Moisture Sorption and Isosteric Heat of Sorption properties








- Slides: 21

Moisture Sorption and Isosteric Heat of Sorption properties of PVP-CMC Hydrogel based Food Packaging Material Nabanita Sahaa, Dipali S. Shindeb, Madhusweta Dasb, Petr Saha a a. Centre of Polymer Systems, University Institute, Tomas Bata University in Zlin, Tř. T. Bati 5678, Zlin 760 01, Czech Republic b. Department of Agricultural and Food Engineering, Indian Institute of Technology, Kharagpur-721302, India * Contact email ID: nabanita@ft. utb. cz / madhu@agfe. iitkgp. ernet. in Tuesday, August 11, 2015 Biopolymers and Bioplastics-2015 1

Outline v. INTRODUCTION ü ü ü Polymer in packaging Hydrogel food packaging Unique properties of hydrogel Application of hydrogels Preparation techniques of hydrogel v. MOTIVATION OF RESEARCH v EXPERIMEMTATION v. RESULTS üVisual Images of PVP-CMC hydrogel food packaging material üAFM image of PVP-CMC hydrogel film üWater activity of PVP-CMC hydrogel at different temperature üMoisture sorption isotherm of PVP-CMC hydrogel film üComparison of PVP-CMC hydrogel film at different temperature üEffect of temperature on moisture sorption isotherm üIsosteric heat of sorption of PVP-CMC hydrogel v. CONCLUSION Tuesday, August 11, 2015 Biopolymers and Bioplastics-2015 2

Introduction Polymers in Packaging Polymeric materials play a dominate role in the food packaging industry Tuesday, August 11, 2015 3

