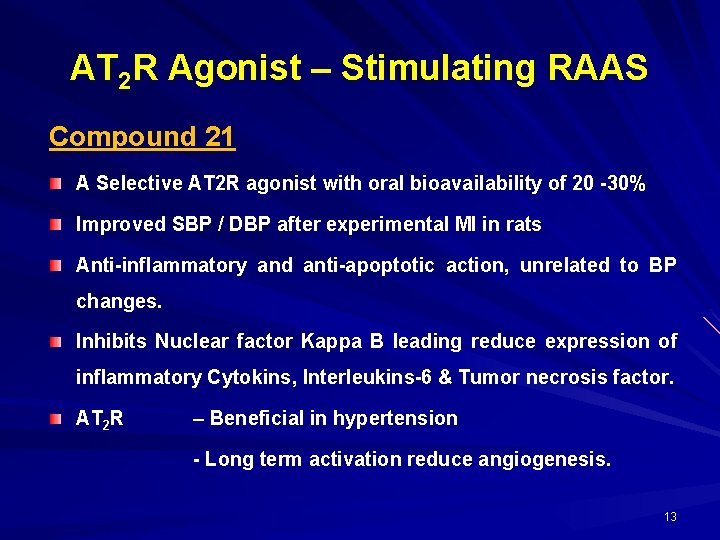
AT 2 R Agonist – Stimulating RAAS Compound 21 A Selective AT 2 R agonist with oral bioavailability of 20 -30% Improved SBP / DBP after experimental MI in rats Anti-inflammatory and anti-apoptotic action, unrelated to BP changes. Inhibits Nuclear factor Kappa B leading reduce expression of inflammatory Cytokins, Interleukins-6 & Tumor necrosis factor. AT 2 R – Beneficial in hypertension - Long term activation reduce angiogenesis. 13

Dual Vasopeptidase Inhibitors Inhibition of Neprilysin, potentiates: - Diuretics - Natriuresis - Vaso-relaxant effects of endogenous natriuretic peptide. Increase concentration of vasoconstrictor peptides (Angiotensin 2 and Endothelin-1), which are metabolized by Neprilysin. 14

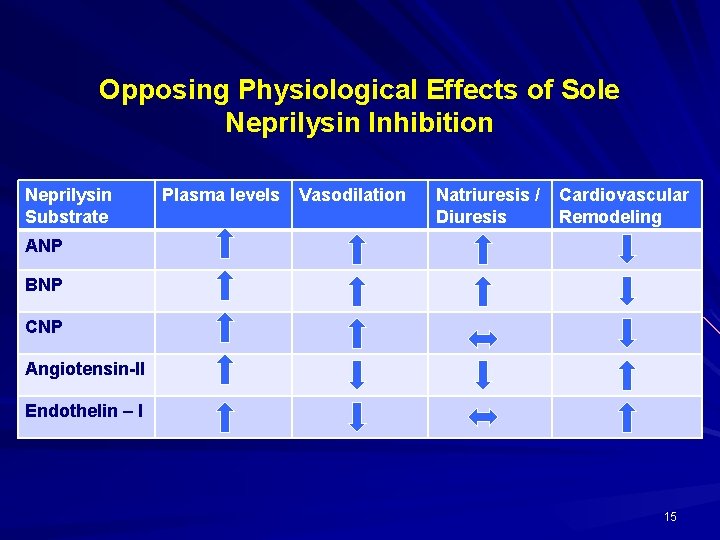
Opposing Physiological Effects of Sole Neprilysin Inhibition Neprilysin Substrate Plasma levels Vasodilation Natriuresis / Diuresis Cardiovascular Remodeling ANP BNP CNP Angiotensin-II Endothelin – I 15

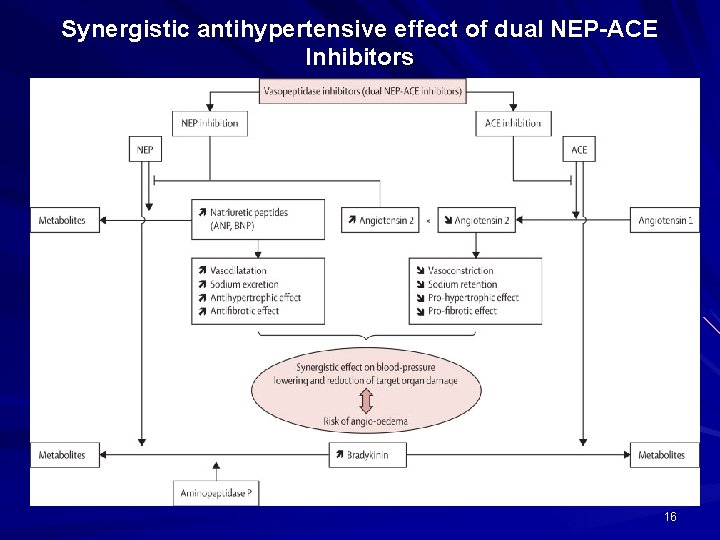
Synergistic antihypertensive effect of dual NEP-ACE Inhibitors 16

