Multicomponent Separations Involving HighRecovery or Sharp Product Streams













- Slides: 62

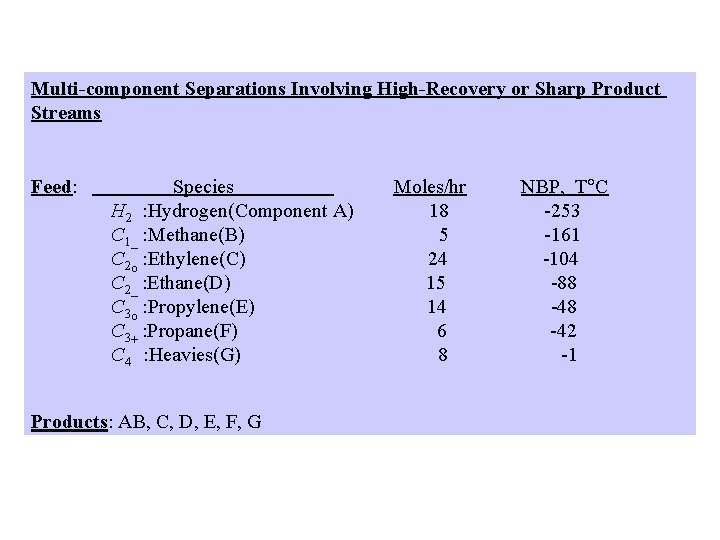
Multi-component Separations Involving High-Recovery or Sharp Product Streams Feed: Species H 2 : Hydrogen(Component A) C 1_ : Methane(B) C 2 o : Ethylene(C) C 2_ : Ethane(D) C 3 o : Propylene(E) C 3+ : Propane(F) C 4 : Heavies(G) Products: AB, C, D, E, F, G Moles/hr 18 5 24 15 14 6 8 NBP, T C -253 -161 -104 -88 -42 -1

Definitions: (1) For sharp product streams, we normally use allsharp or high-recovery separation sequences to separate the feed into products. In such sequences, each component being separated appears almost completely in one and only one product. (2) Key component in an all-sharp separation are commonly defined by: The light key (LK) is the lightest component in the bottoms and the heavy key (HK) is the heaviest component in the overhead.

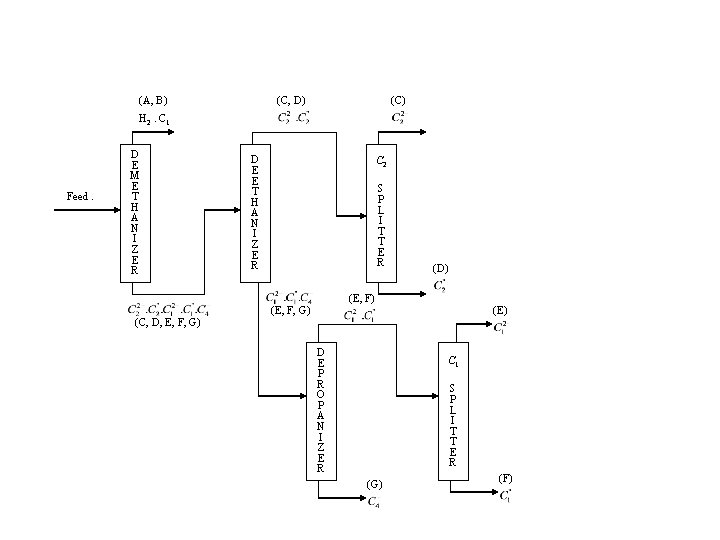
(A, B) (C, D) (C) H 2. C 1 Feed. D E M E T H A N I Z E R (C, D, E, F, G) D E E T H A N I Z E R C 2 S P L I T T E R (D) (E, F, G) D E P R O P A N I Z E R (E) C 1 S P L I T T E R (G) (F)

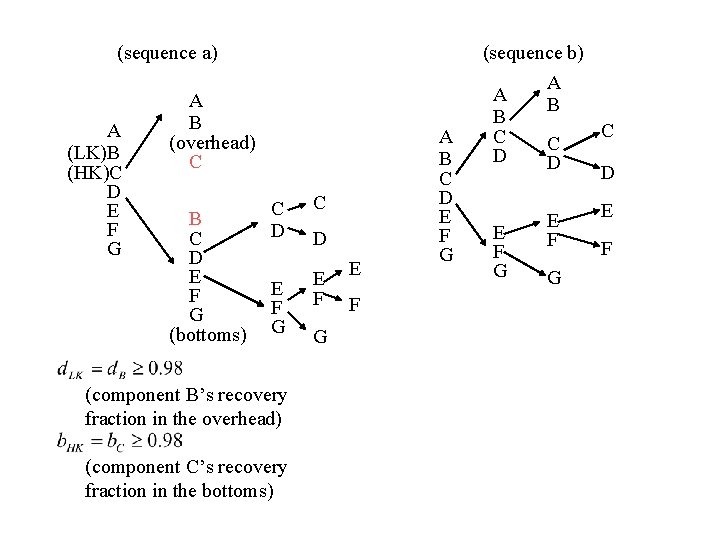
(sequence a) A (LK)B (HK)C D E F G A B (overhead) C B C D E F G (bottoms) C D C E F G E F (component B’s recovery fraction in the overhead) (component C’s recovery fraction in the bottoms) D G E F A B C D E F G (sequence b) A A B B C C C D D D E F G E F

Questions: How do you synthesize these two industrial separation sequences ? Which sequence is “better” ? Does your ranking vary with feed conditions ?

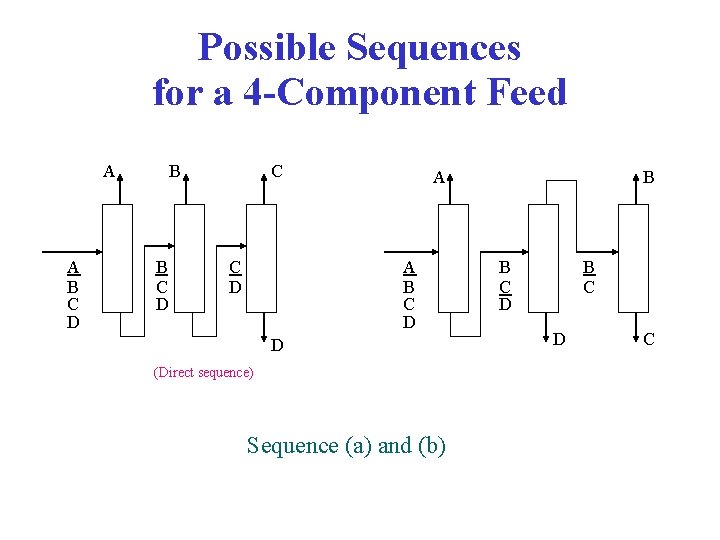
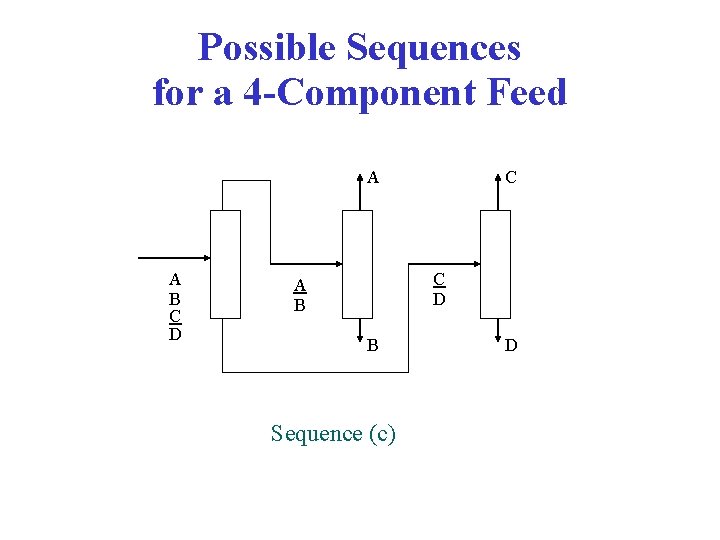
Possible Sequences for a 4 -Component Feed A A B C D B B C D C C D A A B C D D (Direct sequence) Sequence (a) and (b) B B C D C

Possible Sequences for a 4 -Component Feed A A B C D C C D A B B Sequence (c) D

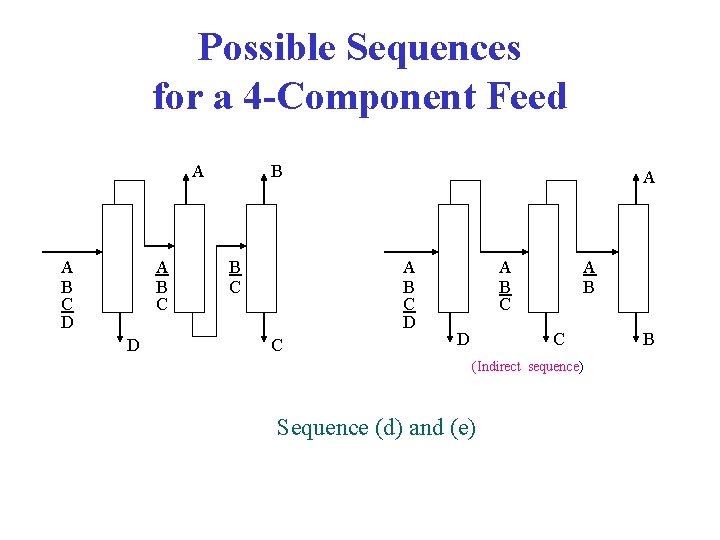
Possible Sequences for a 4 -Component Feed A A B C D B B C A A B C D C A B C D A B C (Indirect sequence) Sequence (d) and (e) B

