Nanomaterials Nathan Liang Paul Maynard Wei Li Nanotechnology




























![Larson’s Lattice Model - typical trial moves l Pair interchange [5] l l Chain Larson’s Lattice Model - typical trial moves l Pair interchange [5] l l Chain](https://slidetodoc.com/presentation_image/559dd8a1c951a4fb2e35a2be58d80ab2/image-45.jpg)





- Slides: 60

Nanomaterials Nathan Liang Paul Maynard Wei Li

Nanotechnology

What is Nanotechnology? It comprises any technological developments on the nanometer scale, usually 0. 1 to 100 nm. l One nanometer equals one thousandth of a micrometer or one millionth of a millimeter. l It is also referred as microscopic technology. l

Molecular Nanotechnology l The term nanotechnology is often used interchangeably with molecular nanotechnology (MNT) l MNT includes the concept of mechanosynthesis. l MNT is a technology based on positionallycontrolled mechanosynthesis guided by molecular machine systems.

Nanotechnology in Field of Electronics Miniaturization l Device Density l

History l Richard Feynman l l 1959, entitled ‘There's Plenty of Room at the Bottom’ Manipulate atoms and molecules directly 1/10 th scale machine to help to develop the next generation of 1/100 th scale machine, and so forth. As things get smaller, gravity would become less important, surface tension molecule attraction would become more important.

History l Tokyo Science University professor Norio Taniguchi l l 1974 to describe the precision manufacture of materials with nanometre tolerances. K Eric Drexler l l l 1980 s the term was reinvented 1986 book Engines of Creation: The Coming Era of Nanotechnology. He expanded the term into Nanosystems: Molecular Machinery, Manufacturing, and Computation

Nanomaterial and Devices l Small Scales l Extreme Properties l Nanobots

Self-Assemble l Nanodevices build themselves from the bottom up. l Scanning probe microscopy l l l Atomic force microscopes scanning tunneling microscopes scanning the probe over the surface and measuring the current, one can thus reconstruct the surface structure of the material

Problems in Nanotechnology l how to assemble atoms and molecules into smart materials and working devices? l Supramolecular chemistry l self-assemble into larger structures

Current Nanotechnology l Stanford University l extremely small transistor two nanometers wide and regulates electric current through a l channel that is just one to three nanometers long ultra-low-power l

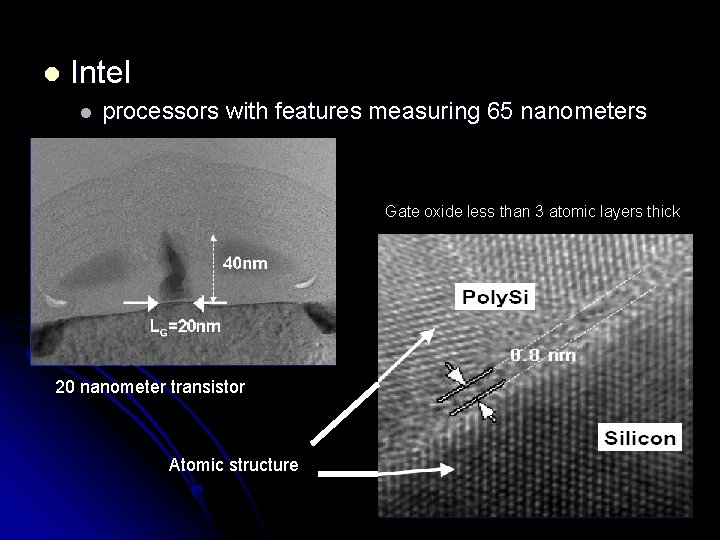
l Intel l processors with features measuring 65 nanometers Gate oxide less than 3 atomic layers thick 20 nanometer transistor Atomic structure

Plasmons l l Waves of electrons traveling along the surface of metals They have the same frequency and electromagnetic field as light. Their sub-wavelength require less space. With the use of plasmons information can be transferred through chips at an incredible speed

Nanomaterial modeling and simulation types

What I will cover Carbon Nanotubes l Bio-Nano-Materials l Thermoelectric Nanomaterials l What is happening at UK l

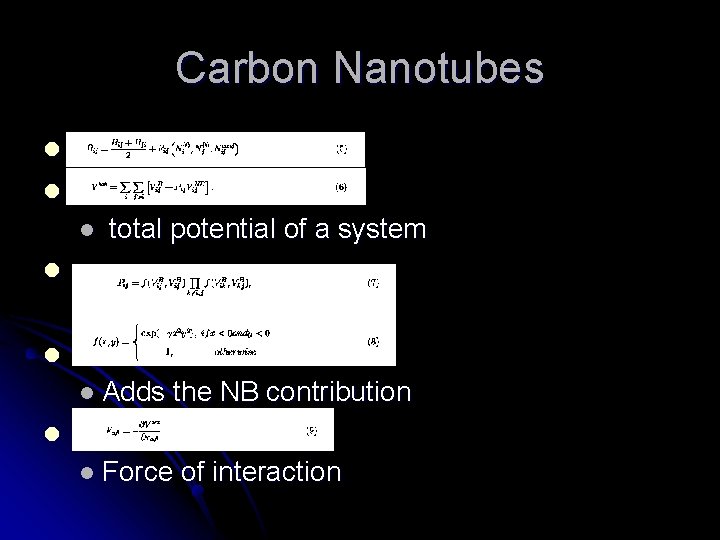
Carbon Nanotubes l What are they? l Carbon molecules aligned in cylinder formation l Who discovered them? l Researchers l at NEC in 1991 What are some of their uses? l Minuscule wires l Extremely small devices


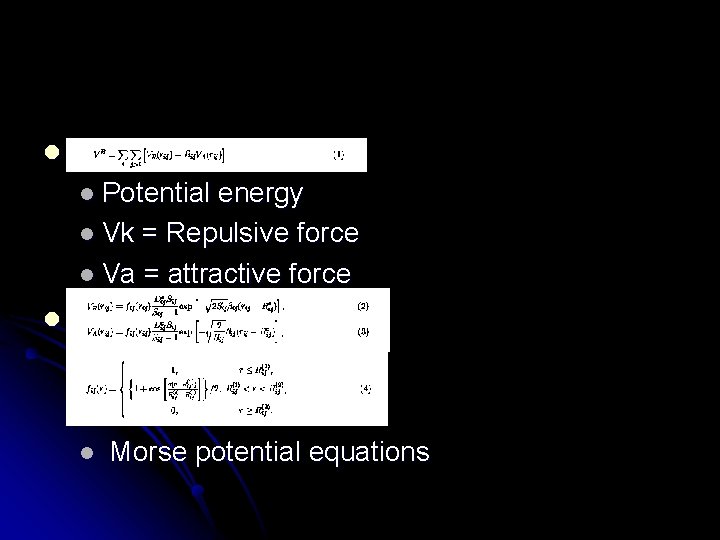
l l Potential energy l Vk = Repulsive force l Va = attractive force l l Morse potential equations