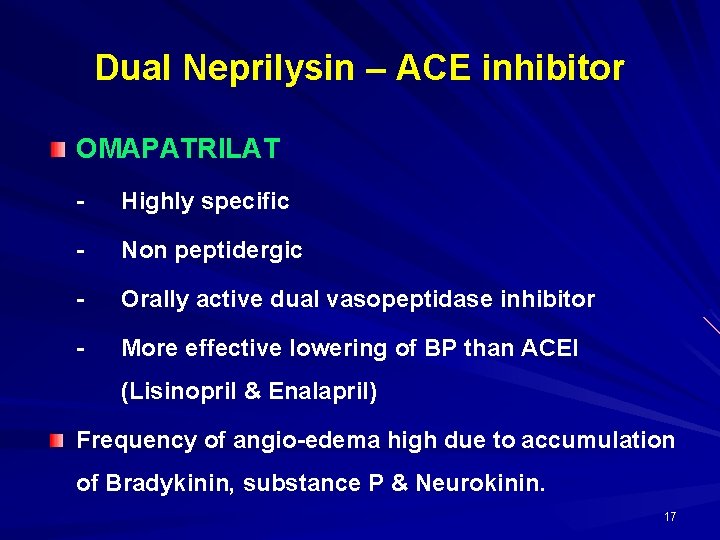
Dual Neprilysin – ACE inhibitor OMAPATRILAT - Highly specific - Non peptidergic - Orally active dual vasopeptidase inhibitor - More effective lowering of BP than ACEI (Lisinopril & Enalapril) Frequency of angio-edema high due to accumulation of Bradykinin, substance P & Neurokinin. 17

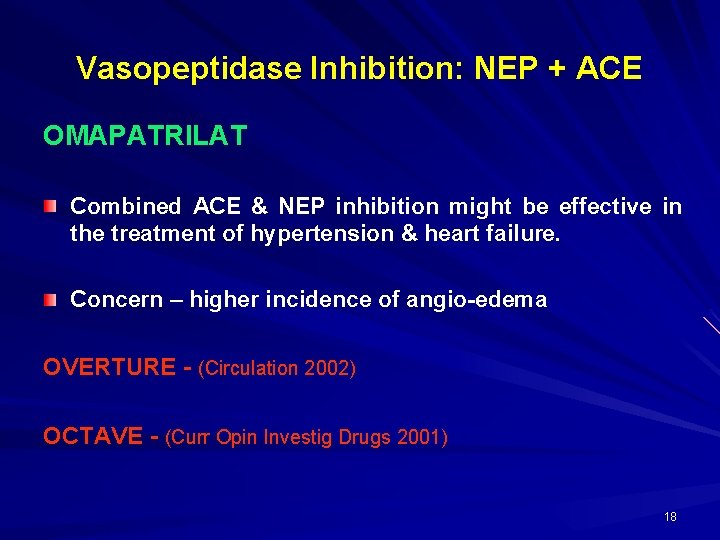
Vasopeptidase Inhibition: NEP + ACE OMAPATRILAT Combined ACE & NEP inhibition might be effective in the treatment of hypertension & heart failure. Concern – higher incidence of angio-edema OVERTURE - (Circulation 2002) OCTAVE - (Curr Opin Investig Drugs 2001) 18