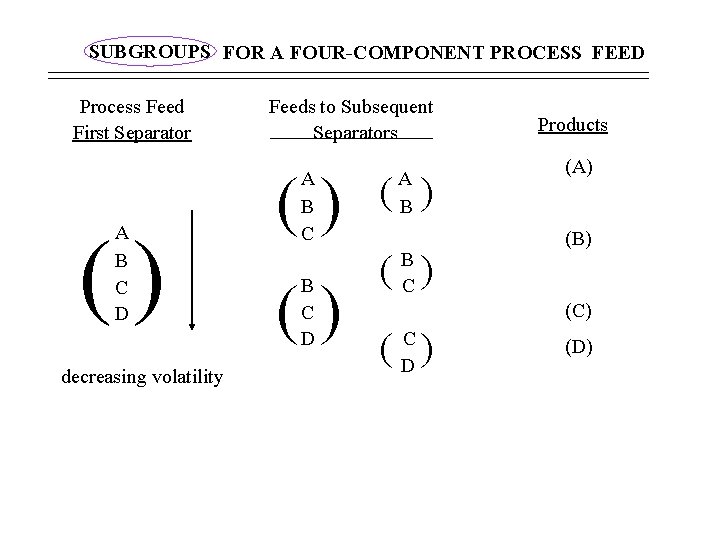
SUBGROUPS FOR A FOUR-COMPONENT PROCESS FEED Process Feed First Separator () A B C D decreasing volatility Feeds to Subsequent Separators () A B C () B C D A B ( ) B C Products (A) (B) (C) ( ) C D (D)

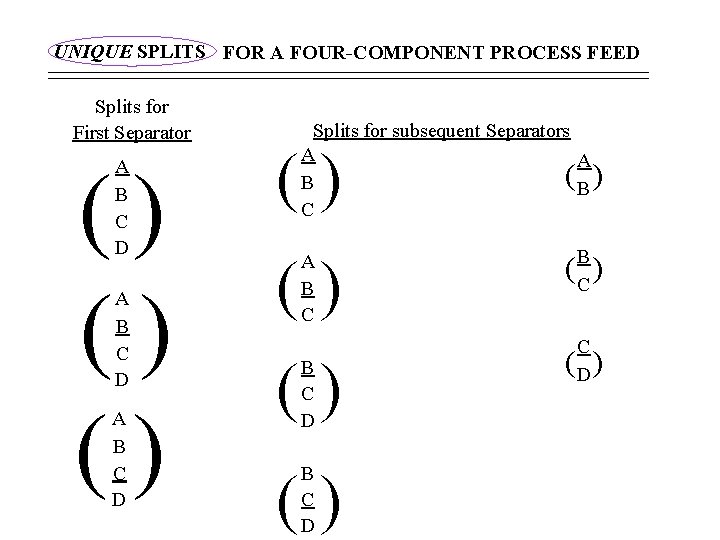
UNIQUE SPLITS FOR A FOUR-COMPONENT PROCESS FEED Splits for First Separator () () () A B C D Splits for subsequent Separators A A B B C () () A B C () B C D () ( ) B C ( ) C D ( )

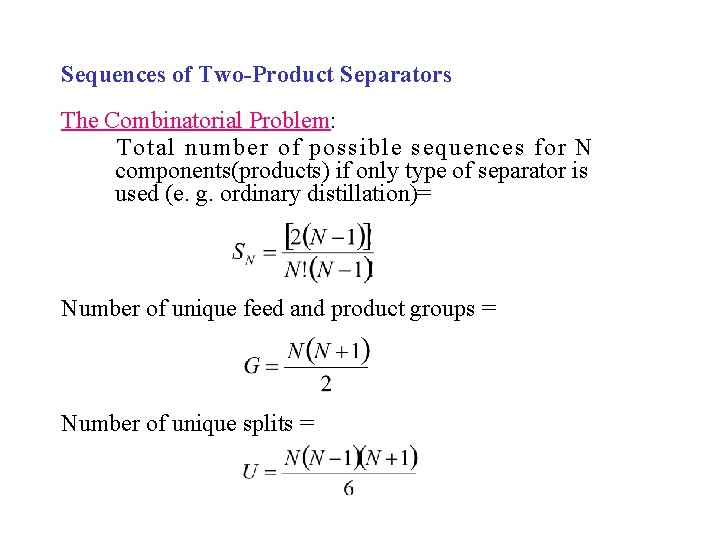
Sequences of Two-Product Separators The Combinatorial Problem: Total number of possible sequences for N components(products) if only type of separator is used (e. g. ordinary distillation)= Number of unique feed and product groups = Number of unique splits =

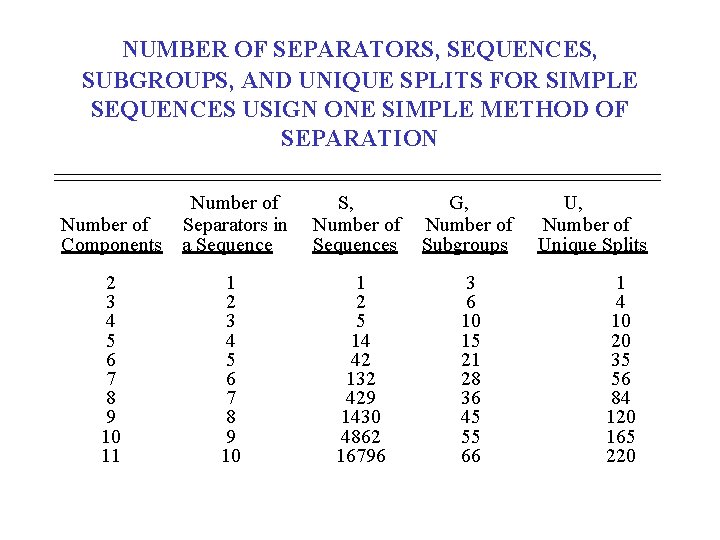
NUMBER OF SEPARATORS, SEQUENCES, SUBGROUPS, AND UNIQUE SPLITS FOR SIMPLE SEQUENCES USIGN ONE SIMPLE METHOD OF SEPARATION Number of Components Number of Separators in a Sequence S, Number of Sequences G, Number of Subgroups 2 3 4 5 6 7 8 9 10 11 1 2 3 4 5 6 7 8 9 10 1 2 5 14 42 132 429 1430 4862 16796 3 6 10 15 21 28 36 45 55 66 U, Number of Unique Splits 1 4 10 20 35 56 84 120 165 220

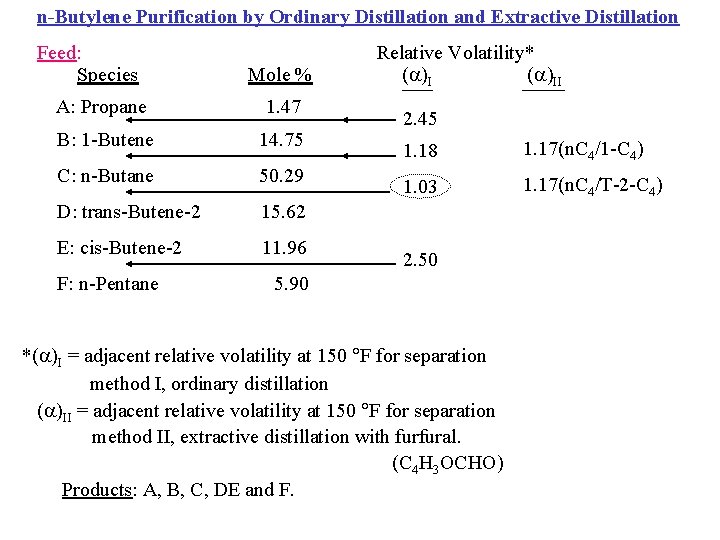
n-Butylene Purification by Ordinary Distillation and Extractive Distillation Feed: Species Mole % A: Propane 1. 47 B: 1 -Butene 14. 75 C: n-Butane 50. 29 D: trans-Butene-2 15. 62 E: cis-Butene-2 11. 96 F: n-Pentane Relative Volatility* ( )II 2. 45 1. 18 1. 17(n. C 4/1 -C 4) 1. 03 1. 17(n. C 4/T-2 -C 4) 2. 50 5. 90 *( )I = adjacent relative volatility at 150 F for separation method I, ordinary distillation ( )II = adjacent relative volatility at 150 F for separation method II, extractive distillation with furfural. (C 4 H 3 OCHO) Products: A, B, C, DE and F.

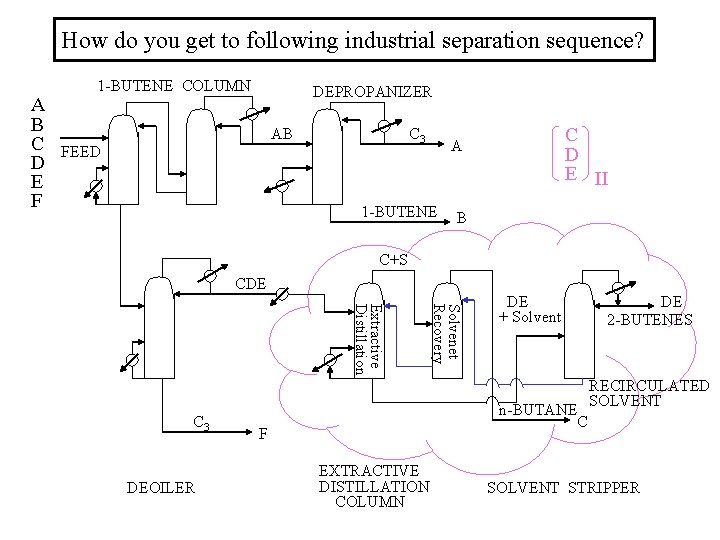
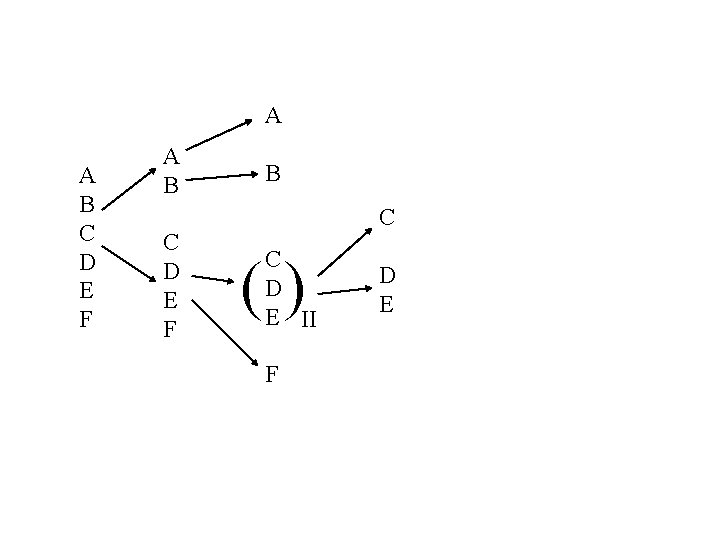
How do you get to following industrial separation sequence? A B C D E F 1 -BUTENE COLUMN DEPROPANIZER AB C 3 FEED C D E II A 1 -BUTENE B C+S CDE DEOILER Solvenet Recovery Extractive Distillation C 3 DE + Solvent n-BUTANE C F EXTRACTIVE DISTILLATION COLUMN DE 2 -BUTENES RECIRCULATED SOLVENT STRIPPER

A A B C D E F B C C D E II () F D E

Definition: Extractive distillation is a form of distillation involving the addition of a solvent which modifies the vapor-liquid equilibria of the components to be separated such the separation becomes easier. The added solvent has a volatility lower than the components to be separated (i. e. , the added solvent has a boiling point higher than those of the components to be separated). Also, it is usually introduced near the top of a column.

