MILI 6990 Using Insurance Claims Data for Health


















- Slides: 32

MILI 6990: Using Insurance Claims Data for Health Market Opportunity Analysis AKA - Steve called in a favor Adrine Chung, MBA and Stephan Dunning, MBA Chronic Disease Research Group, Minneapolis Medical Research Foundation

Agenda I. III. IV. V. Our Background and CDRG Introduction to Claims Data Utilization of Claims Data Market Opportunities MILI Program – Students and Affiliates

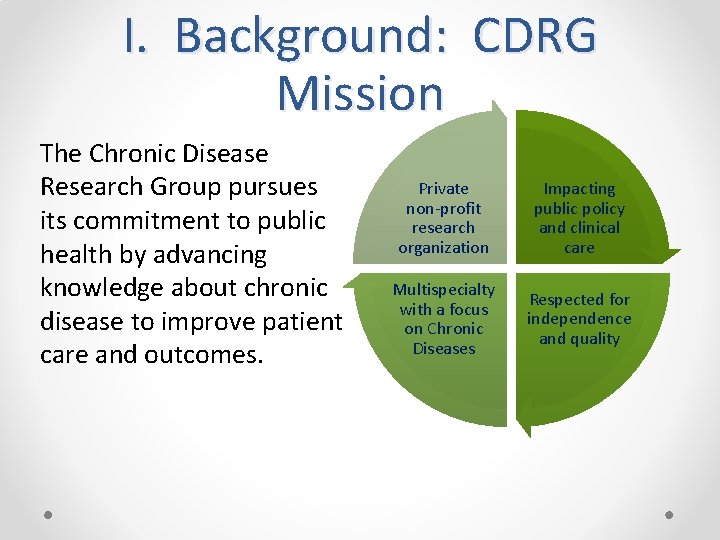
I. Background: CDRG Mission The Chronic Disease Research Group pursues its commitment to public health by advancing knowledge about chronic disease to improve patient care and outcomes. Private non-profit research organization Impacting public policy and clinical care Multispecialty with a focus on Chronic Diseases Respected for independence and quality

I. Background: CDRG Organizational Hierarchy Hennepin Healthcare System, Inc. : Operating Hennepin County Medical Center in Minneapolis, MN, a nationally recognized academic medical center employing 400+ healthcare providers. The physicians also have faculty appointments at the University of Minnesota. Minneapolis Medical Research Foundation (MMRF): Private, non-profit research subsidiary of Hennepin Healthcare System, Inc. Chronic Disease Research Group (CDRG): Operational division within MMRF employing more than 65 staff.

I. Background: CDRG Programs Scientific Registry of Transplant Recipients Health Resources and Services Administration (HRSA) Contract Analyzes data and simulates for policy development, creates reports of programs, and provides data for evaluation of solid organ transplantation in U. S. United States Renal Data System Chronic Disease Research Group National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) Various (sponsored, grants, independent) Collects, analyzes, and distributes information about end-stage renal disease (ESRD) in the United States Public health research in nephrology, cardiology, oncology, pharmacoepidemiology, and geriatric medicine

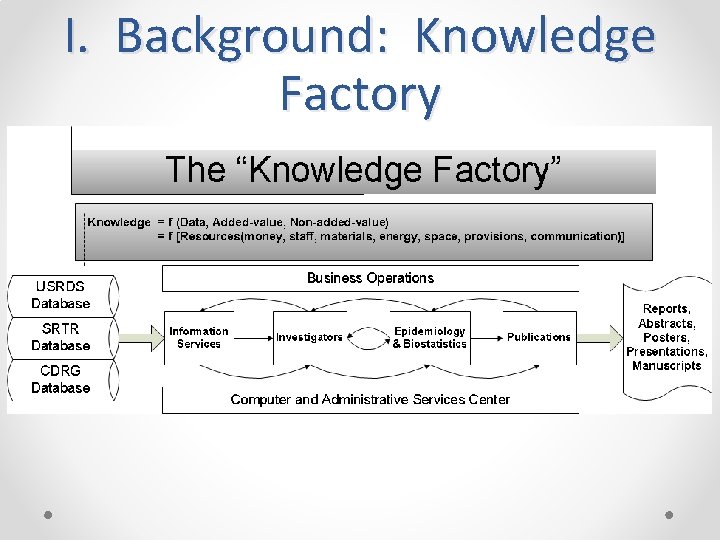
I. Background: Knowledge Factory

• II. Intro to Claims Data: Overview Claims – billable interactions between: • covered patients and the healthcare delivery • health care or service provider and the payer