Carbon Nanotubes l l l total potential of a system l l l Adds the NB contribution l l Force of interaction

Carbon Nanotubes l l Leonard – Jones potential with von der Waals interaction l l Geen l - Kudo relation






Bio-Nanomaterials l What is Bio-Nanomaterials? l l Putting DNA inside of carbon nanotubes What can this research give us? l There are lots of chemical and biological applications

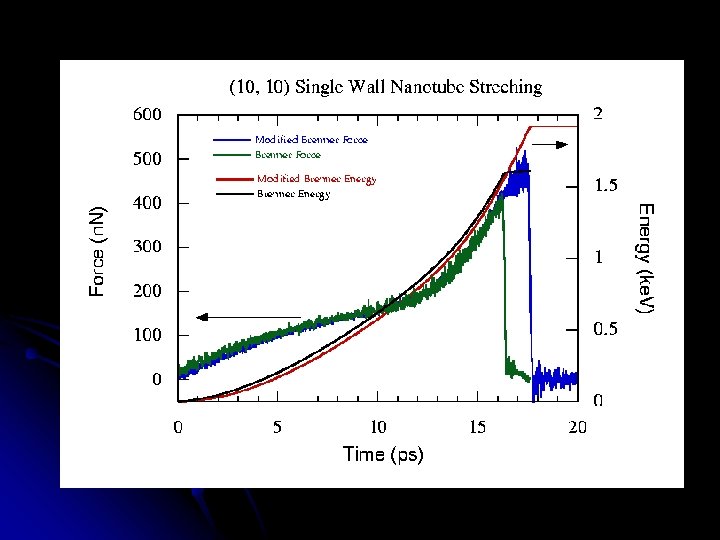
Distances over time

Van der waals engery

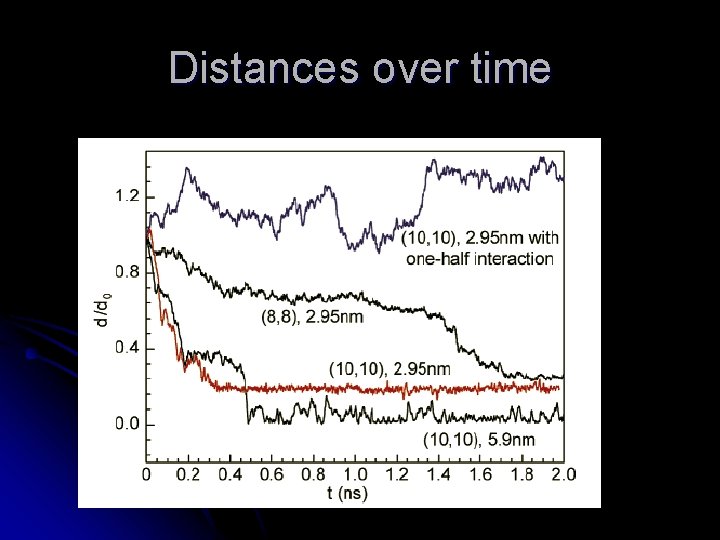
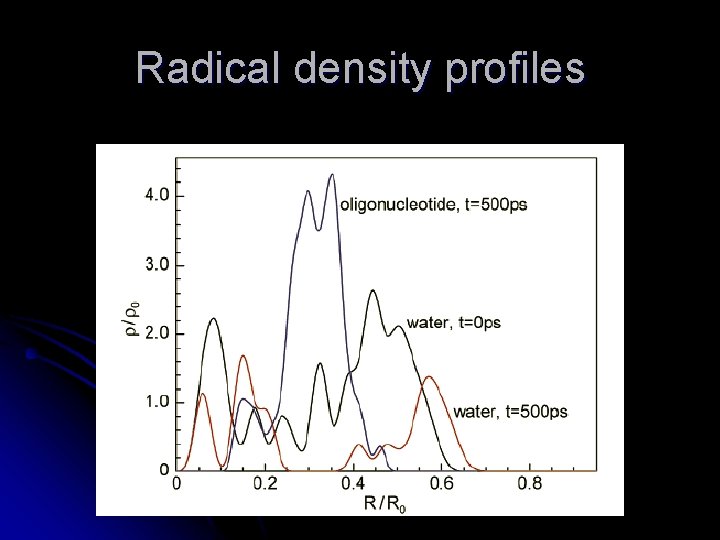
Radical density profiles

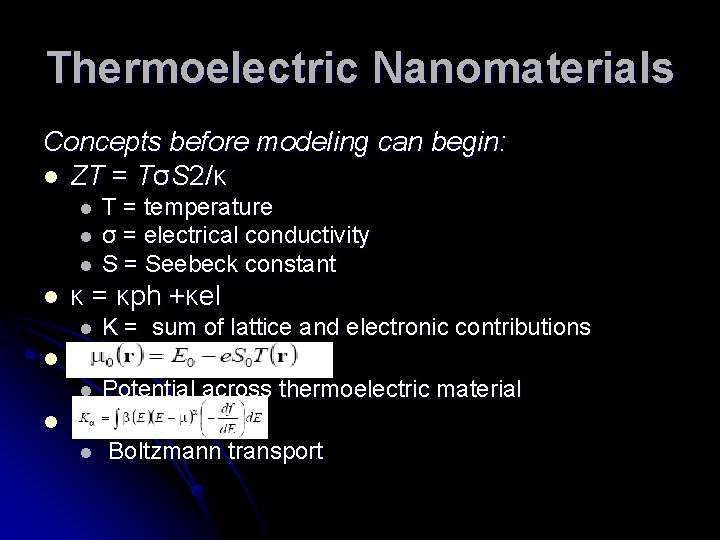
Thermoelectric Nanomaterials Concepts before modeling can begin: l ZT = TσS 2/κ l l T = temperature σ = electrical conductivity S = Seebeck constant κ = κph +κel l K = sum of lattice and electronic contributions l Potential across thermoelectric material l Boltzmann transport