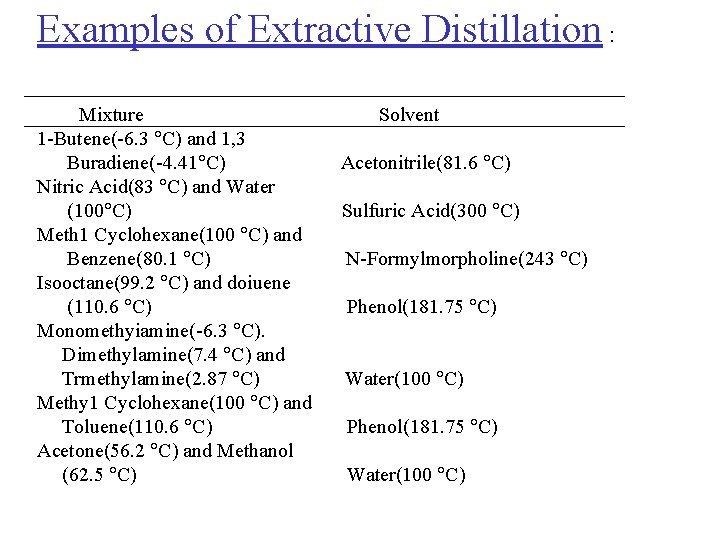
Examples of Extractive Distillation : Mixture 1 -Butene(-6. 3 C) and 1, 3 Buradiene(-4. 41 C) Nitric Acid(83 C) and Water (100 C) Meth 1 Cyclohexane(100 C) and Benzene(80. 1 C) Isooctane(99. 2 C) and doiuene (110. 6 C) Monomethyiamine(-6. 3 C). Dimethylamine(7. 4 C) and Trmethylamine(2. 87 C) Methy 1 Cyclohexane(100 C) and Toluene(110. 6 C) Acetone(56. 2 C) and Methanol (62. 5 C) Solvent Acetonitrile(81. 6 C) Sulfuric Acid(300 C) N-Formylmorpholine(243 C) Phenol(181. 75 C) Water(100 C)

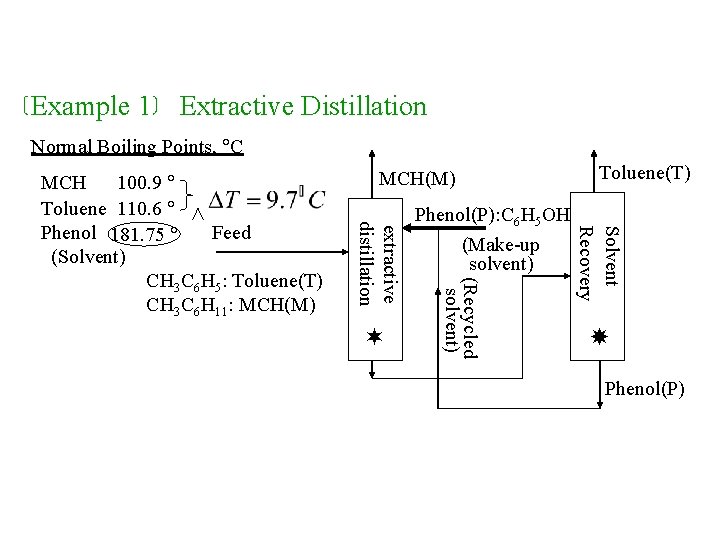
Example 1 Extractive Distillation Normal Boiling Points, C MCH(M) Toluene(T) Solvent Recovery extractive distillation Phenol(P): C 6 H 5 OH (Make-up solvent) (Recycled solvent) MCH 100. 9 Toluene 110. 6 Phenol 181. 75 Feed (Solvent) CH 3 C 6 H 5: Toluene(T) CH 3 C 6 H 11: MCH(M) Phenol(P)


If : 1. T type of two-product separators are allowed. 2. Any mass-separating agent is recovered for recycle in the separator following the one into which it is introduced. Then : For example : N = 4 components Ordinary distillation only gives SN = 5 Ordinary distillation plus extractive distillation with phenol gives

HEURISTICS Heuristics Used in Heuristic and Evolutionary Strategies for Synthesis of Separation Sequences Reference Lockhart (1947) Harbert (1957) Rod & Marek (1959) Heaven (1969) Rudd and his coworkers (1971 -73) King (1971) and Thomp son and King (1972 a, b) Stephanopoulos (1974), and Stephanopoulos and Westerberg (1976) Type Separators Applied Distillation General Heuristics Used 1, 6 2, 3 4 1, 2, 3, 5 1, 2, 3, 6, 8 12, 13 1, 2, 3, 7, 11 7 plus evol. rules

HEURISTICS Heuristics Used in Heuristic and Evolutionary Strategies for Synthesis of Separation Sequences Reference Freshwater & Henry (1975) Mahaec (1976) and Mahalec & Motard (1977 a, b) Seader & Weaterberg (1977) Type Separators Applied Distillation General Nath & Motard (1978) General Doukas & Luyben (1978) Hartmann (1979) and Hartmann and Hacker (1979) Distillation General Heuristics Used 1, 2, 3, 5, 6 6, 11 plus evol. rules 1, 2, 3, 9, 11, 12, 13, 18 plus evol. rules 9, 10, 11, 14, 15 17, 19 plus evol. Rules 1 1, 2, 3, 6, 8, 16 19

Heuristic Rules. 1. Remove components one-by-one as overhead products. 2. Save the most difficult separation for last. 3. Favor 50 -50 splits. 4. Sequence with the minimum total vapor flow. 5. Make high recovery fractions last. 6. Separate the more plentiful components first. 7. Choose the cheapest as the next separator. 8. Remove thermally unstable and corrosive material early. 9. Perform least-tight separation first. 10. Favor sequences with the smallest product set. 11. Avoid separations using a mass-separating agent (MSA). 12. Remove a MSA from one of the products in another, subsequent separation product.

Heuristic Rules 13. A separation method using a MSA cannot be used to isolate. 14. Favor distillation. 15. Separate first the components which might undergo undesirable reactions. 16. Set splits fractions of the key components to pre-specified values. 17. Avoid extreme processing conditions. 18. Favor ambient operating pressure. Nishida, Stephanopoulos, Westerberg(1980)

Heuristic Synthesis of High-Recovery or Sharp, Multi-component Separation Sequences (Nadgir and Liu, 1983; Liu, 1987) Classification of Heuristics Method Heuristics (M Heuristics) : Favor the use of certain separation methods under given problem specifications Design Heuristics (D Heuristics) : Favor specific separation sequences with certain desirable properties Species Heuristics (S Heuristics) : Based on the property differences between the species to be separated Composition Heuristics (C Heuristics) : Related to the effects of feed and product composition on separation costs Nadgir, V. M. and Y. A. Liu, “Studies in Chemical Process Design and Synthesis. 5. A Simple Heuristic Method for Systematic Synthesis of Initial Sequences for Multi-component Separation, ” AICh. E Journal, 29, 926 -934 (1983). Liu, Y. A. , “Process Synthesis: Some Simple and Practical Developments, ” Chapter 6, in Recent Development in Chemical Process and Plant Design, Y. A. Mebee, Jr. and W. R. Epperly, editor, Wiley, NY (1987), pp. 147 -168 and 245 -260.

A Simple, “Rank-Ordered, ” Heuristic Method (Nadgir and Liu, 1983) a. Decide the separation method to be used : M 1: Favor ordinary distillation M 2: Avoid vacuum and refrigeration b. Identify the forbidden splits, and essential first and last separations : D 1: Favor smallest product set S 1: Remove corrosive and hazardous components first (also : reactive component, monomer) S 2: Perform difficult separations last c. Synthesize the initial separation sequences : C 1: Remove most plentiful component first C 2: Favor 50/50 split d. Remove products and recycle stream as distillates. If not possible, take vapor from reboiler.

“Rank-Ordered” Heuristics: (1) The heuristics are to be applied one by one in the given order. Higher-ranked heuristics appear first. (2) If any heuristic is not important in, or not applicable to, the synthesis problem, the next one in the method is considered. (3) If two heuristics give different recommendations regarding the next split, we should follow the guideline suggested by the higher-ranked heuristic. For example, heuristic C 1 overrules heuristic C 2.