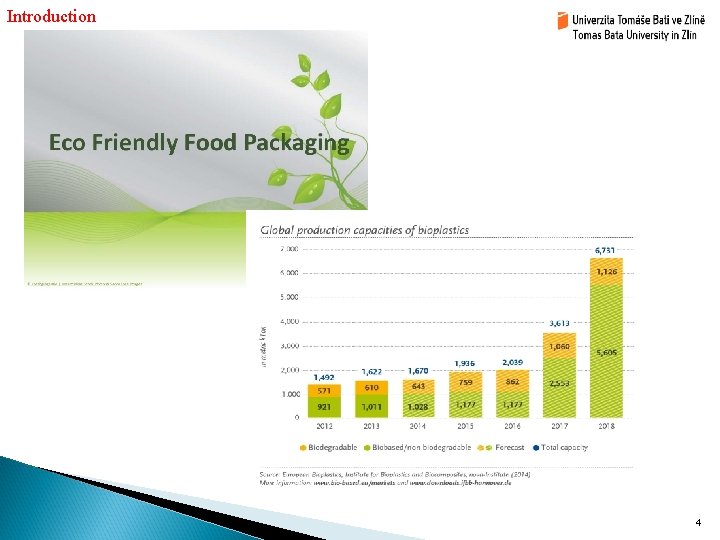
Introduction 4

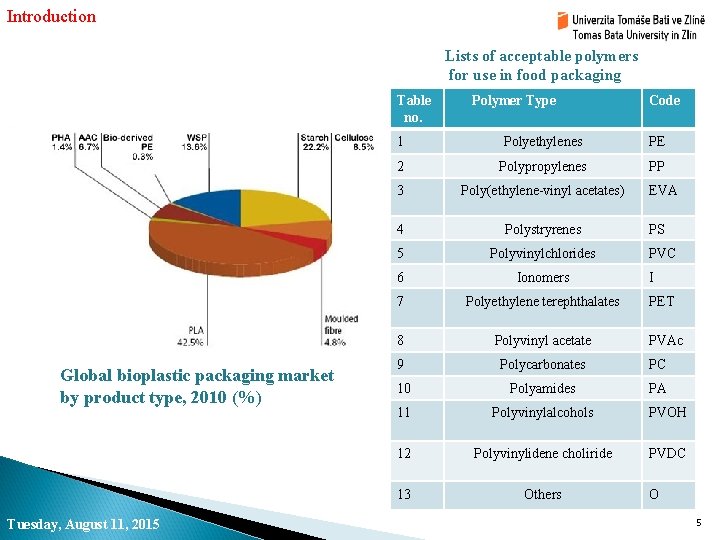
Introduction Lists of acceptable polymers for use in food packaging Table no. Global bioplastic packaging market by product type, 2010 (%) Tuesday, August 11, 2015 Polymer Type Code 1 Polyethylenes PE 2 Polypropylenes PP 3 Poly(ethylene-vinyl acetates) 4 Polystryrenes 5 Polyvinylchlorides 6 Ionomers 7 Polyethylene terephthalates 8 Polyvinyl acetate 9 Polycarbonates PC 10 Polyamides PA 11 Polyvinylalcohols PVOH 12 Polyvinylidene choliride PVDC 13 Others EVA PS PVC I PET PVAc O 5

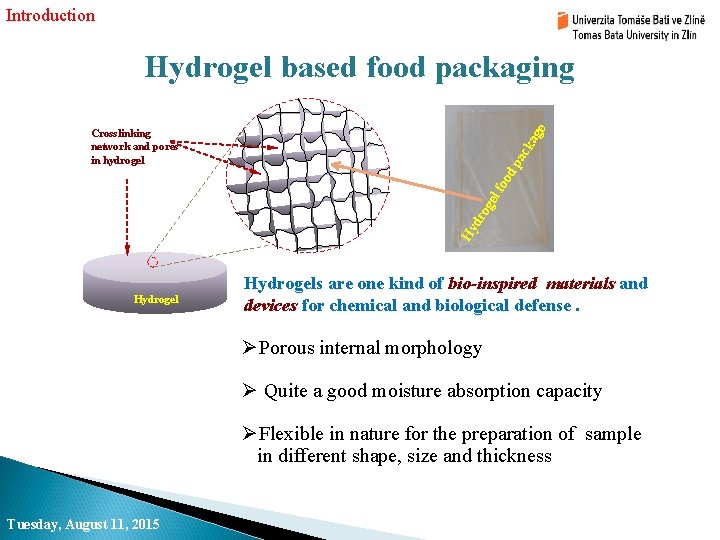
Introduction Hydrogel based food packaging Hy dr og el foo dp ac ka ge Crosslinking network and pores in hydrogel Hydrogels are one kind of bio-inspired materials and devices for chemical and biological defense. ØPorous internal morphology Ø Quite a good moisture absorption capacity ØFlexible in nature for the preparation of sample in different shape, size and thickness Tuesday, August 11, 2015

Introduction Unique properties of hydrogels v Hydrogels provide suitable semiwet, three-dimensional environments for molecular-level biological interactions. v Provide inert surfaces that prevent nonspecific adsorption of proteins, a property known as antifouling v Biological molecules can be covalently incorporated into hydrogel structures using a range of well-established chemistries v Hydrogels can be designed to change properties (e. g. swelling/collapse or solution-to-gel transitions) in response to externally applied triggers, such as temperature, ionic strength, solvent polarity, electric/magnetic field, light, or small (bio)molecules. Tuesday, August 11, 2015 R. V. Ulijn. , et al. Materialstoday, Vol 10, p-40 -48, 2007 7