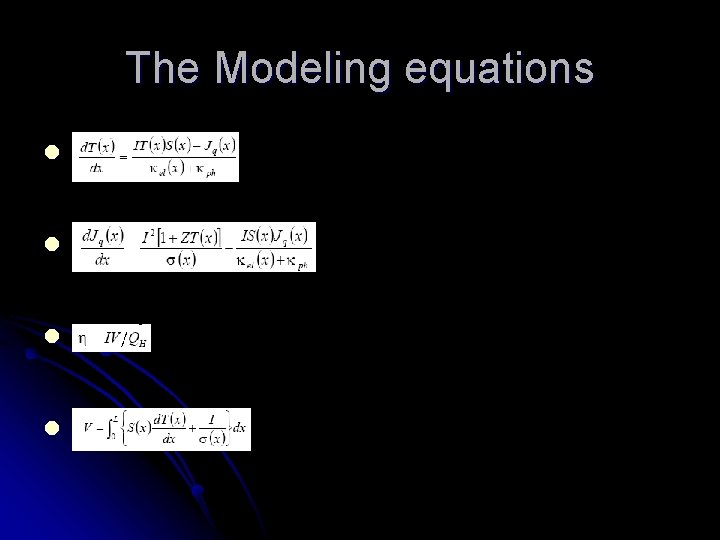
The Modeling equations l l

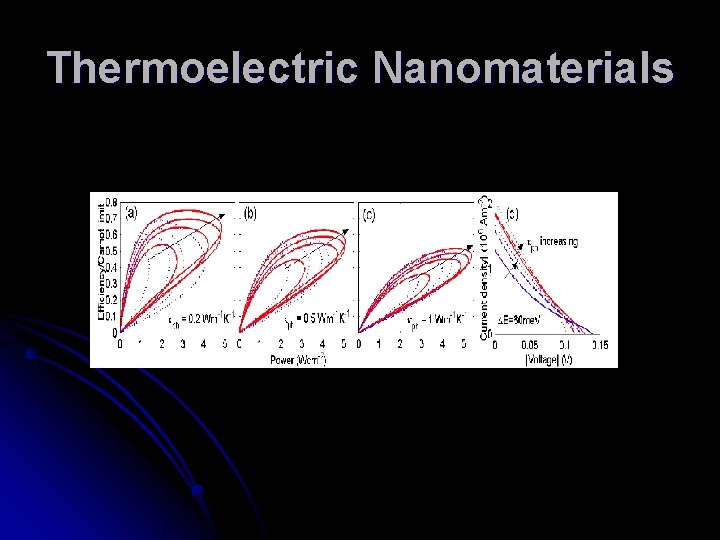
Thermoelectric Nanomaterials

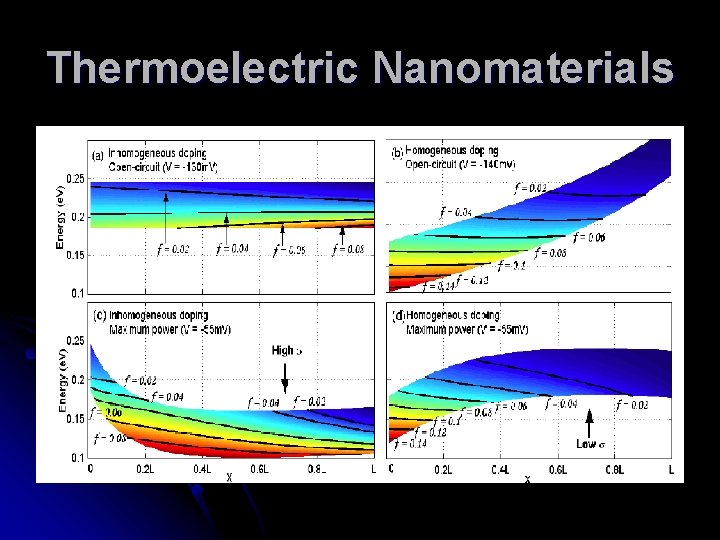
Thermoelectric Nanomaterials

Thermoelectric Nanomaterials

Nanomaterials at UK l l l l l Deformation Mechanisms of Nanostructured Materials Synthesis of Nanoporous Ceramics by Engineered Molecular Assembly Carbon Nanotubes Optical-based Nano-Manufacturing The Grand Quest: CMOS High-k Gate Insulators Self-assembled metal alloy nanostructures Rare-earth Monosulfides: From Bulk Samples to Nanowires Thermionic Emission and Energy Conversion with Quantum Wires Resonance-Coupled Photoconductive Decay

Computer Simulation of Fluorinated Surfactants

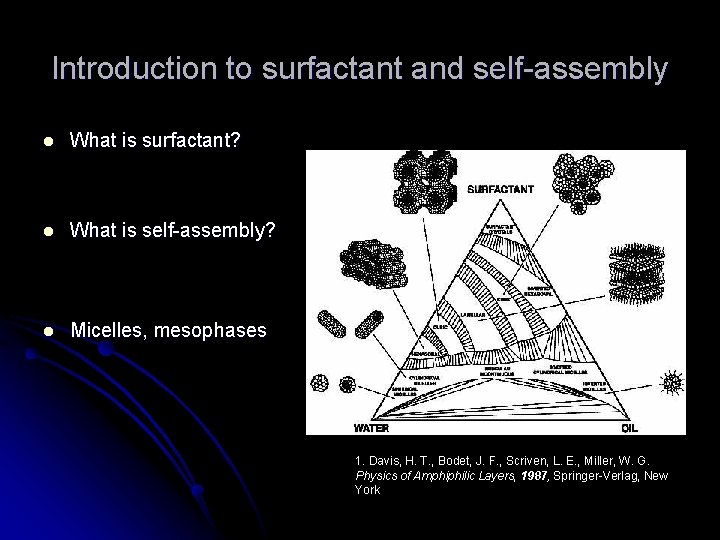
Introduction to surfactant and self-assembly l What is surfactant? l What is self-assembly? l Micelles, mesophases 1. Davis, H. T. , Bodet, J. F. , Scriven, L. E. , Miller, W. G. Physics of Amphiphilic Layers, 1987, Springer-Verlag, New York