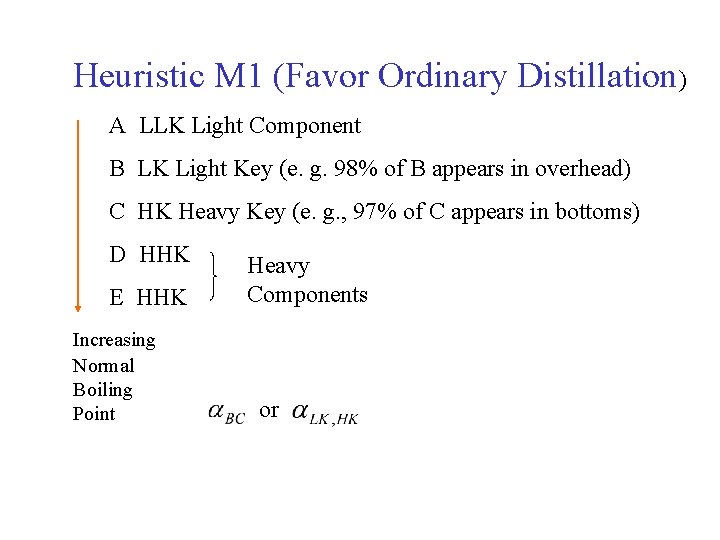
Heuristic M 1 (Favor Ordinary Distillation) a. All other things being equal, favor separation methods using only energy separating agent (e. g. , ordinary distillation), and avoid using separation methods (e. g. , extractive distillation) which require the use of species not normally present in the processing, i. e. , the mass separating agent (MSA). b. If the separation factor or relative volatility of the key components < 1. 10, then the use of ordinary distillation is not recommended. or c. An MSA may be used provided it improves . d. When an MSA is used, remove it immediately follow the separator into which it is used. In other words, always try to remove MSA early.

Heuristic M 1 (Favor Ordinary Distillation) A LLK Light Component B LK Light Key (e. g. 98% of B appears in overhead) C HK Heavy Key (e. g. , 97% of C appears in bottoms) D HHK E HHK Increasing Normal Boiling Point Heavy Components or

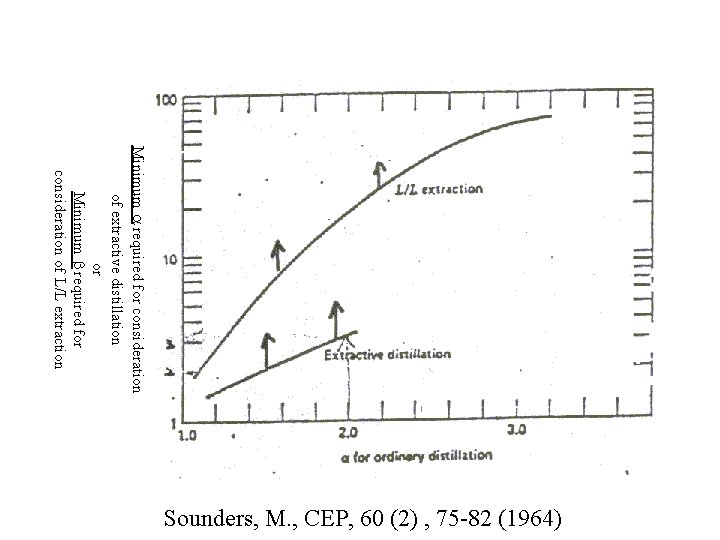
Minimum required for consideration of extractive distillation or Minimum required for consideration of L/L extraction Sounders, M. , CEP, 60 (2) , 75 -82 (1964)

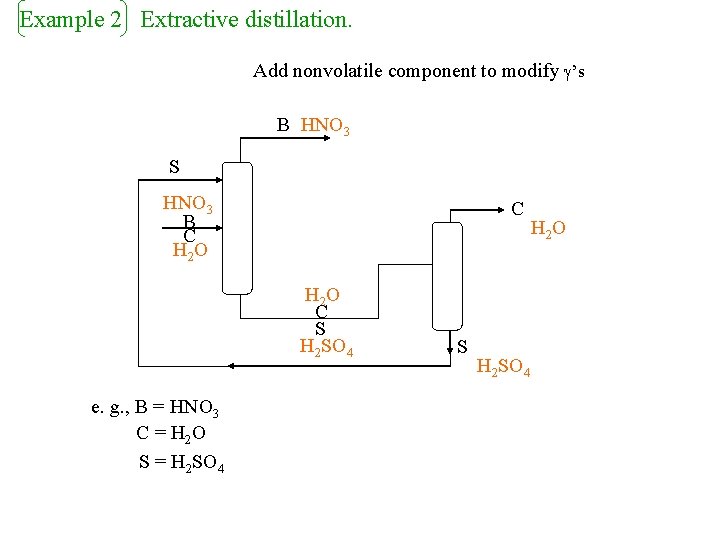
Example 2 Extractive distillation. Add nonvolatile component to modify ’s B HNO 3 S HNO 3 B C H 2 O C S H 2 SO 4 e. g. , B = HNO 3 C = H 2 O S = H 2 SO 4 S H 2 SO 4 H 2 O

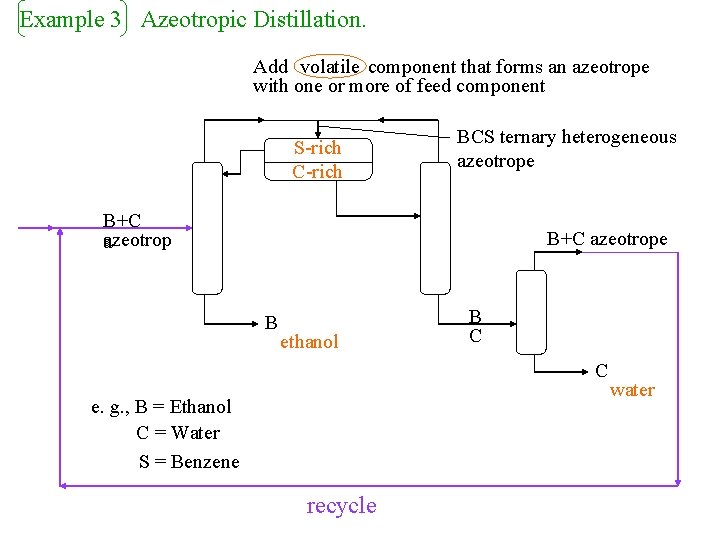
Example 3 Azeotropic Distillation. Add volatile component that forms an azeotrope with one or more of feed component S-rich C-rich BCS ternary heterogeneous azeotrope B+C azeotrop a B+C azeotrope B ethanol B C C e. g. , B = Ethanol C = Water S = Benzene recycle water

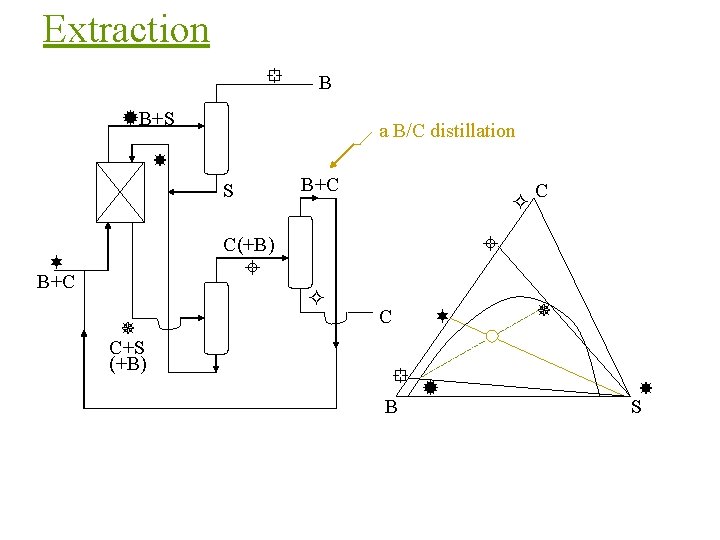
Extraction B B+S a B/C distillation S B+C C(+B) B+C C+S (+B) C C B S

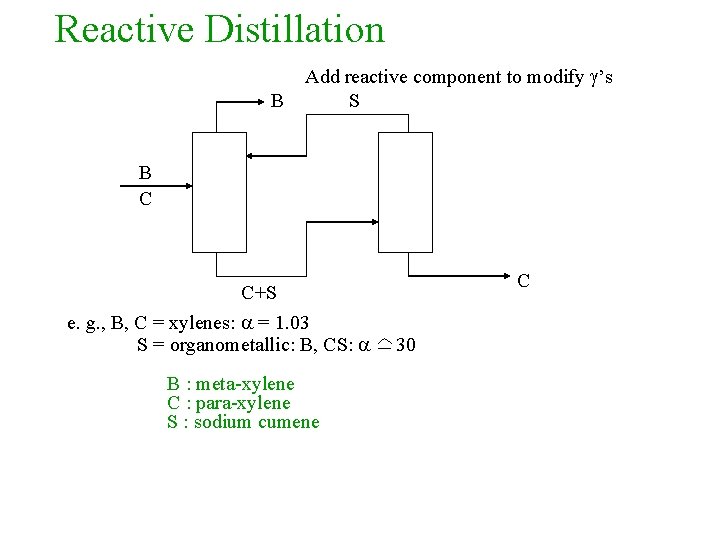
Reactive Distillation B Add reactive component to modify ’s S B C C+S e. g. , B, C = xylenes: = 1. 03 S = organometallic: B, CS: B : meta-xylene C : para-xylene S : sodium cumene C 30

Heuristic M 2 (Avoid Vacuum and Refrigeration) a. All other things being equal, avoid excursions in temperature and pressure, but aim high rather than low. b. If vacuum operation of ordinary distillation is required, liquid extraction with various solvents might be considered. c. If refrigeration is required, cheaper alternatives to distillation such as absorption might be considered.