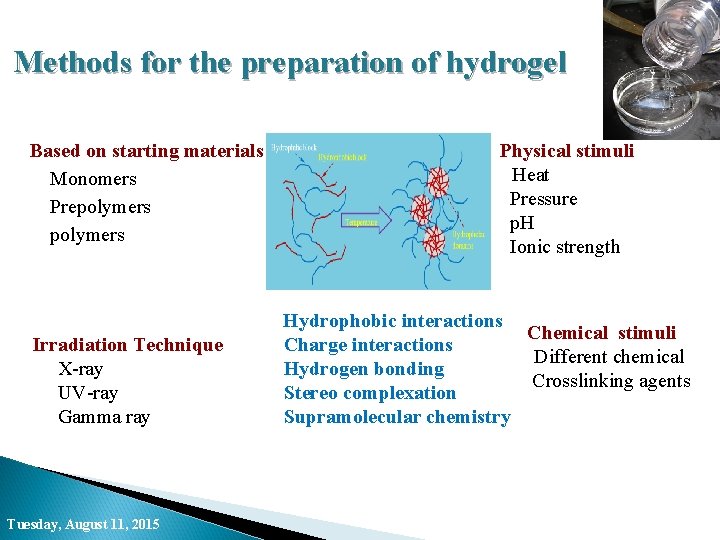
Methods for the preparation of hydrogel Based on starting materials • Monomers • Prepolymers • polymers Physical stimuli • Heat Pressure p. H Ionic strength Hydrophobic interactions Chemical stimuli • Charge interactions • Different chemical • Hydrogen bonding Crosslinking agents • Stereo complexation • Supramolecular chemistry • Irradiation Technique X-ray UV-ray Gamma ray Tuesday, August 11, 2015

Experimental Preparation of “PVP-CMC Hydrogel” for Food Packaging Tuesday, August 11, 2015 SEM images of hydrogels: PVP/CMC (a. i) surface (a. ii) cross section Tuesday, August 11, 2015 9

MOTIVATION OF RESEARCH v It is known that macromolecular network determining the properties of biopolymer based polymeric films where this macromolecular network is dependent on moisture content. v. On the other hand, moisture sorption isotherm (MSI) provides information on the moisture holding capacity of the films at variable relative humidity (water activity, a w). v. Water activity (aw) is a measure of the energy status of the moisture content in a system, and controls several properties of biopolymer based materials; high water activity leads to chemical and microbial instability. v. The equilibrium relationship between water activity (aw, ranging within 0. 0 -1. 0) and the corresponding moisture content at any particular temperature is an essential tool for design of drying, packaging and storage systems of food. TBU researcher reported that PVP-CMC hydrogel based food packaging material has capacity to absorb moisture, therefore, we are motivated to pursue the research to find the moisture sorption isotherm and isosteric heat sorption properties of PVP-CMC hydrogel. Tuesday, August 11, 2015 10

Visual images of PVP-CMC hydrogel based food packaging material ü Sealable Hy dr og e l fo od pa c ka ge ü Transparent ü Printable ü Able to absorb moisture ü Breathable and ü Biodegradable Tuesday, August 11, 2015 11

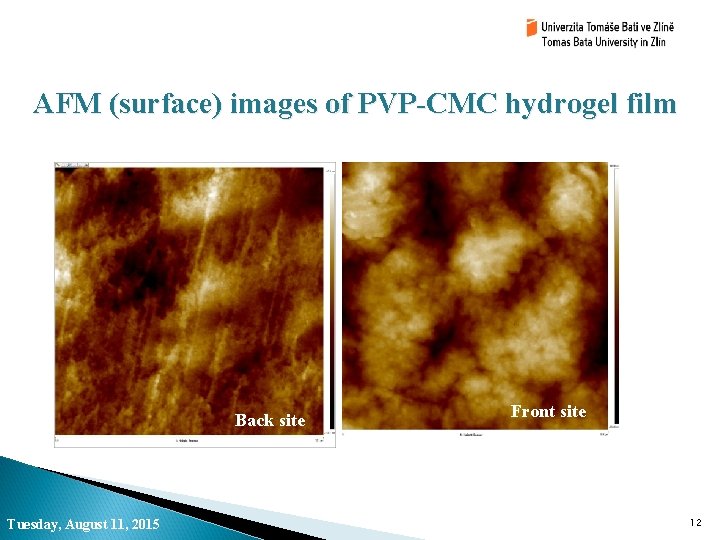
AFM (surface) images of PVP-CMC hydrogel film Back site Tuesday, August 11, 2015 Front site 12