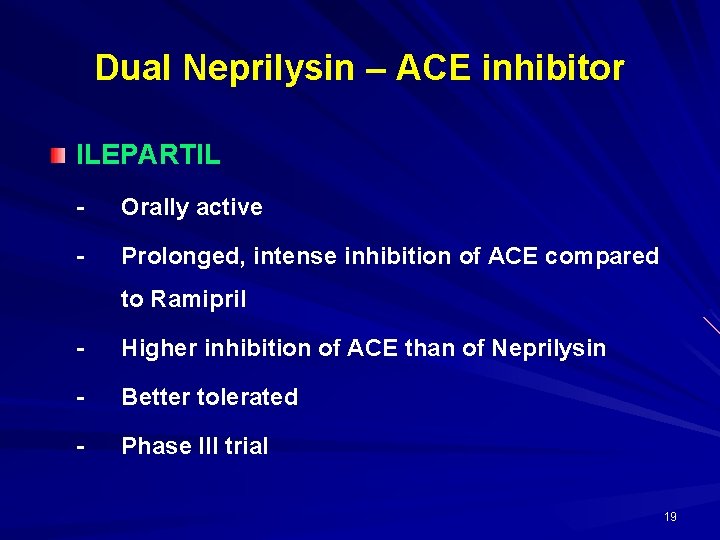
Dual Neprilysin – ACE inhibitor ILEPARTIL - Orally active - Prolonged, intense inhibition of ACE compared to Ramipril - Higher inhibition of ACE than of Neprilysin - Better tolerated - Phase III trial 19

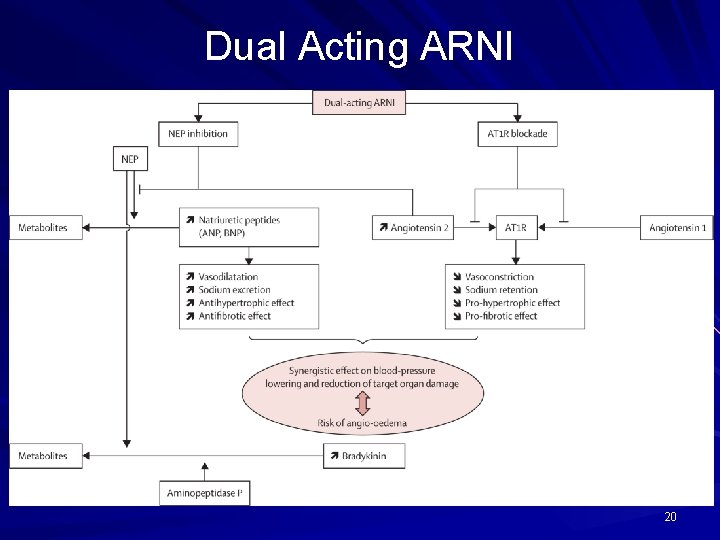
Dual Acting ARNI 20

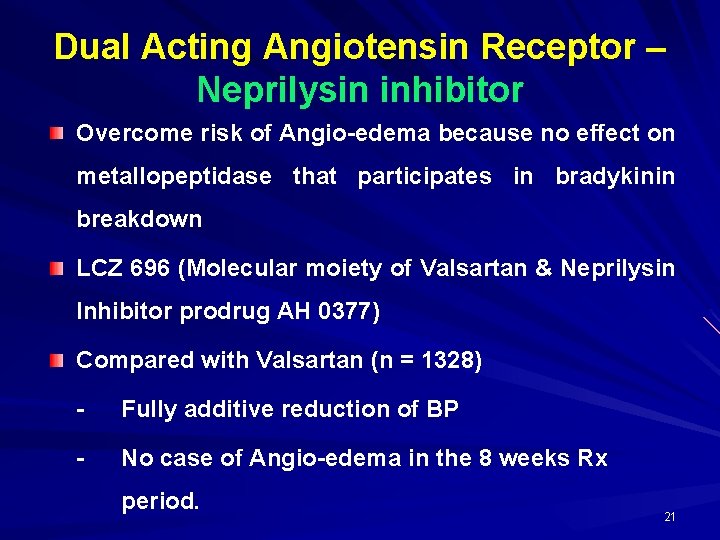
Dual Acting Angiotensin Receptor – Neprilysin inhibitor Overcome risk of Angio-edema because no effect on metallopeptidase that participates in bradykinin breakdown LCZ 696 (Molecular moiety of Valsartan & Neprilysin Inhibitor prodrug AH 0377) Compared with Valsartan (n = 1328) - Fully additive reduction of BP - No case of Angio-edema in the 8 weeks Rx period. 21