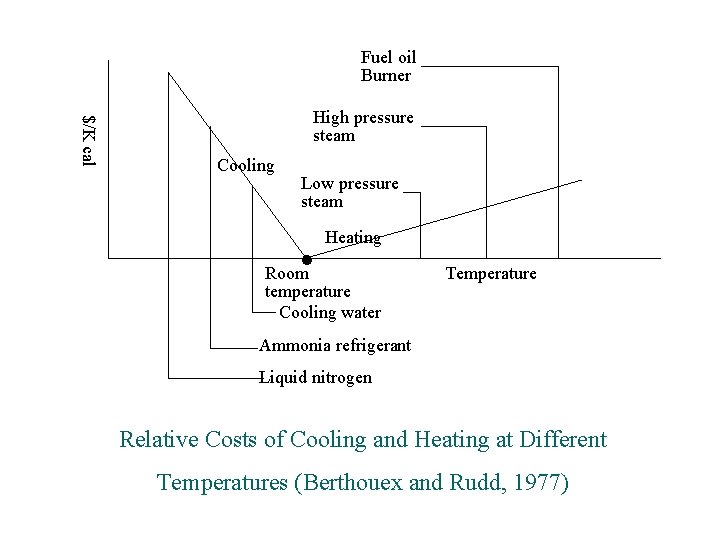
Fuel oil Burner $/K cal High pressure steam Cooling Low pressure steam Heating Room temperature Cooling water Temperature Ammonia refrigerant Liquid nitrogen Relative Costs of Cooling and Heating at Different Temperatures (Berthouex and Rudd, 1977)

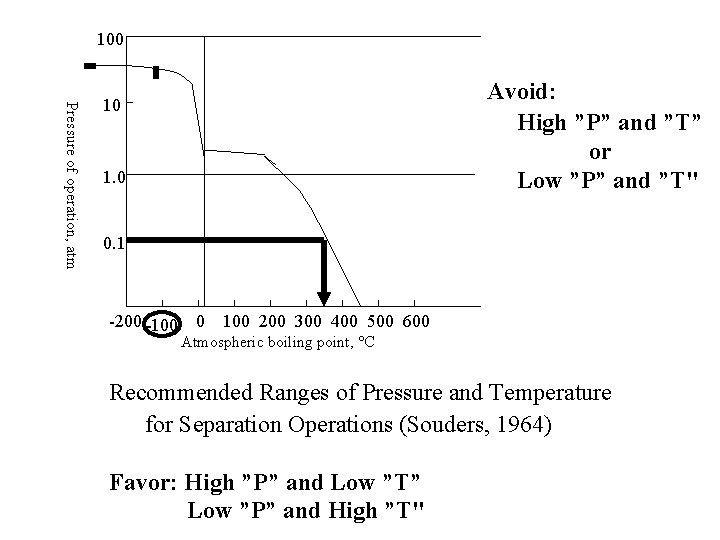
100 Pressure of operation, atm Avoid: High ”P” and ”T” or Low ”P” and ”T" 10 1. 0 0. 1 -200 -100 0 100 200 300 400 500 600 Atmospheric boiling point, C Recommended Ranges of Pressure and Temperature for Separation Operations (Souders, 1964) Favor: High ”P” and Low ”T” Low ”P” and High ”T"

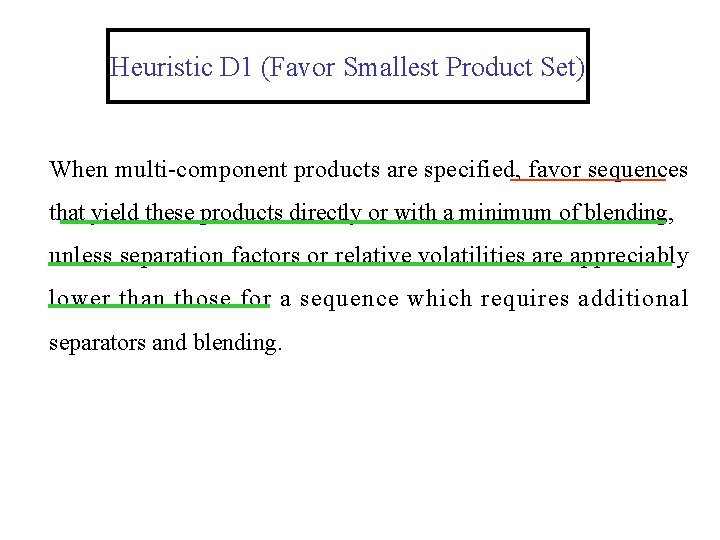
Heuristic D 1 (Favor Smallest Product Set) When multi-component products are specified, favor sequences that yield these products directly or with a minimum of blending, unless separation factors or relative volatilities are appreciably lower than those for a sequence which requires additional separators and blending.

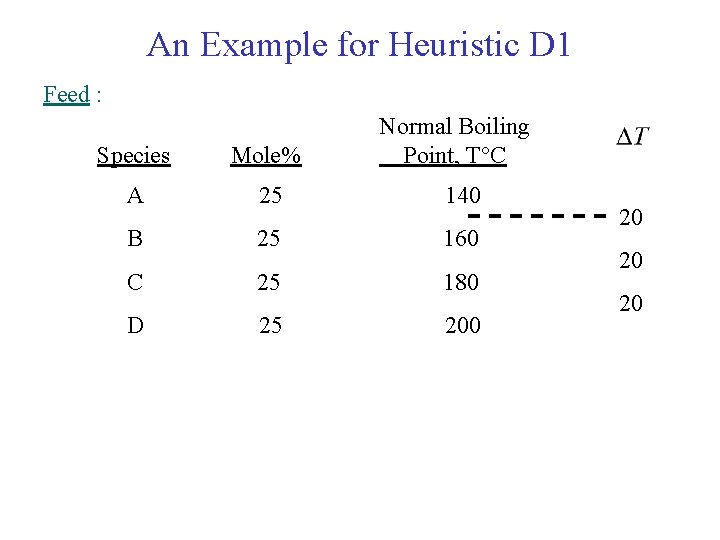
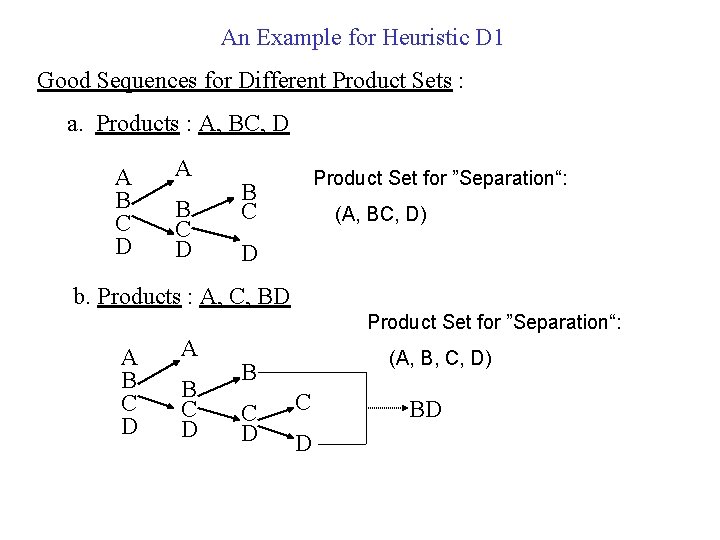
An Example for Heuristic D 1 Feed : Normal Boiling Point, T C Species Mole% A 25 140 B 25 160 C 25 180 D 25 200 20 20 20

An Example for Heuristic D 1 Good Sequences for Different Product Sets : a. Products : A, BC, D A B C D Product Set for ”Separation“: B C (A, BC, D) D b. Products : A, C, BD Product Set for ”Separation“: A B C D (A, B, C, D) B C D BD

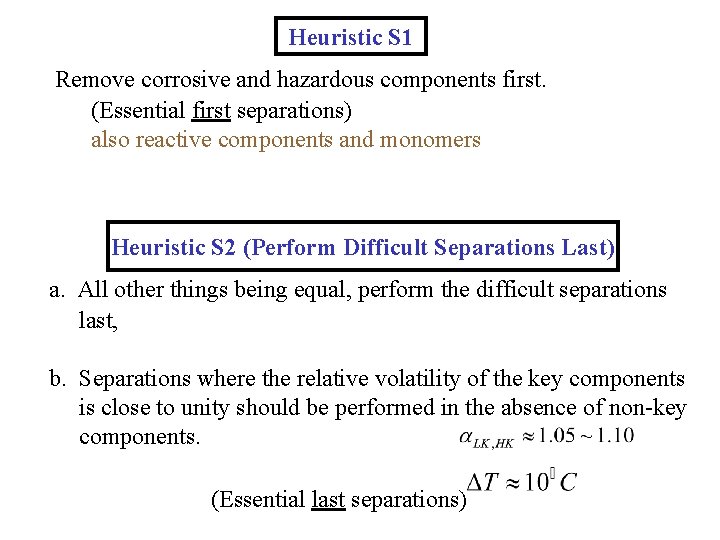
Heuristic S 1 Remove corrosive and hazardous components first. (Essential first separations) also reactive components and monomers Heuristic S 2 (Perform Difficult Separations Last) a. All other things being equal, perform the difficult separations last, b. Separations where the relative volatility of the key components is close to unity should be performed in the absence of non-key components. (Essential last separations)

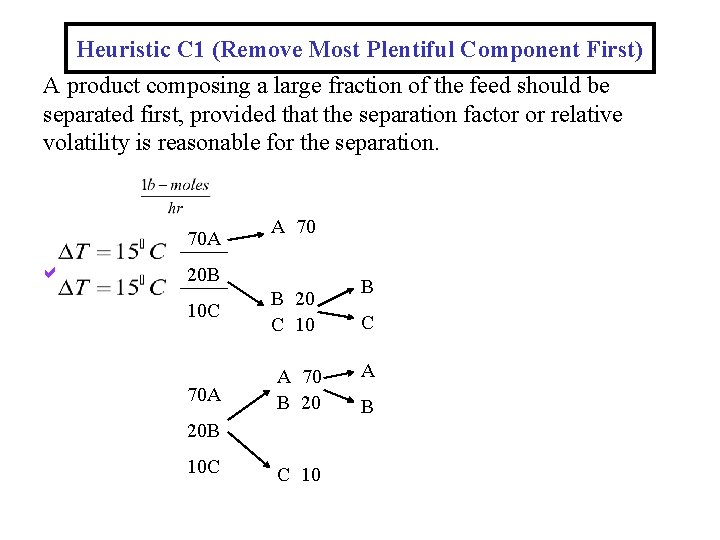
Heuristic C 1 (Remove Most Plentiful Component First) A product composing a large fraction of the feed should be separated first, provided that the separation factor or relative volatility is reasonable for the separation. 70 A A 70 20 B 10 C B 20 C 10 70 A A 70 B 20 20 B 10 C C 10 B C A B