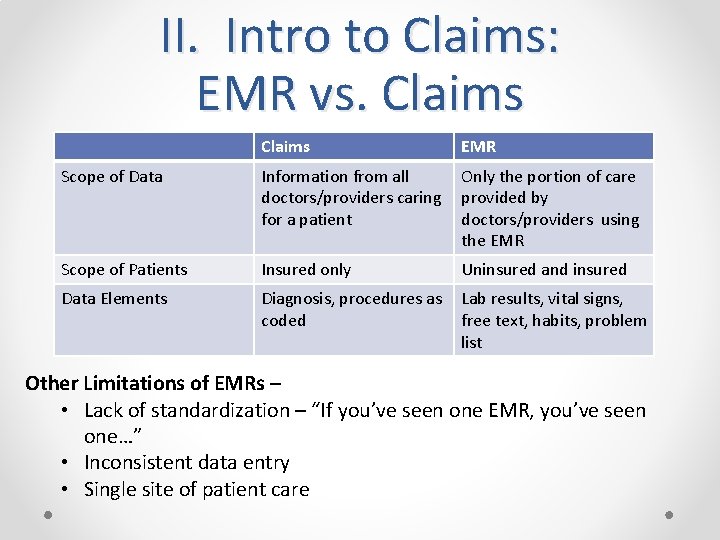
II. Intro to Claims: EMR vs. Claims EMR Scope of Data Information from all Only the portion of care doctors/providers caring provided by for a patient doctors/providers using the EMR Scope of Patients Insured only Data Elements Diagnosis, procedures as Lab results, vital signs, coded free text, habits, problem list Uninsured and insured Other Limitations of EMRs – • Lack of standardization – “If you’ve seen one EMR, you’ve seen one…” • Inconsistent data entry • Single site of patient care

II. Intro to Claims: Source of Claims Data • Commercial Claims (i. e. United Health, Market. Scan) • Medicare o Limited (LDS) o Research Identifiable (RIF) o USRDS (ESRD) • Medicaid • Linked Datasets (i. e. SEER-Medicare)

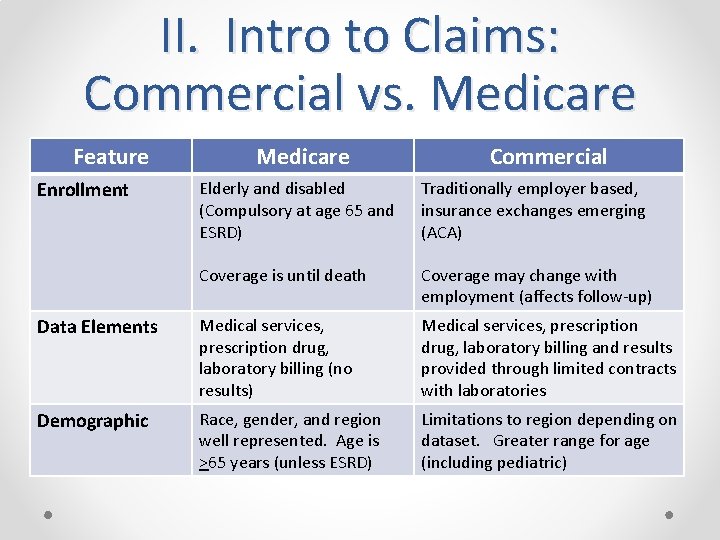
II. Intro to Claims: Commercial vs. Medicare Feature Enrollment Medicare Commercial Elderly and disabled (Compulsory at age 65 and ESRD) Traditionally employer based, insurance exchanges emerging (ACA) Coverage is until death Coverage may change with employment (affects follow-up) Data Elements Medical services, prescription drug, laboratory billing (no results) Medical services, prescription drug, laboratory billing and results provided through limited contracts with laboratories Demographic Race, gender, and region well represented. Age is >65 years (unless ESRD) Limitations to region depending on dataset. Greater range for age (including pediatric)