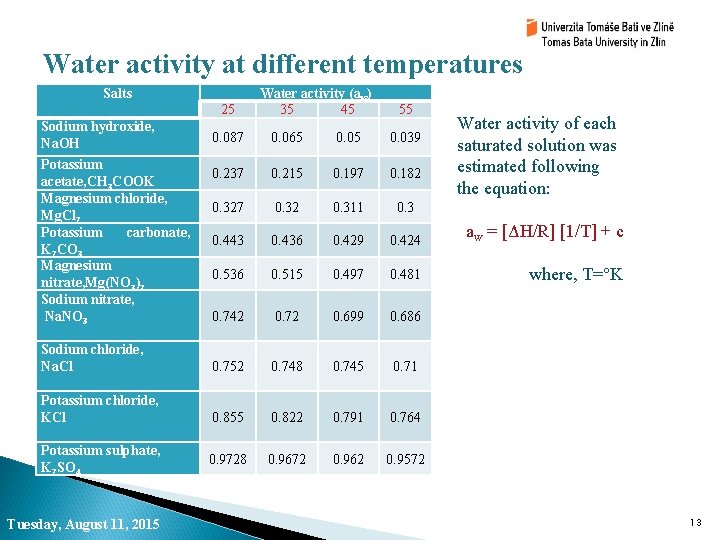
Water activity at different temperatures Salts 25 Sodium hydroxide, Na. OH Water activity (aw) 35 45 55 Water activity of each saturated solution was estimated following the equation: 0. 087 0. 065 0. 039 0. 237 0. 215 0. 197 0. 182 0. 327 0. 32 0. 311 0. 3 0. 443 0. 436 0. 429 0. 424 aw = [ΔH/R] [1/T] + c 0. 536 0. 515 0. 497 0. 481 where, T=°K 0. 742 0. 72 0. 699 0. 686 Sodium chloride, Na. Cl 0. 752 0. 748 0. 745 0. 71 Potassium chloride, KCl 0. 855 0. 822 0. 791 0. 764 0. 9728 0. 9672 0. 962 0. 9572 Potassium acetate, CH 3 COOK Magnesium chloride, Mg. Cl 2 Potassium carbonate, K 2 CO 3 Magnesium nitrate, Mg(NO 3)2 Sodium nitrate, Na. NO 3 Potassium sulphate, K 2 SO 4 Tuesday, August 11, 2015 13