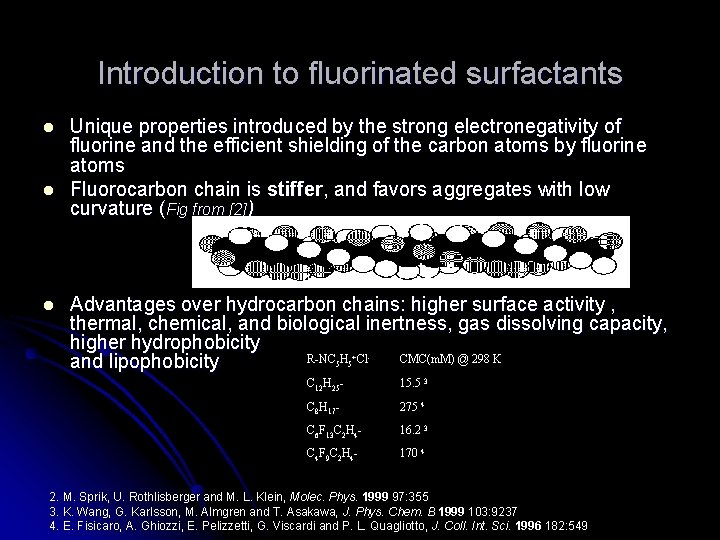
Introduction to fluorinated surfactants l l l Unique properties introduced by the strong electronegativity of fluorine and the efficient shielding of the carbon atoms by fluorine atoms Fluorocarbon chain is stiffer, and favors aggregates with low curvature (Fig from [2]) Advantages over hydrocarbon chains: higher surface activity , thermal, chemical, and biological inertness, gas dissolving capacity, higher hydrophobicity R-NC H Cl CMC(m. M) @ 298 K and lipophobicity 5 5 + - C 12 H 25 - 15. 5 3 C 8 H 17 - 275 4 C 6 F 13 C 2 H 4 - 16. 2 3 C 4 F 9 C 2 H 4 - 170 4 2. M. Sprik, U. Rothlisberger and M. L. Klein, Molec. Phys. 1999 97: 355 3. K. Wang, G. Karlsson, M. Almgren and T. Asakawa, J. Phys. Chem. B 1999 103: 9237 4. E. Fisicaro, A. Ghiozzi, E. Pelizzetti, G. Viscardi and P. L. Quagliotto, J. Coll. Int. Sci. 1996 182: 549

Motivations for the computer simulation of fluorinated surfactants l Simulations can be treated as computer experiments that serve as adjuncts to theory and real experiments l Experiment is a viable way to study the effect of chain stiffness, yet it might be expensive to do a systematic study on this topic. l Computer simulations might help selecting surfactants for the right type of mesophase, which provides a guideline for experimental study.

Monte Carlo techniques for the simulation of surfactant solutions l Off-lattice atomistic simulation l All atoms (or small group of atoms, e. g. CH 2 ) are explicitly represented l Most interactions are included, more realistic, yet hard to model Can simulates molecular trajectories on a time-scale of nanoseconds Can’t simulation the self-assembly phenomena l l l Off-lattice coarse-grain l l A number of atoms are grouped together and represented in a simplified manner Electrostatic and dihedral angle potentials are usually absent Can simulate process happening on a time-scale of microseconds, e. g. micelle formation Can’t simulate equilibrium self-assembly structure at higher concentration

Monte Carlo techniques for the simulation of surfactant solutions (continued) l Lattice coarse-grain l l l replacing the continuous space with a discretized lattice of suitable geometry Electrostatic and intra-molecular potentials are absent Fast, efficient, can simulate process happening on a time-scale of a few hours, e. g. mesophase formation Based on Flory-Huggins Theory. Proven to be successful in polymer science for many years for investigating universal properties of single chains, polymer layers and solutions and melts Utility of the model is limited

Choosing the right model for our simulation purpose – lattice coarse-grain l l Most time-consuming part in a MC simulation is the evaluation of inter and intra-molecular potentials after each trial move The speed of off-lattice models is limited, because l l Lattice models are fast, because l l l It has to reevaluate the potential functions explicitly when calculate the energy change after each move The speed of the simulation is determined by the complexity of the potential functions Off-lattice can at most simulate the formation of a few micelles Atoms (united atoms) are moving on the lattice, intra and inter-molecular distance, bond angles are thus discretized It’s possible to precalculate the potentials corresponding to certain distance and angles and build look-up tables When calculate the energy change, only need to look up the tables Can simulate the mesophase formation efficiently Our targeted system: mesophase formation in surfactant solutions

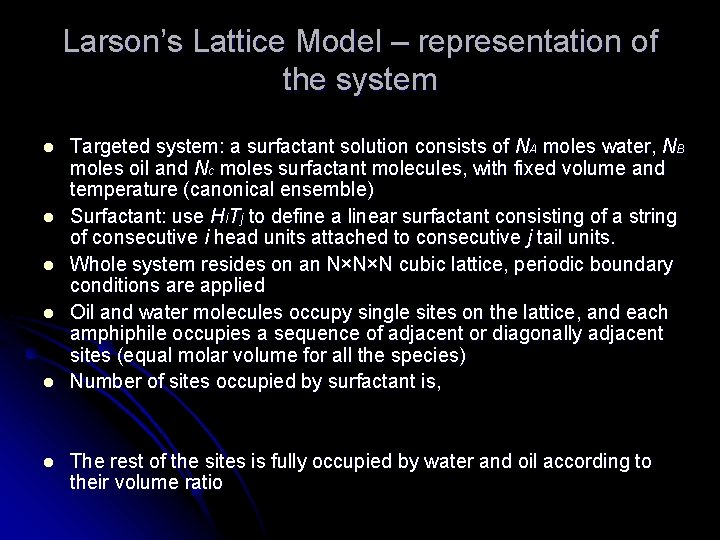
Larson’s Lattice Model – representation of the system l l l Targeted system: a surfactant solution consists of NA moles water, NB moles oil and Nc moles surfactant molecules, with fixed volume and temperature (canonical ensemble) Surfactant: use Hi. Tj to define a linear surfactant consisting of a string of consecutive i head units attached to consecutive j tail units. Whole system resides on an N×N×N cubic lattice, periodic boundary conditions are applied Oil and water molecules occupy single sites on the lattice, and each amphiphile occupies a sequence of adjacent or diagonally adjacent sites (equal molar volume for all the species) Number of sites occupied by surfactant is, The rest of the sites is fully occupied by water and oil according to their volume ratio