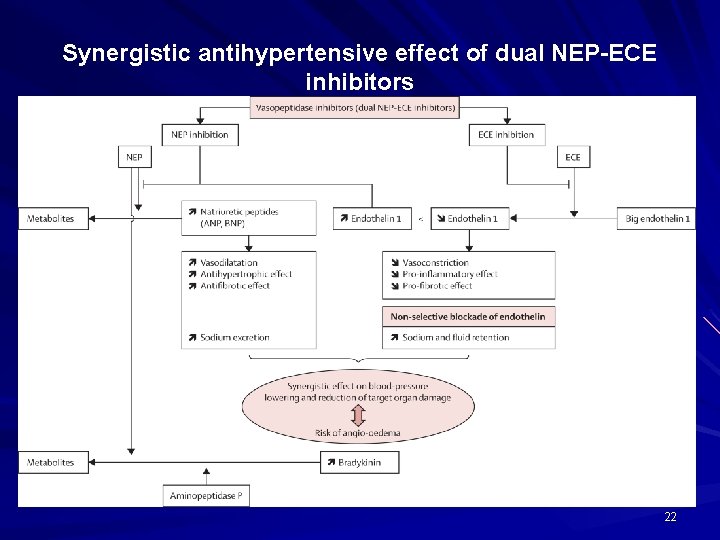
Synergistic antihypertensive effect of dual NEP-ECE inhibitors 22

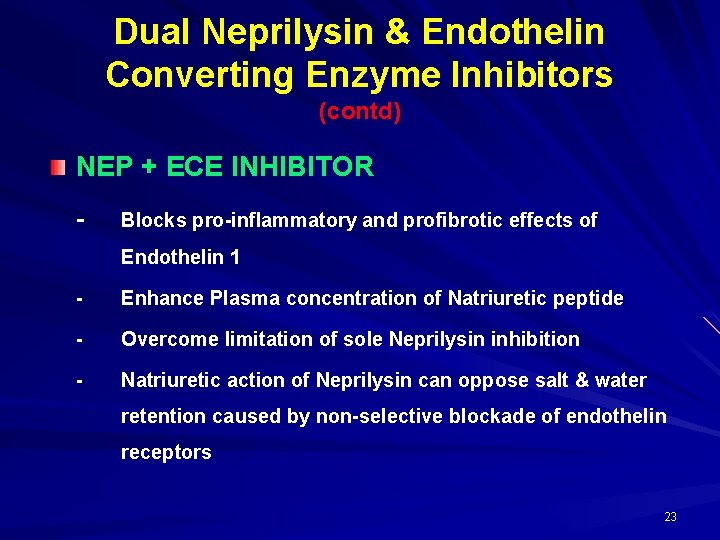
Dual Neprilysin & Endothelin Converting Enzyme Inhibitors (contd) NEP + ECE INHIBITOR - Blocks pro-inflammatory and profibrotic effects of Endothelin 1 - Enhance Plasma concentration of Natriuretic peptide - Overcome limitation of sole Neprilysin inhibition - Natriuretic action of Neprilysin can oppose salt & water retention caused by non-selective blockade of endothelin receptors 23

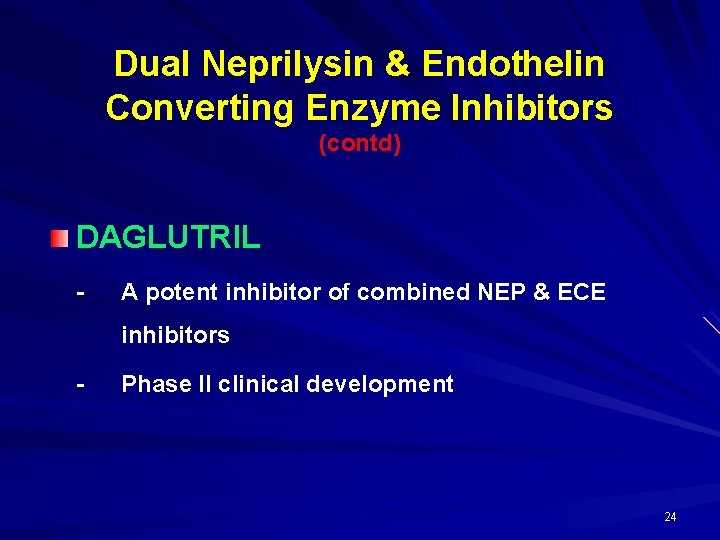
Dual Neprilysin & Endothelin Converting Enzyme Inhibitors (contd) DAGLUTRIL - A potent inhibitor of combined NEP & ECE inhibitors - Phase II clinical development 24