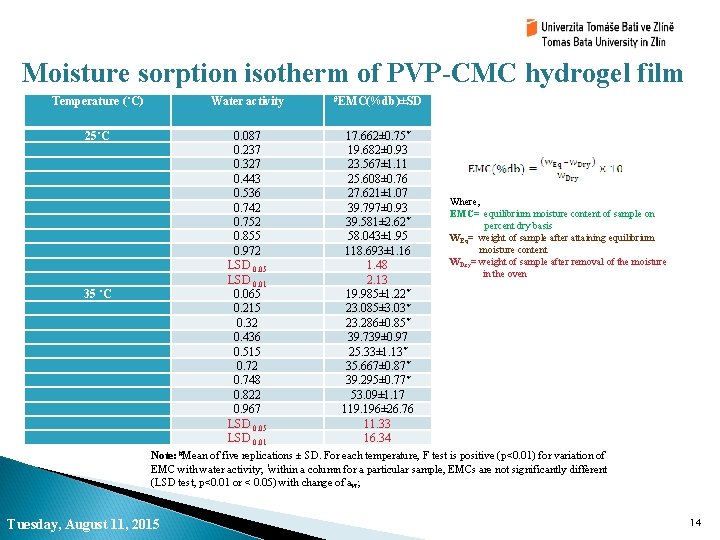
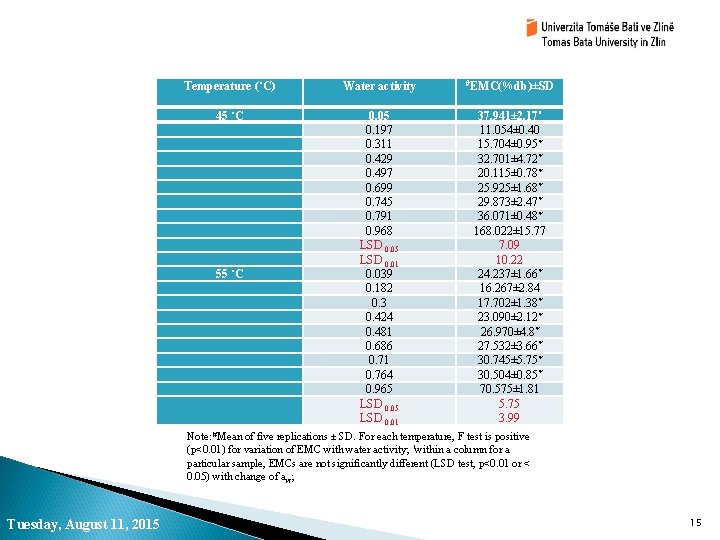
Moisture sorption isotherm of PVP-CMC hydrogel film Temperature (˚C) Water activity #EMC(%db)±SD 25˚C 0. 087 0. 237 0. 327 0. 443 0. 536 0. 742 0. 752 0. 855 0. 972 LSD 0. 05 LSD 0. 01 0. 065 0. 215 0. 32 0. 436 0. 515 0. 72 0. 748 0. 822 0. 967 LSD 0. 05 LSD 0. 01 17. 662± 0. 75* 19. 682± 0. 93 23. 567± 1. 11 25. 608± 0. 76 27. 621± 1. 07 39. 797± 0. 93 39. 581± 2. 62* 58. 043± 1. 95 118. 693± 1. 16 1. 48 2. 13 19. 985± 1. 22* 23. 085± 3. 03* 23. 286± 0. 85* 39. 739± 0. 97 25. 33± 1. 13* 35. 667± 0. 87* 39. 295± 0. 77* 53. 09± 1. 17 119. 196± 26. 76 11. 33 16. 34 35 ˚C Where, EMC= equilibrium moisture content of sample on percent dry basis WEq= weight of sample after attaining equilibrium moisture content WDry= weight of sample after removal of the moisture in the oven Note: #Mean of five replications ± SD. For each temperature, F test is positive (p<0. 01) for variation of EMC with water activity; *within a column for a particular sample, EMCs are not significantly different (LSD test, p<0. 01 or < 0. 05) with change of aw; Tuesday, August 11, 2015 14

Temperature (˚C) Water activity #EMC(%db)±SD 45 ˚C 0. 05 0. 197 0. 311 0. 429 0. 497 0. 699 0. 745 0. 791 0. 968 LSD 0. 05 LSD 0. 01 0. 039 0. 182 0. 3 0. 424 0. 481 0. 686 0. 71 0. 764 0. 965 LSD 0. 01 37. 941± 2. 17* 11. 054± 0. 40 15. 704± 0. 95* 32. 701± 4. 72* 20. 115± 0. 78* 25. 925± 1. 68* 29. 873± 2. 47* 36. 071± 0. 48* 168. 022± 15. 77 7. 09 10. 22 24. 237± 1. 66* 16. 267± 2. 84 17. 702± 1. 38* 23. 090± 2. 12* 26. 970± 4. 8* 27. 532± 3. 66* 30. 745± 5. 75* 30. 504± 0. 85* 70. 575± 1. 81 5. 75 3. 99 55 ˚C Note: #Mean of five replications ± SD. For each temperature, F test is positive (p<0. 01) for variation of EMC with water activity; *within a column for a particular sample, EMCs are not significantly different (LSD test, p<0. 01 or < 0. 05) with change of aw; Tuesday, August 11, 2015 15