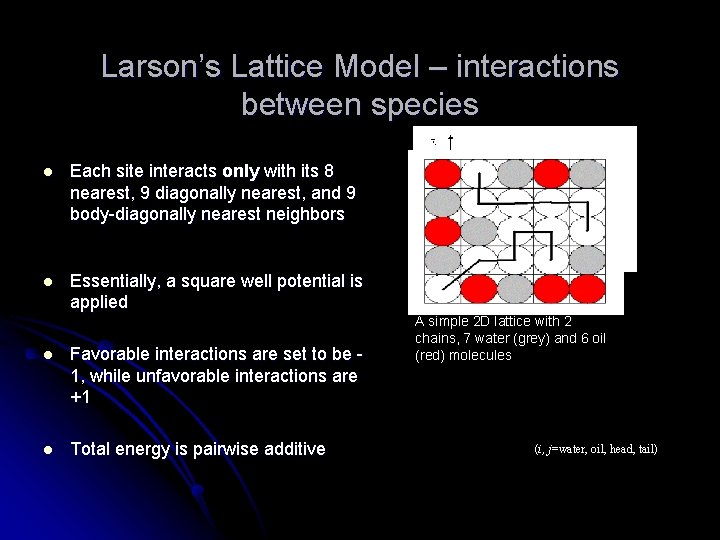
Larson’s Lattice Model – interactions between species l Each site interacts only with its 8 nearest, 9 diagonally nearest, and 9 body-diagonally nearest neighbors l Essentially, a square well potential is applied l Favorable interactions are set to be 1, while unfavorable interactions are +1 l Total energy is pairwise additive Square-well potential A simple 2 D lattice with 2 chains, 7 water (grey) and 6 oil (red) molecules (i, j=water, oil, head, tail)
![Larsons Lattice Model typical trial moves l Pair interchange 5 l l Chain Larson’s Lattice Model - typical trial moves l Pair interchange [5] l l Chain](https://slidetodoc.com/presentation_image/559dd8a1c951a4fb2e35a2be58d80ab2/image-45.jpg)
Larson’s Lattice Model - typical trial moves l Pair interchange [5] l l Chain kink [5] l l A surfactant segment exchanges position with its neighbor without breaking the surfactant chain Chain reptation [5] l l Exchange of positions of two simple molecules One chain end moves to a neighboring site, and the rest of that chain slithers a unit to keep the chain connectivity Chain multiple kink [6] l If a kink move creates a single break in the chain, the simple molecule will continue to exchange with subsequent beads along the chain until beads on the chain are close enough to reconnect. 5. R. G. Larson, L. E. Scriven and H. T. Davis, J. Chem. Phys. , 1985, 83, 2411 6. K. R. Haire, T. J. Carver, A. H. Windle, Computational and Theoretical Polymer Science, 2001, 17

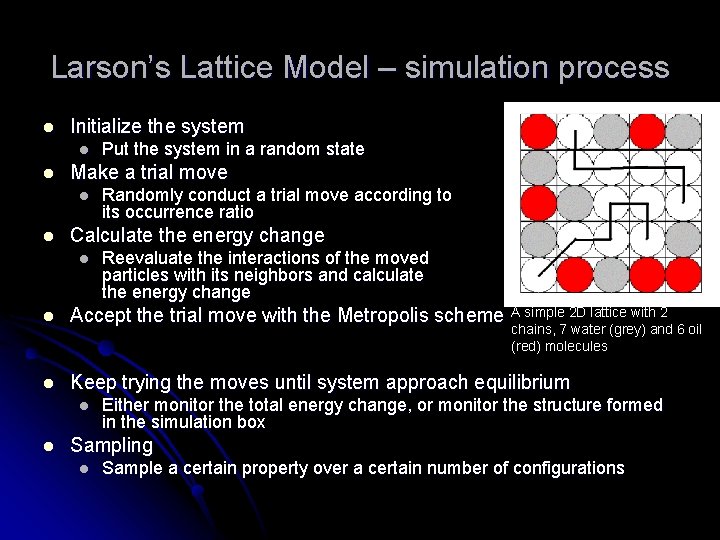
Larson’s Lattice Model – simulation process l Initialize the system l l Make a trial move l l Put the system in a random state Randomly conduct a trial move according to its occurrence ratio Calculate the energy change l Reevaluate the interactions of the moved particles with its neighbors and calculate the energy change l Accept the trial move with the Metropolis scheme A simple 2 D lattice with 2 l Keep trying the moves until system approach equilibrium chains, 7 water (grey) and 6 oil (red) molecules l l Either monitor the total energy change, or monitor the structure formed in the simulation box Sampling l Sample a certain property over a certain number of configurations

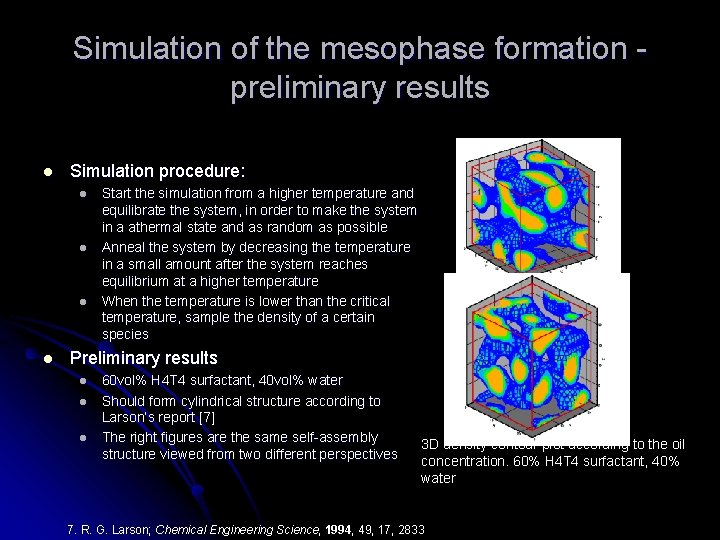
Simulation of the mesophase formation preliminary results l Simulation procedure: l l Start the simulation from a higher temperature and equilibrate the system, in order to make the system in a athermal state and as random as possible Anneal the system by decreasing the temperature in a small amount after the system reaches equilibrium at a higher temperature When the temperature is lower than the critical temperature, sample the density of a certain species Preliminary results l l l 60 vol% H 4 T 4 surfactant, 40 vol% water Should form cylindrical structure according to Larson’s report [7] The right figures are the same self-assembly structure viewed from two different perspectives 3 D density contour plot according to the oil concentration. 60% H 4 T 4 surfactant, 40% water 7. R. G. Larson; Chemical Engineering Science, 1994, 49, 17, 2833