II. Intro to Claims Data: Medicare • Part A – hospital care, skilled nursing facility care, nursing home care, hospice, and home health services • Part B – physician visits, ambulance services, durable medical equipment, mental health, preventative services • Part D – prescription drug coverage (70%)

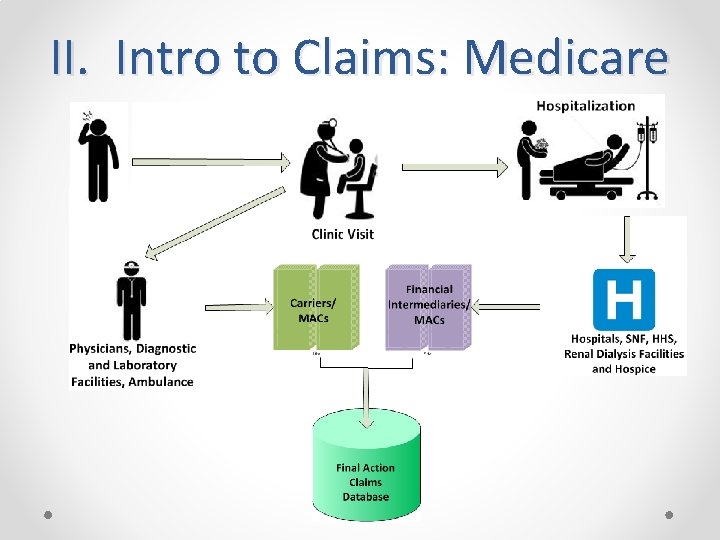
II. Intro to Claims: Medicare

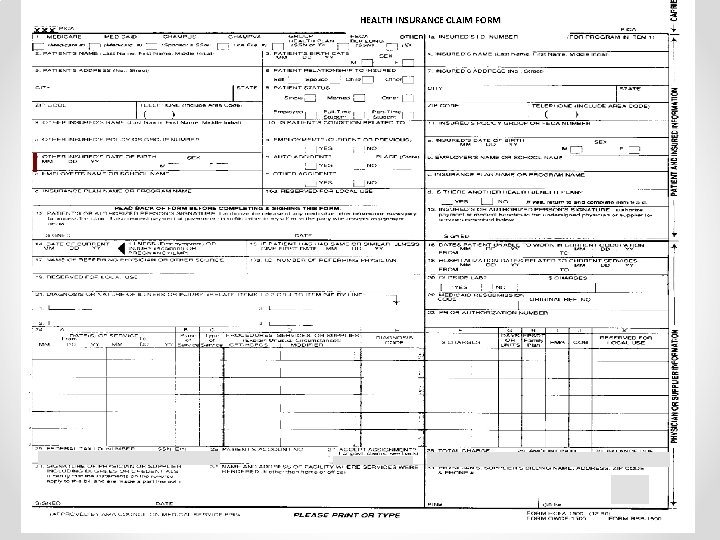
HEALTH INSURANCE CLAIM FORM

II. Intro to Claims Data: Coding • ICD 9 – International Classification of Diseases, Version 9 (diagnoses) • XXX. XX – AMI 410. X, PTCA 00. 66 • X matters • CPT 4 – Current Procedural Terminology, Version 4 (procedures) • 5 digits, 0 matters • i. e. PTCA 92982 • NDC - Food and Drug Administration’s Nation Drug Code directory (Drugs) • 10 digit number with 3 segments

II. Intro to Claims: DRGs • Part A Hospital Claims o ICD-9 and CPT codes associated with the hospitalization episode are processed through “grouping” algorithms to result in a single Diagnosis Related Group (DRG) for payment from CMS. o The position of codes matters for payment. That is, not all diagnosis and procedure code are created equal.