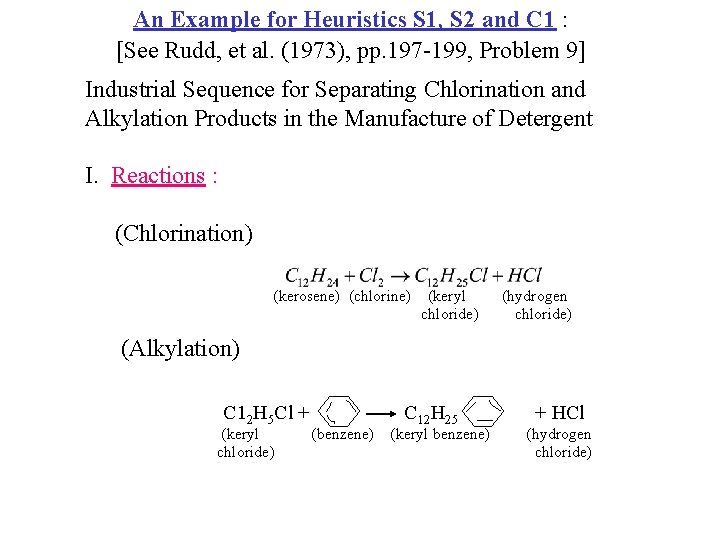
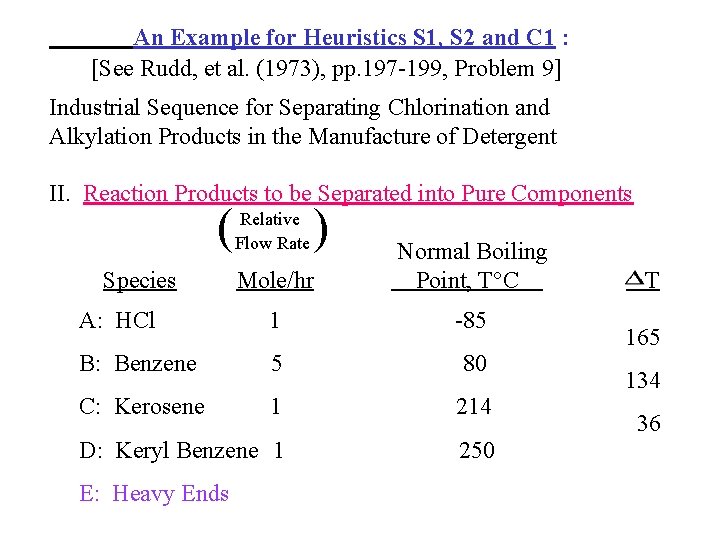
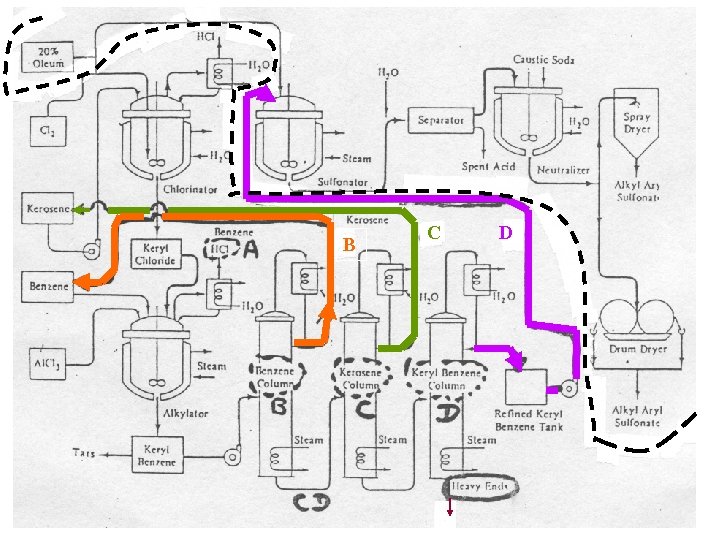
An Example for Heuristics S 1, S 2 and C 1 : [See Rudd, et al. (1973), pp. 197 -199, Problem 9] Industrial Sequence for Separating Chlorination and Alkylation Products in the Manufacture of Detergent I. Reactions : (Chlorination) (kerosene) (chlorine) (keryl chloride) (hydrogen chloride) (Alkylation) C 12 H 5 Cl + (keryl chloride) (benzene) C 12 H 25 - (keryl benzene) + HCl (hydrogen chloride)

An Example for Heuristics S 1, S 2 and C 1 : [See Rudd, et al. (1973), pp. 197 -199, Problem 9] Industrial Sequence for Separating Chlorination and Alkylation Products in the Manufacture of Detergent II. Reaction Products to be Separated into Pure Components ( Relative Flow Rate ) Mole/hr Normal Boiling Point, T C A: HCl 1 -85 B: Benzene 5 80 C: Kerosene 1 214 D: Keryl Benzene 1 250 Species E: Heavy Ends T 165 134 36

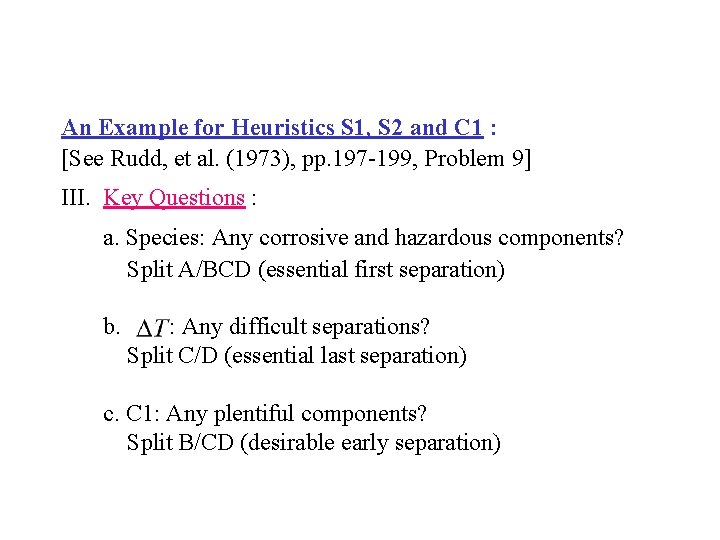
An Example for Heuristics S 1, S 2 and C 1 : [See Rudd, et al. (1973), pp. 197 -199, Problem 9] III. Key Questions : a. Species: Any corrosive and hazardous components? Split A/BCD (essential first separation) b. : Any difficult separations? Split C/D (essential last separation) c. C 1: Any plentiful components? Split B/CD (desirable early separation)

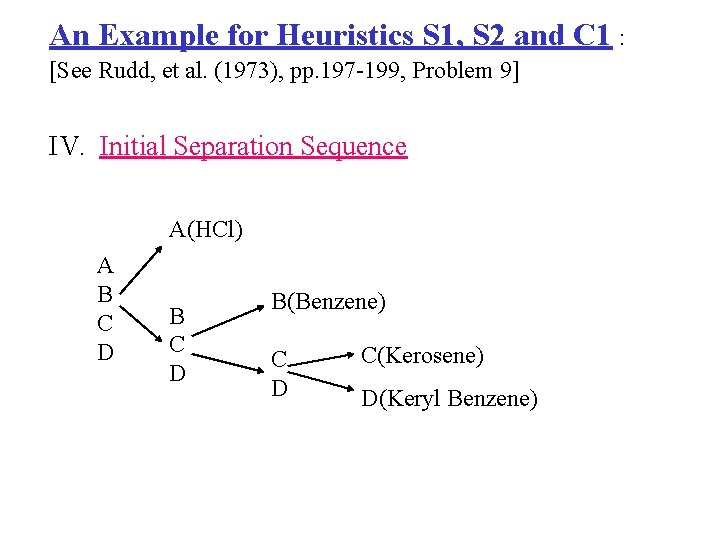
An Example for Heuristics S 1, S 2 and C 1 : [See Rudd, et al. (1973), pp. 197 -199, Problem 9] IV. Initial Separation Sequence A(HCl) A B C D B(Benzene) C D C(Kerosene) D(Keryl Benzene)

B C D

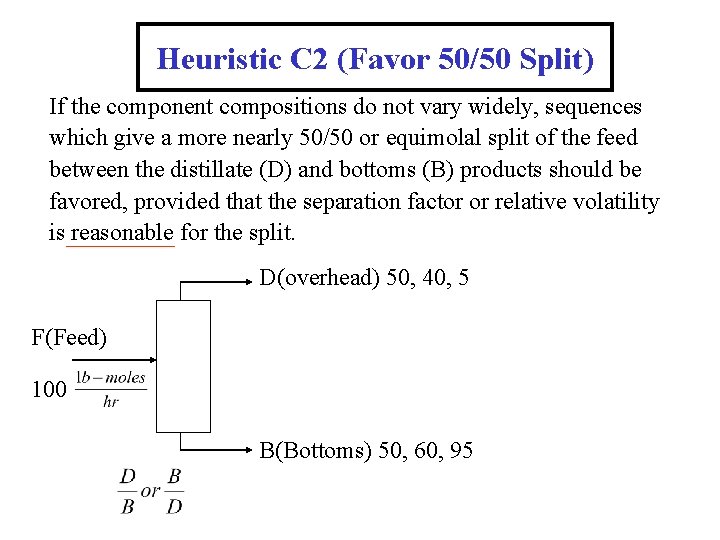
Heuristic C 2 (Favor 50/50 Split) If the component compositions do not vary widely, sequences which give a more nearly 50/50 or equimolal split of the feed between the distillate (D) and bottoms (B) products should be favored, provided that the separation factor or relative volatility is reasonable for the split. D(overhead) 50, 40, 5 F(Feed) 100 B(Bottoms) 50, 60, 95

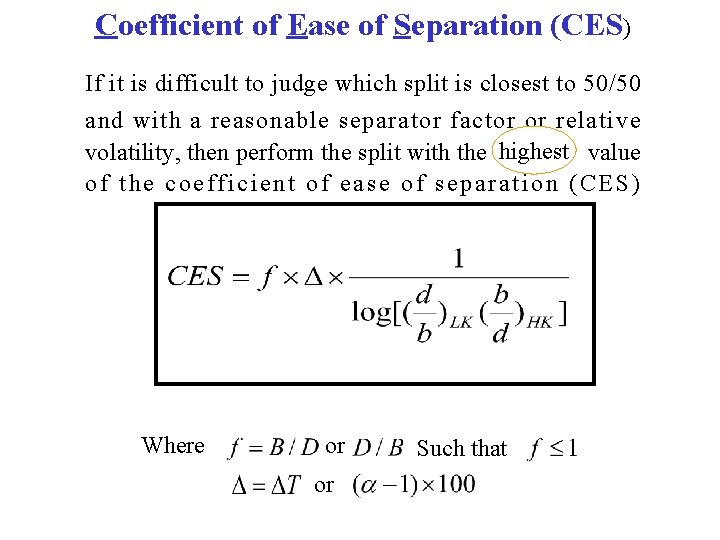
Coefficient of Ease of Separation (CES) If it is difficult to judge which split is closest to 50/50 and with a reasonable separator factor or relative volatility, then perform the split with the highest value of the coefficient of ease of separation (CES) Where or or Such that