Add the bond overlapping constraint l l Bond overlapping may occurred in the system, which is unrealistic Simulation results after adding the bond overlapping constrain (other conditions are the same). Perfect hexagonal close packing cylindrical structure is formed. Two chains overlaps with each other 3 D density contour plot according to the oil concentration. 60% H 4 T 4 surfactant, 40% water

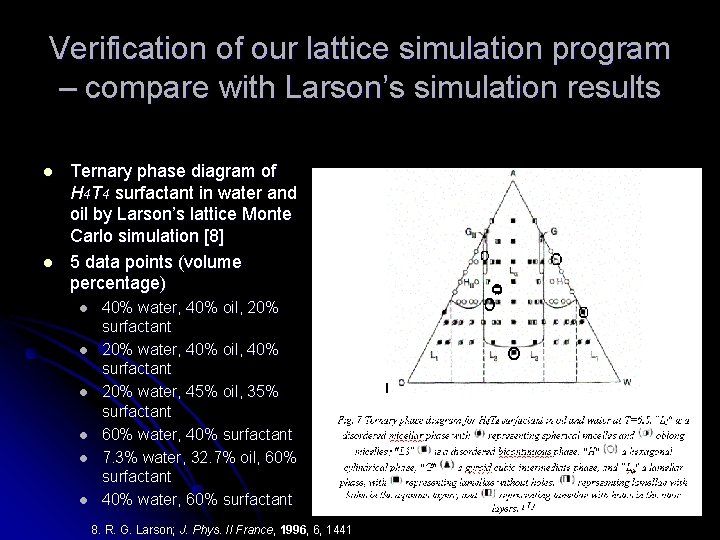
Verification of our lattice simulation program – compare with Larson’s simulation results l l Ternary phase diagram of H 4 T 4 surfactant in water and oil by Larson’s lattice Monte Carlo simulation [8] 5 data points (volume percentage) l l l 40% water, 40% oil, 20% surfactant 20% water, 40% oil, 40% surfactant 20% water, 45% oil, 35% surfactant 60% water, 40% surfactant 7. 3% water, 32. 7% oil, 60% surfactant 40% water, 60% surfactant 8. R. G. Larson; J. Phys. II France, 1996, 6, 1441

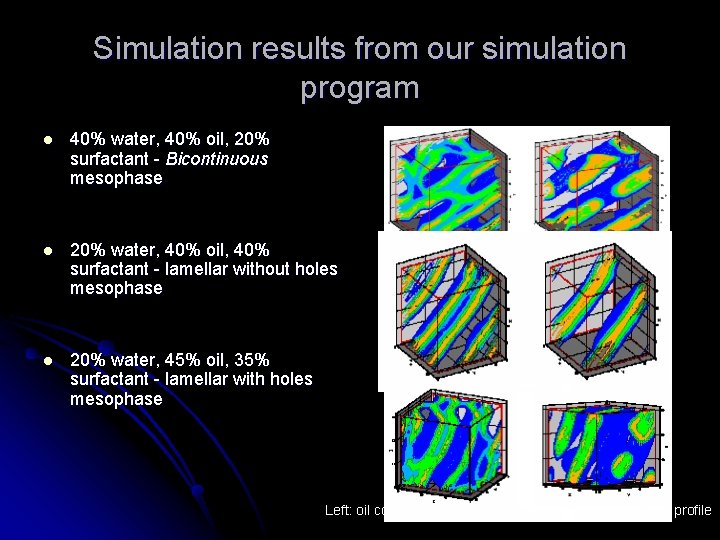
Simulation results from our simulation program l 40% water, 40% oil, 20% surfactant - Bicontinuous mesophase l 20% water, 40% oil, 40% surfactant - lamellar without holes mesophase l 20% water, 45% oil, 35% surfactant - lamellar with holes mesophase Left: oil concentration profile, Right: water concentration profile

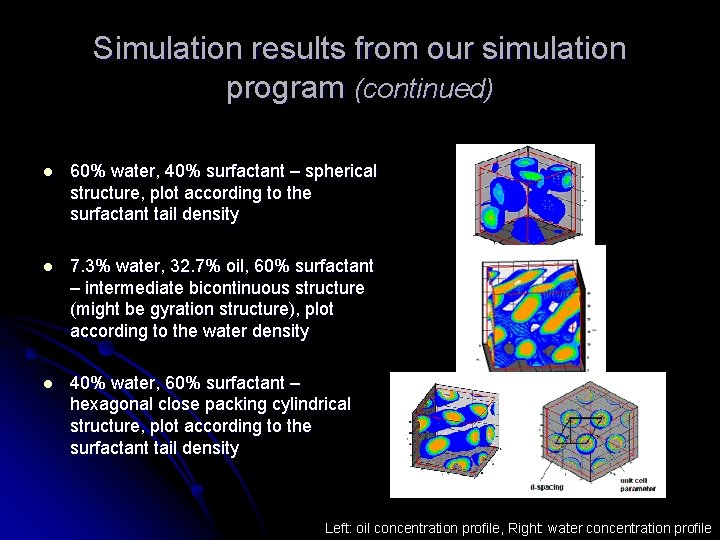
Simulation results from our simulation program (continued) l 60% water, 40% surfactant – spherical structure, plot according to the surfactant tail density l 7. 3% water, 32. 7% oil, 60% surfactant – intermediate bicontinuous structure (might be gyration structure), plot according to the water density l 40% water, 60% surfactant – hexagonal close packing cylindrical structure, plot according to the surfactant tail density Left: oil concentration profile, Right: water concentration profile

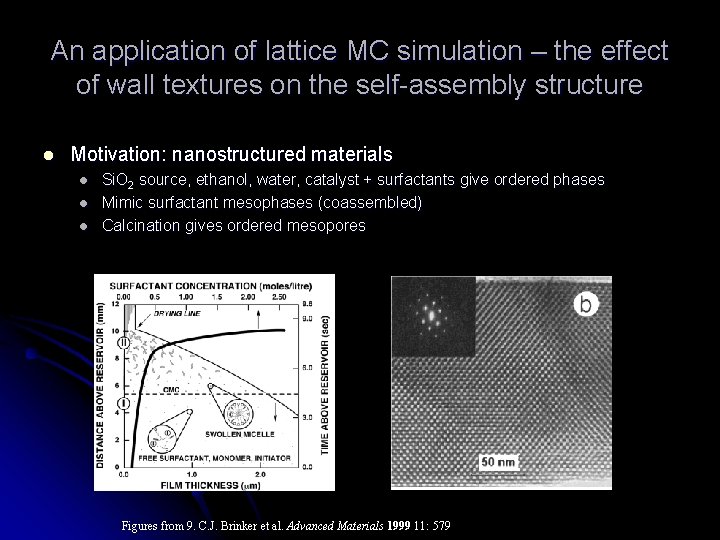
An application of lattice MC simulation – the effect of wall textures on the self-assembly structure l Motivation: nanostructured materials l l l Si. O 2 source, ethanol, water, catalyst + surfactants give ordered phases Mimic surfactant mesophases (coassembled) Calcination gives ordered mesopores Figures from 9. C. J. Brinker et al. Advanced Materials 1999 11: 579