Newer Antihypertensive Drugs – Endothelin Antagonists Endothelin 1 – A potent vasoconstrictor peptide acts through Endothelin A & B receptors Endothelin A receptor Antagonists – Bosentan Endothelin A + B – Arosentan - Enrasentan - Tozesentan Endothelin 1 Antagonist – Resistant Hypertension – Significantly lowered BP compared to Placebo - Water Retention, edema & rise in liver transaminases 25

Newer Antihypertensive Drugs – Endothelin Antagonists (contd) DARUSENTAN (Mixed Endothelin A & B Antagonists – Phase III trial (Gilead Sciences) More effective than placebo in lowering clinic & ABP, in addition to Rx with three or more antihypertensive drugs. 25% patients had fluid retention & edema, managed by Diuretic. Weber MA et al: Lancet 2009 26

ACE 2 / Ang (1 -7) / Mas Receptor axis Agonists Mas receptors “Protective RAAS” Mas receptor effects Antifibrosis Antiinflammation Antiproliferation No release Non-peptide Mas Receptor Agonist (AVE-0991) “Besides BP lowering effects, AVE-0991 seems to exert blood pressure independent renoprotective effect” 27

Newer Antihypertensive Drugs Aldosterone Receptor Antagonists Spiranolactone - Effective BP reduction - Reduce mortality in heart failure - Poor selectivity for mineralocorticoid receptor leading to progesterone & testosterone – dependent effects, loss of lipido, menstrual irregularities, impotence & gynaecomastia 28

Newer Antihypertensive Drugs Selective mineralocorticoid receptor blocker – Eplerenone ---------------------------------------Non-inferior to Amlodipine, Enalapril & Losartan in reducing BP. Reduce all cause mortality in cases with heart failure. Sexual adverse effects are less pronounced with Eplerenone than with Spironolactone. 29

Newer Antihypertensive Drugs Aldosterone Synthetase Inhibitors Inhibits the formation of Aldosterone. FAD 286 / SPP 2745 - Lowered BP in rats - Good specificity - Offer protection to cardiac, renal & vascular system - Compatible with conventional therapy 30

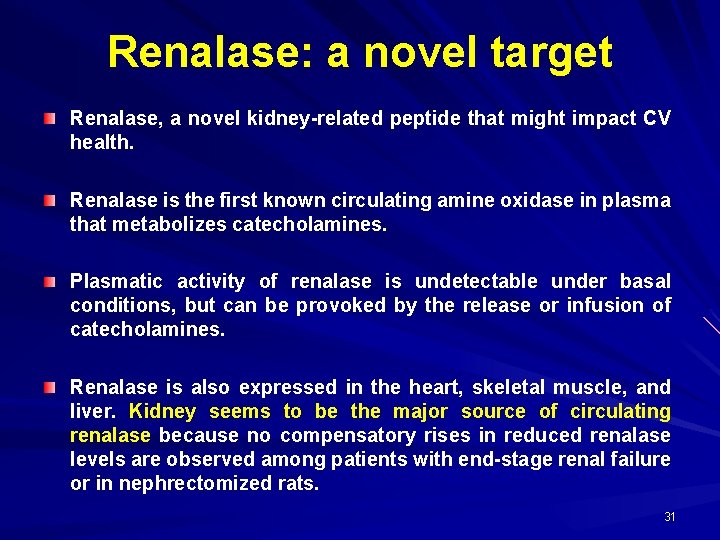
Renalase: a novel target Renalase, a novel kidney-related peptide that might impact CV health. Renalase is the first known circulating amine oxidase in plasma that metabolizes catecholamines. Plasmatic activity of renalase is undetectable under basal conditions, but can be provoked by the release or infusion of catecholamines. Renalase is also expressed in the heart, skeletal muscle, and liver. Kidney seems to be the major source of circulating renalase because no compensatory rises in reduced renalase levels are observed among patients with end-stage renal failure or in nephrectomized rats. 31

Renalase: a novel target (contd) The down regulation, or knock-out, of renalase is associated with elevated BP. Recombinant renalase has been shown to dose-dependently lower BP, heart rate, and contractility & to protect the myocardium against ischemia – reperfusion injury Metabolism of the renal vasodilator, dopamine, by renalase could raise safety concerns and limit its therapeutic potential. 32

Novel Drugs in Preclinical Development Nitric Oxide Donors - Nitrosyl – Cobinamide - Nitric Oxide releasing Pharmacodynamic hybrids of Losartan & Telmisartan - Naproxcinod Orally active Aminopeptidase – A Inhibitors (QGC 001) - Targets aminopeptidase A, the enzyme that generates angiotensin – 3 in the brain, a major effector peptides of Brain Renin – Angiotensin system in control of Arginine – vasopressin release & blood pressure 33