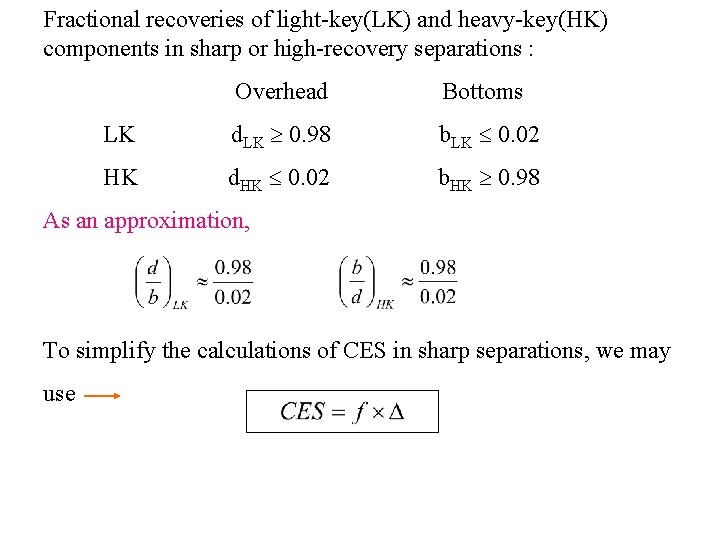
Fractional recoveries of light-key(LK) and heavy-key(HK) components in sharp or high-recovery separations : Overhead Bottoms LK d. LK 0. 98 b. LK 0. 02 HK d. HK 0. 02 b. HK 0. 98 As an approximation, To simplify the calculations of CES in sharp separations, we may use

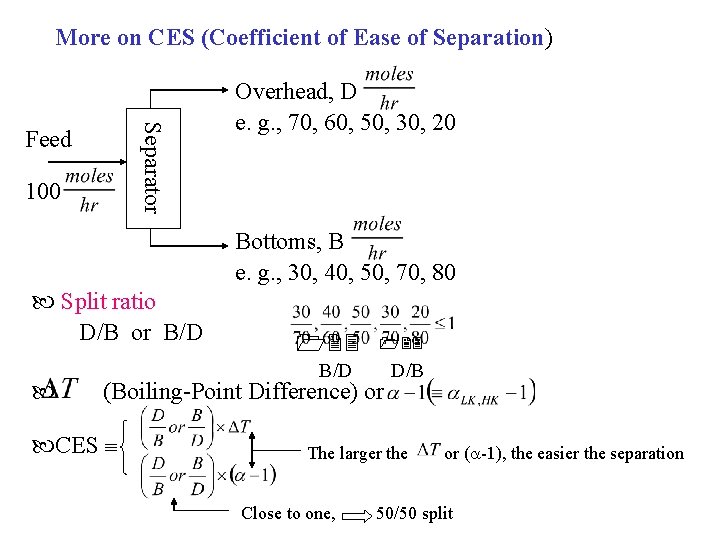
More on CES (Coefficient of Ease of Separation) Separator Feed 100 Overhead, D e. g. , 70, 60, 50, 30, 20 Bottoms, B e. g. , 30, 40, 50, 70, 80 Split ratio D/B or B/D (Boiling-Point Difference) or CES D/B The larger the Close to one, or ( -1), the easier the separation 50/50 split

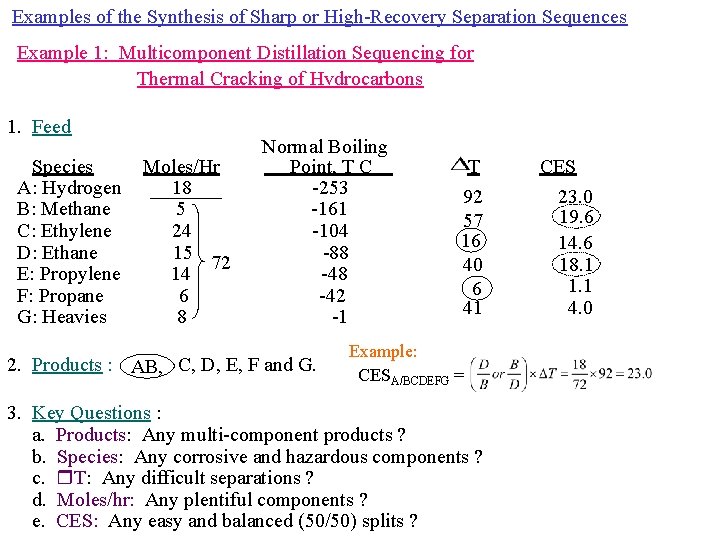
Examples of the Synthesis of Sharp or High-Recovery Separation Sequences Example 1: Multicomponent Distillation Sequencing for Thermal Cracking of Hvdrocarbons 1. Feed Species A: Hydrogen B: Methane C: Ethylene D: Ethane E: Propylene F: Propane G: Heavies Moles/Hr 18 5 24 15 72 14 6 8 Normal Boiling Point, T C -253 -161 -104 -88 -42 -1 2. Products : AB, C, D, E, F and G. T 92 57 16 40 6 41 Example: CESA/BCDEFG = 3. Key Questions : a. Products: Any multi-component products ? b. Species: Any corrosive and hazardous components ? c. T: Any difficult separations ? d. Moles/hr: Any plentiful components ? e. CES: Any easy and balanced (50/50) splits ? CES 23. 0 19. 6 14. 6 18. 1 1. 1 4. 0

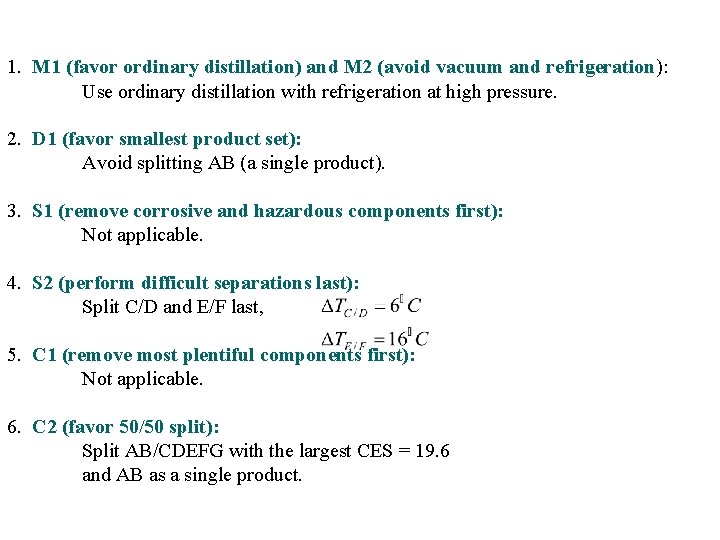
1. M 1 (favor ordinary distillation) and M 2 (avoid vacuum and refrigeration): Use ordinary distillation with refrigeration at high pressure. 2. D 1 (favor smallest product set): Avoid splitting AB (a single product). 3. S 1 (remove corrosive and hazardous components first): Not applicable. 4. S 2 (perform difficult separations last): Split C/D and E/F last, 5. C 1 (remove most plentiful components first): Not applicable. 6. C 2 (favor 50/50 split): Split AB/CDEFG with the largest CES = 19. 6 and AB as a single product.

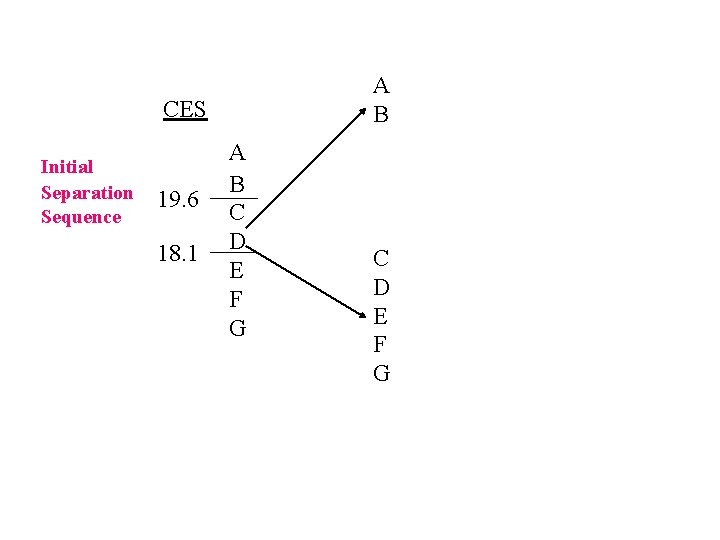
A B CES Initial Separation Sequence 19. 6 18. 1 A B C D E F G

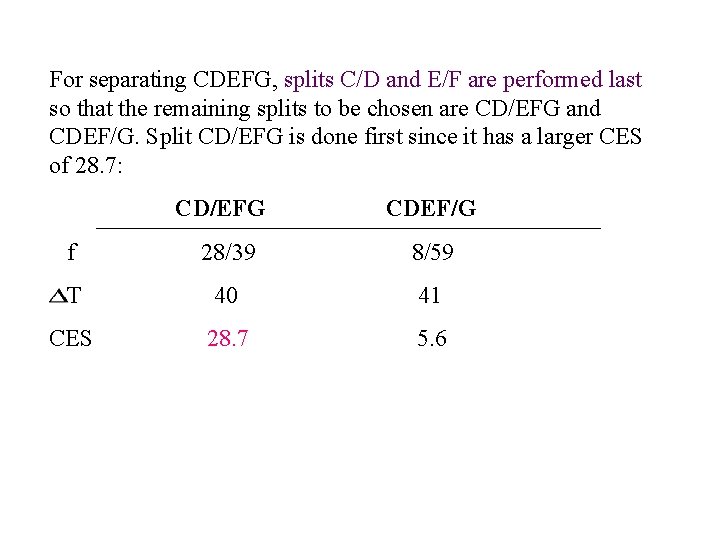
For separating CDEFG, splits C/D and E/F are performed last so that the remaining splits to be chosen are CD/EFG and CDEF/G. Split CD/EFG is done first since it has a larger CES of 28. 7: CD/EFG CDEF/G f 28/39 8/59 T 40 41 CES 28. 7 5. 6