Motivations to study the textured walls l l l Real substrate surface may not be flat For hierarchical materials (macroporous / mesoporous), curved surfaces may be present Design of nanostructure using surface texturing – use nanopatterned substrate to control the orientation of the self-assembly structure l l Mesopores perpendicular to the substrate is desired Use the texture on the substrate to make the mesopores perpendicular to the substrate

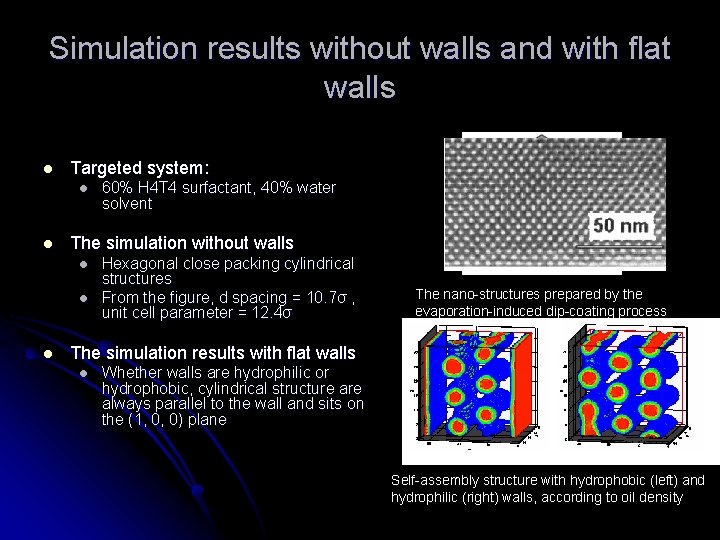
Simulation results without walls and with flat walls l Targeted system: l l The simulation without walls l l l 60% H 4 T 4 surfactant, 40% water solvent Hexagonal close packing cylindrical structures From the figure, d spacing = 10. 7σ , unit cell parameter = 12. 4σ The nano-structures prepared by the evaporation-induced dip-coating process The simulation results with flat walls l Whether walls are hydrophilic or hydrophobic, cylindrical structure always parallel to the wall and sits on the (1, 0, 0) plane Self-assembly structure with hydrophobic (left) and hydrophilic (right) walls, according to oil density

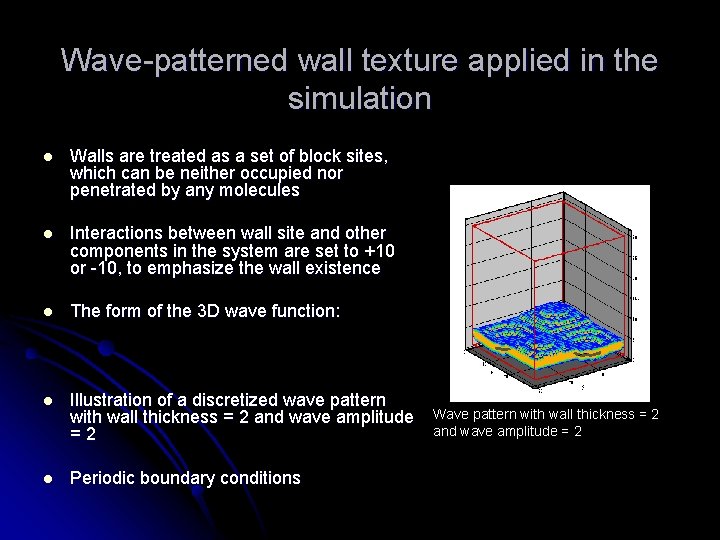
Wave-patterned wall texture applied in the simulation l Walls are treated as a set of block sites, which can be neither occupied nor penetrated by any molecules l Interactions between wall site and other components in the system are set to +10 or -10, to emphasize the wall existence l The form of the 3 D wave function: l Illustration of a discretized wave pattern with wall thickness = 2 and wave amplitude =2 l Periodic boundary conditions Wave pattern with wall thickness = 2 and wave amplitude = 2

Lattice Monte Carlo simulation results for the hydrophilic textured walls l l l Simulation results of 30 x 30 and 30 x 40 simulation box, wave amplitude = 1 Surface pattern doesn’t change the structure much at lower wall spacing. Walls sit on the (2 1 0) plane. A little calculation: l How many layer in the horizontal plane: l Number of layers in the vertical plane: Box size = 30 x 30, wave amplitude = 1, plot according to the oil density Box size = 30 x 40, wave amplitude = 1, plot according to the oil density

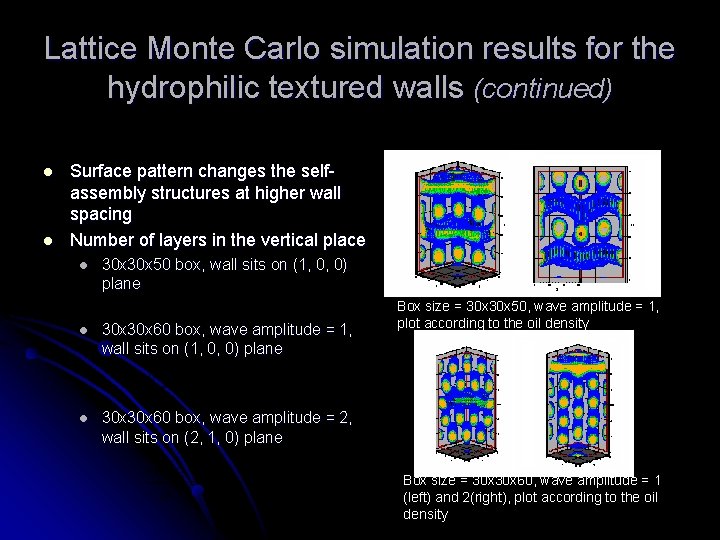
Lattice Monte Carlo simulation results for the hydrophilic textured walls (continued) l l Surface pattern changes the selfassembly structures at higher wall spacing Number of layers in the vertical place l 30 x 50 box, wall sits on (1, 0, 0) plane l 30 x 60 box, wave amplitude = 1, wall sits on (1, 0, 0) plane l 30 x 60 box, wave amplitude = 2, wall sits on (2, 1, 0) plane Box size = 30 x 50, wave amplitude = 1, plot according to the oil density Box size = 30 x 60, wave amplitude = 1 (left) and 2(right), plot according to the oil density