Newer Antihypertensive Drugs INNOVATIVE TARGETING OF ANGIOTENSIN II ANTI-RAAS VACCINATION 1960, Early anti-renin formula was associated with severe autoimmune kidney disease 2000, Immunization against Angiotensin I vaccine (PMD 3117) - Some evidence of RAAS Blockade - Failed to lower BP 34

Newer Antihypertensive Drugs (contd) ANTI-RAAS VACCINATION Anti-Angiotensin II Antigenic peptide conjugated to a virus like particle (CYT 006) - Lowered SBP by up to 21 mm Hg in rats - Well tolerated in phase I study - Modest BP reduction 9/4 mm Hg in a Phase IIa study. 35

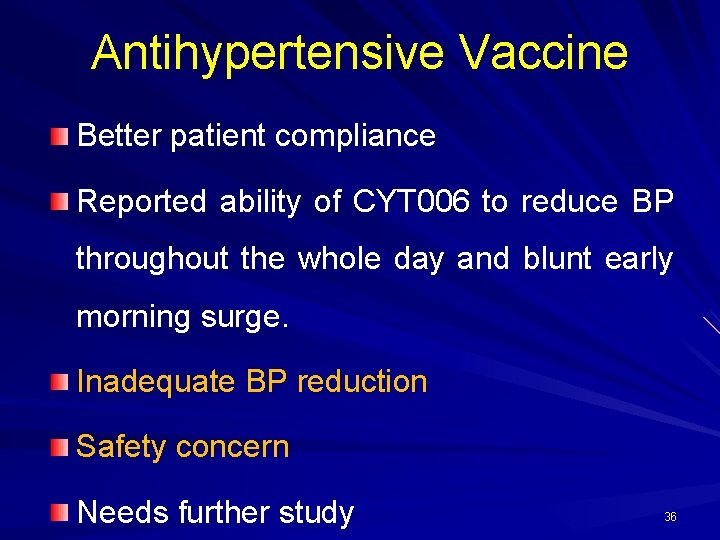
Antihypertensive Vaccine Better patient compliance Reported ability of CYT 006 to reduce BP throughout the whole day and blunt early morning surge. Inadequate BP reduction Safety concern Needs further study 36

37

Human Vaccines & Immunotherapeutics, March 2014 38
 Dr vinod sharma national heart institute
Dr vinod sharma national heart institute Antihypertensive drugs classification
Antihypertensive drugs classification Classification of sympatholytic drugs
Classification of sympatholytic drugs Antihypertensive drugs classification
Antihypertensive drugs classification Left ventricular hypertrophy
Left ventricular hypertrophy Classification of antihypertensive drugs with examples
Classification of antihypertensive drugs with examples Centrally acting sympathoplegic drugs
Centrally acting sympathoplegic drugs Antihypertensive
Antihypertensive Dr adarsh kumar national heart institute
Dr adarsh kumar national heart institute Newer drug delivery system
Newer drug delivery system Optimistic comparative and superlative
Optimistic comparative and superlative Light comparative
Light comparative Translate
Translate Vinod sampat
Vinod sampat Vinod ganapathy
Vinod ganapathy Dma directions
Dma directions Simsonattribut
Simsonattribut Byzantine reliable broadcast
Byzantine reliable broadcast Ca vinod jain
Ca vinod jain Vinod panicker
Vinod panicker Aorldw
Aorldw Vinod bidwaik
Vinod bidwaik Vinod kurup
Vinod kurup Vinod dhall
Vinod dhall Chapter 24 heart failure drugs
Chapter 24 heart failure drugs Idaho heart institute
Idaho heart institute Heart and vascular institute
Heart and vascular institute Heart and vascular institute
Heart and vascular institute Heart and vascular institute
Heart and vascular institute Robert cubeddu md
Robert cubeddu md Unist ulsan national institute of science and technology
Unist ulsan national institute of science and technology National institute of meteorology
National institute of meteorology National assistive technology research institute
National assistive technology research institute National metrology institute of japan
National metrology institute of japan Lvivtech city
Lvivtech city Nawea
Nawea National institute on drug abuse
National institute on drug abuse National institute of standards and technology
National institute of standards and technology National human genome research institute
National human genome research institute