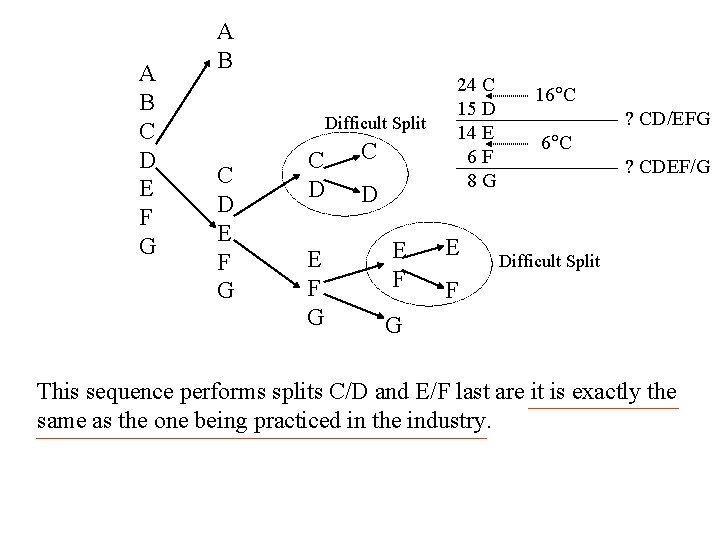
A B C D E F G A B Difficult Split C D E F G C D E F 24 C 15 D 14 E 6 F 8 G E 16 C ? CD/EFG 6 C ? CDEF/G Difficult Split F G This sequence performs splits C/D and E/F last are it is exactly the same as the one being practiced in the industry.

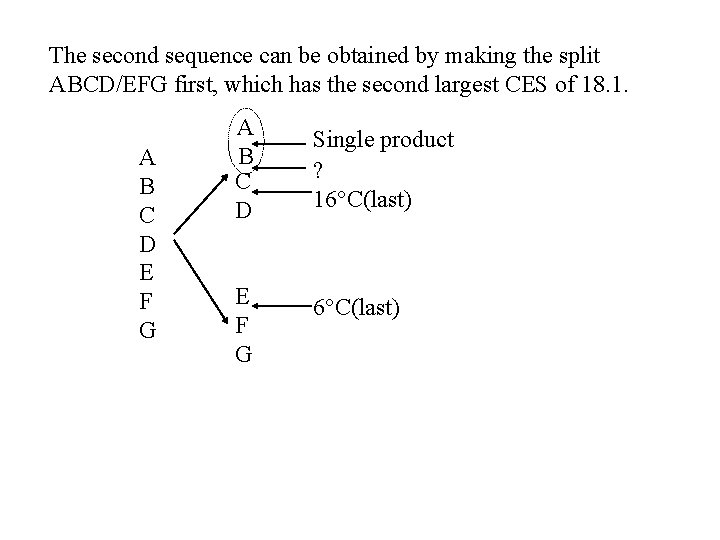
The second sequence can be obtained by making the split ABCD/EFG first, which has the second largest CES of 18. 1. A B C D E F G A B C D Single product ? 16 C(last) E F G 6 C(last)

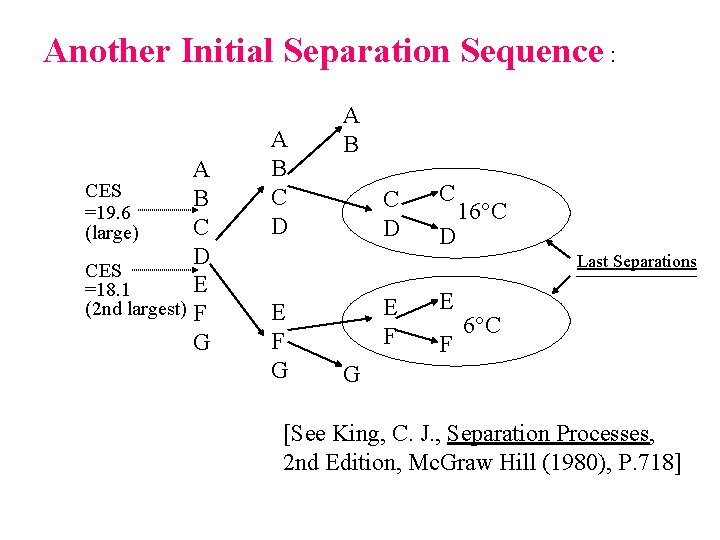
Another Initial Separation Sequence : A CES B =19. 6 C (large) D CES E =18. 1 (2 nd largest) F G A B C D C D 16 C Last Separations E F G E F 6 C G [See King, C. J. , Separation Processes, 2 nd Edition, Mc. Graw Hill (1980), P. 718]

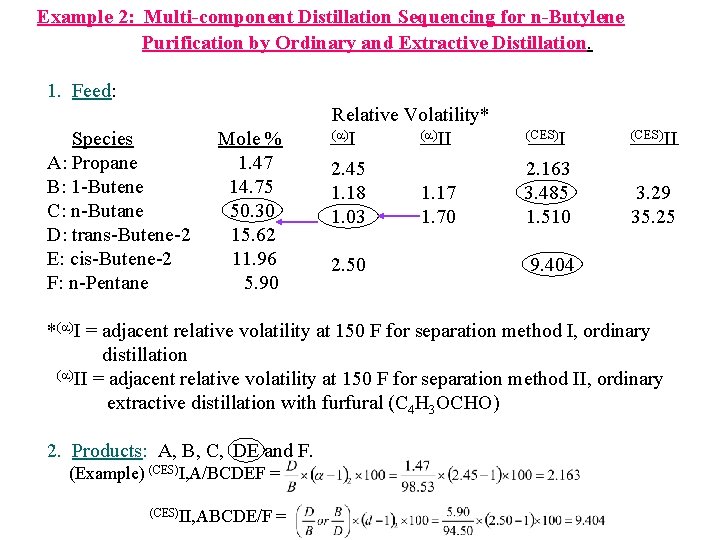
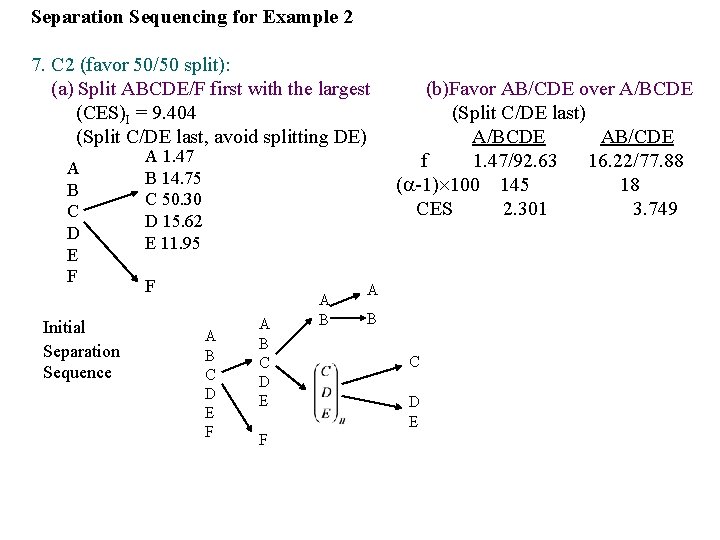
Example 2: Multi-component Distillation Sequencing for n-Butylene Purification by Ordinary and Extractive Distillation. 1. Feed: Species A: Propane B: 1 -Butene C: n-Butane D: trans-Butene-2 E: cis-Butene-2 F: n-Pentane Mole % 1. 47 14. 75 50. 30 15. 62 11. 96 5. 90 Relative Volatility* ( )II (CES)II 2. 45 1. 18 1. 03 2. 163 3. 485 1. 510 3. 29 35. 25 2. 50 1. 17 1. 70 9. 404 *( )I = adjacent relative volatility at 150 F for separation method I, ordinary distillation ( )II = adjacent relative volatility at 150 F for separation method II, ordinary extractive distillation with furfural (C 4 H 3 OCHO) 2. Products: A, B, C, DE and F. (Example) (CES)I, A/BCDEF = (CES)II, ABCDE/F =

3. Key Question : a. Products: Any multi-component products ? b. Species: Any corrosive and hazardous components ? c. Mole%: Any plentiful components ? d. Relative Volatilities: Any difficult separations ? e. (CES): Any easy and balanced (50/50) splits ?

1. M 1 (favor ordinary distillation): Use extractive distillation for C/D and ordinary distillation for all other splits. 2. M 2 (avoid vacuum and refrigeration): Use refrigeration at high pressure. 3. D 1 (favor smallest product set): Avoid splitting DE (a single product) 4. S 1 (remove corrosive and hazardous components first): Not applicable. 5. S 2 (perform difficult separations last): Split C/DE last (difficult extractive distillation), so that the added solvent (furfural) can be recovered at the end without having to worry about its presence as a contaminant in earlier splits. 6. C 1 (remove most plentiful components first): C(50. 30 mole %) dominates the feed, but it is not separated first due to S 2. Note: Heuristics are ranked in their relative importance according to their given order, e. g. , Heuristic S 2 overrides Heuristic C 1

Separation Sequencing for Example 2 7. C 2 (favor 50/50 split): (a) Split ABCDE/F first with the largest (CES)I = 9. 404 (Split C/DE last, avoid splitting DE) A B C D E F Initial Separation Sequence A 1. 47 B 14. 75 C 50. 30 D 15. 62 E 11. 95 F A B C D E F A B (b)Favor AB/CDE over A/BCDE (Split C/DE last) A/BCDE AB/CDE f 1. 47/92. 63 16. 22/77. 88 ( -1) 100 145 18 CES 2. 301 3. 749 A B C D E