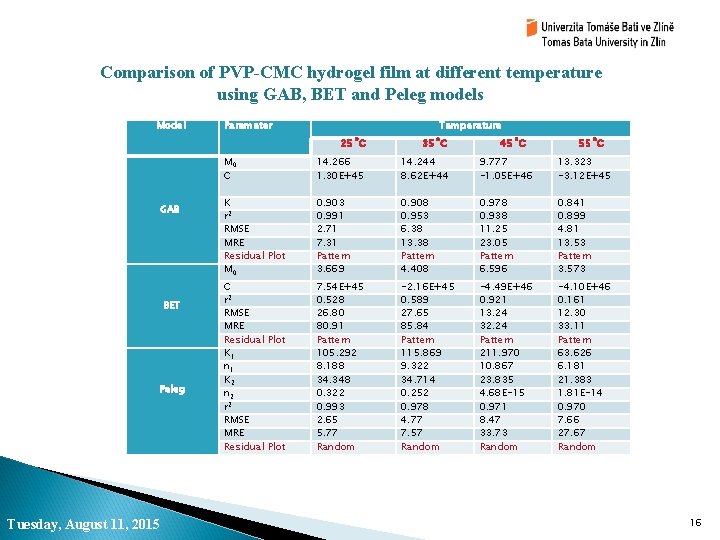
Comparison of PVP-CMC hydrogel film at different temperature using GAB, BET and Peleg models Model GAB BET Peleg Tuesday, August 11, 2015 Parameter Temperature 25˚C 35˚C 45˚C 55˚C M 0 C 14. 266 1. 30 E+45 14. 244 8. 62 E+44 9. 777 -1. 05 E+46 13. 323 -3. 12 E+45 K r 2 RMSE MRE Residual Plot M 0 0. 903 0. 991 2. 71 7. 31 Pattern 3. 669 0. 908 0. 953 6. 38 13. 38 Pattern 4. 408 0. 978 0. 938 11. 25 23. 05 Pattern 6. 596 0. 841 0. 899 4. 81 13. 53 Pattern 3. 573 C r 2 RMSE MRE Residual Plot K 1 n 1 K 2 n 2 r 2 RMSE MRE Residual Plot 7. 54 E+45 0. 528 26. 80 80. 91 Pattern 105. 292 8. 188 34. 348 0. 322 0. 993 2. 65 5. 77 Random -2. 16 E+45 0. 589 27. 65 85. 84 Pattern 115. 869 9. 322 34. 714 0. 252 0. 978 4. 77 7. 57 Random -4. 49 E+46 0. 921 13. 24 32. 24 Pattern 211. 970 10. 867 23. 835 4. 68 E-15 0. 971 8. 47 33. 73 Random -4. 10 E+46 0. 161 12. 30 33. 11 Pattern 63. 626 6. 181 21. 383 1. 81 E-14 0. 970 7. 66 27. 67 Random 16