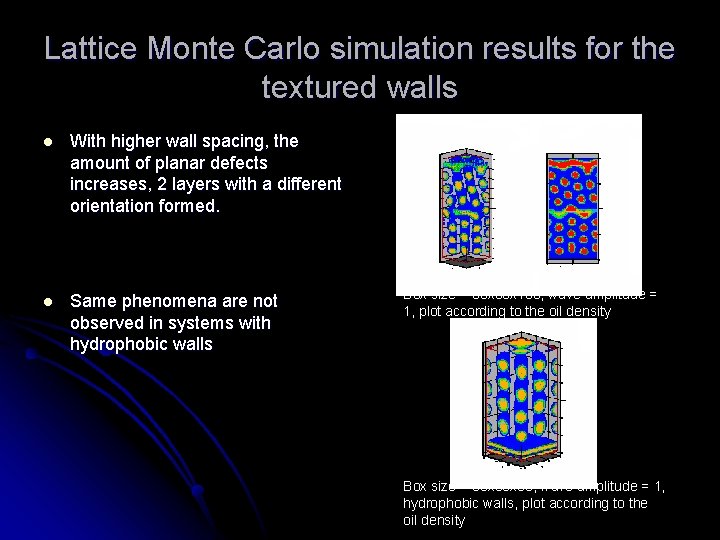
Lattice Monte Carlo simulation results for the textured walls l With higher wall spacing, the amount of planar defects increases, 2 layers with a different orientation formed. l Same phenomena are not observed in systems with hydrophobic walls Box size = 30 x 100, wave amplitude = 1, plot according to the oil density Box size = 30 x 60, wave amplitude = 1, hydrophobic walls, plot according to the oil density

Conclusions l Cylinders always align along diagonal of texture, even with small wave amplitude l For hydrophilic walls, small wall spacing with small wave amplitude only distorts structure l For hydrophilic walls, large wall spacing with small wave amplitude promotes (1 0 0) orientation l For hydrophilic walls, planar defects may be more likely if wall spacing > space needed for # of layers l systems with hydrophobic walls may avoid planar defects, because l l the deposition of a monolayer of surfactant on the wall. The chain softness mitigates the pattern

Referances l l l l http: //shasta. mpistuttgart. mpg. de/research/bionano/modeling%2 0 and%20 simulation%20 of%20 bio-nano-materials. htm http: //www. foresight. org/Conferences/MNT 6/Papers/Cagi n 3/ http: //www. humphrey. id. au/papers/ITC 2004. pdf http: //www. engr. uky. edu/%7 Emenguc/NECP_Sems/ http: //pubs. acs. org/cgibin/article. cgi/nalefd/2003/3/i 04/pdf/nl 025967 a. pdf http: //www. foresight. org/Conferences/MNT 6/Papers/Cagi n 3/ http: //www. research. ibm. com/topics/popups/serious/nan o/html/nanotubes. html
 Magnetic properties of nanomaterials
Magnetic properties of nanomaterials Simple cubic unit cell
Simple cubic unit cell Hardness of nanomaterials
Hardness of nanomaterials Bachelor of science la trobe
Bachelor of science la trobe Conclusion of nanotechnology
Conclusion of nanotechnology Nanotechnology in sports
Nanotechnology in sports Journal of nanoscience and nanotechnology sci
Journal of nanoscience and nanotechnology sci Nanotechnology
Nanotechnology Timeline of nanotechnology
Timeline of nanotechnology Plasma arcing
Plasma arcing Interpretations of moore's law assert that:
Interpretations of moore's law assert that: Nanotechnology fact or myth
Nanotechnology fact or myth Examples of discourse communities
Examples of discourse communities What is the nanotechnology
What is the nanotechnology What is nanotechnology
What is nanotechnology Nanotechnology definition
Nanotechnology definition Disadvantages of nanotechnology
Disadvantages of nanotechnology Difference between nanoscience and nanotechnology
Difference between nanoscience and nanotechnology Nanotechnology for kids
Nanotechnology for kids What is nanotechnology
What is nanotechnology Zong-liang yang
Zong-liang yang Mengindeks sistem abjad
Mengindeks sistem abjad How was ma liang like?
How was ma liang like? Elementary programming in java
Elementary programming in java Anticorps anti nucléaire moucheté 1/160
Anticorps anti nucléaire moucheté 1/160 Liang stoma
Liang stoma Chia liang cheng
Chia liang cheng Amber liang
Amber liang Siemens sans bold
Siemens sans bold Zong-liang yang
Zong-liang yang Zachary hensley
Zachary hensley Zhuo shi wo li liang
Zhuo shi wo li liang Bob liang
Bob liang Kathleen liang
Kathleen liang Administrasi keuangan kelas 11
Administrasi keuangan kelas 11 Liang fontdemo.java
Liang fontdemo.java Dr liang hao nan
Dr liang hao nan Liang jianhui
Liang jianhui Zhong hanliang
Zhong hanliang Diana liang faa
Diana liang faa Edmund liang
Edmund liang Liang zhu umbc
Liang zhu umbc Liang fontdemo.java
Liang fontdemo.java David teo choon liang
David teo choon liang Richard seow yung liang
Richard seow yung liang Pengertian arsip dan kearsipan
Pengertian arsip dan kearsipan Nba schedule maker
Nba schedule maker Yuanxing liang
Yuanxing liang Cnc in construction contract
Cnc in construction contract Maynard cpa
Maynard cpa Predetermined time study
Predetermined time study Contoh time sequence
Contoh time sequence Maynard jackson was born in ___.
Maynard jackson was born in ___. What did the sibley commission do
What did the sibley commission do Popyt po angielsku
Popyt po angielsku Maynard operation sequence technique chart pdf
Maynard operation sequence technique chart pdf Maynard hill
Maynard hill Maynard g. krebs quotes
Maynard g. krebs quotes Diana maynard
Diana maynard Maynard jackson drawing
Maynard jackson drawing Nearman maynard vallez cpas
Nearman maynard vallez cpas