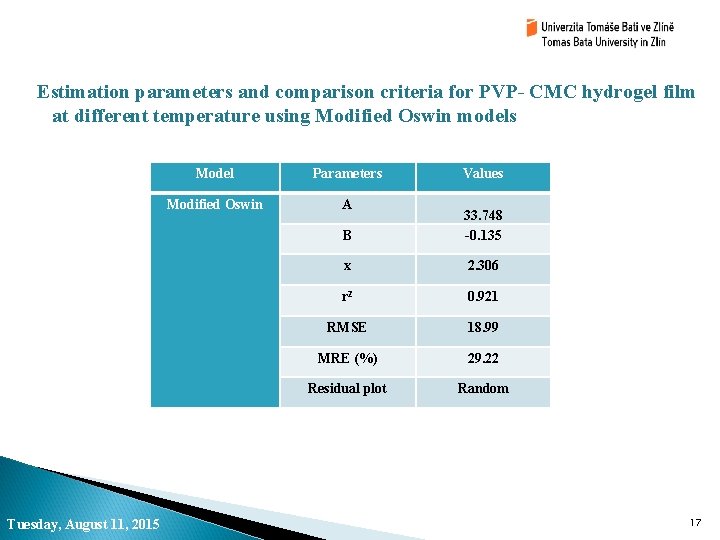
Estimation parameters and comparison criteria for PVP- CMC hydrogel film at different temperature using Modified Oswin models Tuesday, August 11, 2015 Model Parameters Modified Oswin A Values B 33. 748 -0. 135 x 2. 306 r 2 0. 921 RMSE 18. 99 MRE (%) 29. 22 Residual plot Random 17

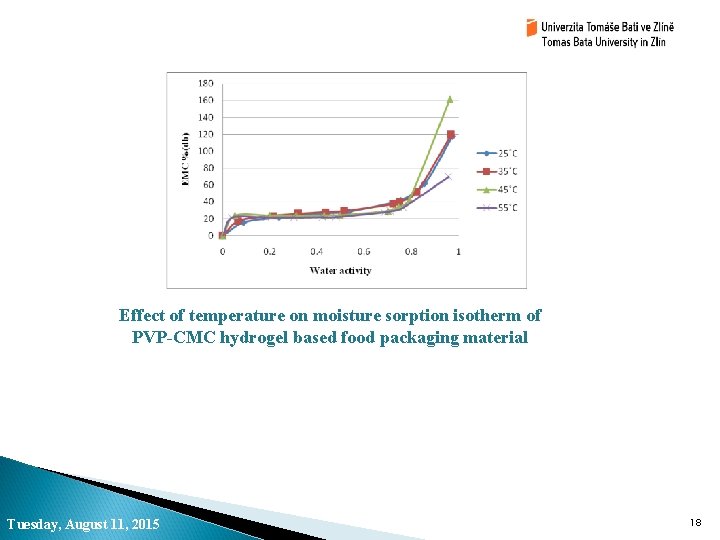
Effect of temperature on moisture sorption isotherm of PVP-CMC hydrogel based food packaging material Tuesday, August 11, 2015 18

Isosteric heat of sorption of PVP-CMC hydrogel based food packaging material at different moisture content Tuesday, August 11, 2015 19

Acknowledgement Authors are thankful for the financial support provided by the Ministry of Education , Youth and Sports of the Czech Republic - Program NPU I (LO 1504) Tuesday, August 11, 2015 20

Thank you for your attention! 21
 Moisture sorption isotherm
Moisture sorption isotherm Sorption definition
Sorption definition Sorption definition
Sorption definition On in
On in Sorption
Sorption Latent heat problem
Latent heat problem Love of cloud and rain chapter 18
Love of cloud and rain chapter 18 Localized convective lifting
Localized convective lifting Moisture and total solids analysis
Moisture and total solids analysis Soft gelatin capsules
Soft gelatin capsules Estimating soil moisture by feel and appearance
Estimating soil moisture by feel and appearance Moisture clouds and precipitation
Moisture clouds and precipitation Bonide leaf shine and moisture guard
Bonide leaf shine and moisture guard Extensive vs intensive properties
Extensive vs intensive properties Chemical and physical properties
Chemical and physical properties Properties of heat
Properties of heat Define dry heat cooking methods
Define dry heat cooking methods Lab 5 atmospheric moisture
Lab 5 atmospheric moisture Scs cn method of runoff estimation
Scs cn method of runoff estimation Define equilibrium moisture content
Define equilibrium moisture content Goddess of moisture
Goddess of moisture Moisture equation
Moisture equation