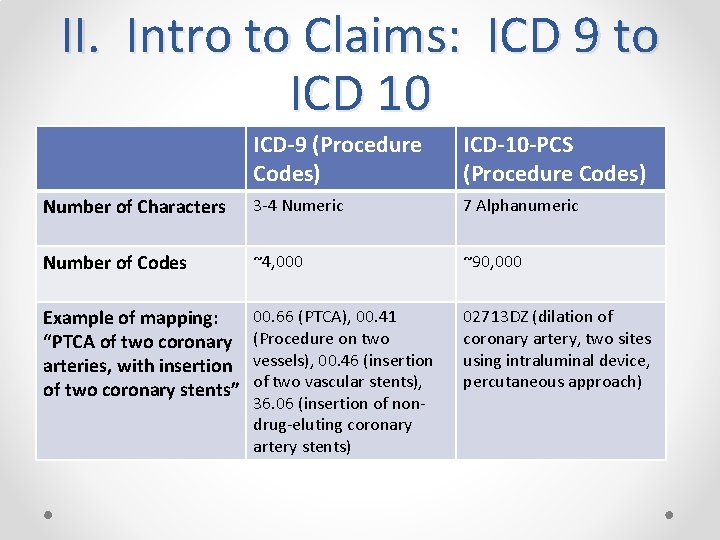
II. Intro to Claims: ICD 9 to ICD 10 ICD-9 (Procedure Codes) ICD-10 -PCS (Procedure Codes) Number of Characters 3 -4 Numeric 7 Alphanumeric Number of Codes ~4, 000 ~90, 000 Example of mapping: “PTCA of two coronary arteries, with insertion of two coronary stents” 00. 66 (PTCA), 00. 41 (Procedure on two vessels), 00. 46 (insertion of two vascular stents), 36. 06 (insertion of nondrug-eluting coronary artery stents) 02713 DZ (dilation of coronary artery, two sites using intraluminal device, percutaneous approach)

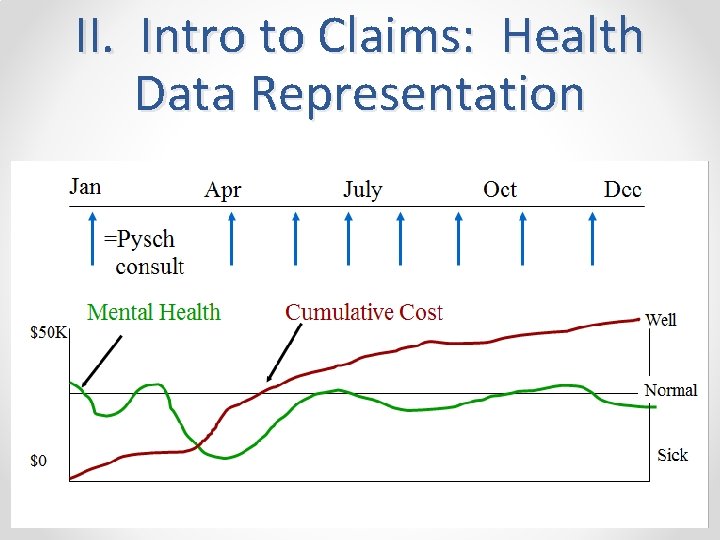
II. Intro to Claims: Health Data Representation

II. Intro to Claims: Strengths and Limitations Strengths • Clinical validity – information about covered services • Demographic information (if available) • Population Coverage (different strengths for different datasets) • Cost effective in comparison to chart reviews or clinical trials Limitations • Underdiagnosed diseases (diabetes, depression, hypertension) • Incomprehensive disease and severity information • Incidence vs. prevalence • Limited clinical information • Limit to reimbursed services • Limit to number of codes reported • Primary source of all clinical insight but codes are at times“ questionable accuracy, completeness, meaningfulness and clinical scope” • “…codes are not meant to tell stories, rather to generate reimbursement…” (Iezzoni 2002: 348)

II. Intro to Claims: Access to Data • Medicare & Medicaid: o Research Data Assistance Center (Res. DAC) o Aggregate-level data through private research groups that use CMS with approval (i. e. CDRG and University of Minnesota) o Direct for federally funded contracts o Data lag: 9 months for Part A/Part B and 15 for Part D • Commercially-insured claims data: o Optum. Insights, Market. Scan, Medco, Phar. Metrics o Data updated quarterly

III. Utilization of Claims Data • • • Market Research Quality Improvement- QIP Fraud Detection Drug Safety Signal Detection (FDA Sentinel) Post-market Safety and Surveillance Health Economics and Outcome Research (CDRG’s Core) o Comparative Effectiveness • Clinical • Economic • Value o Clinical Trial Supplement

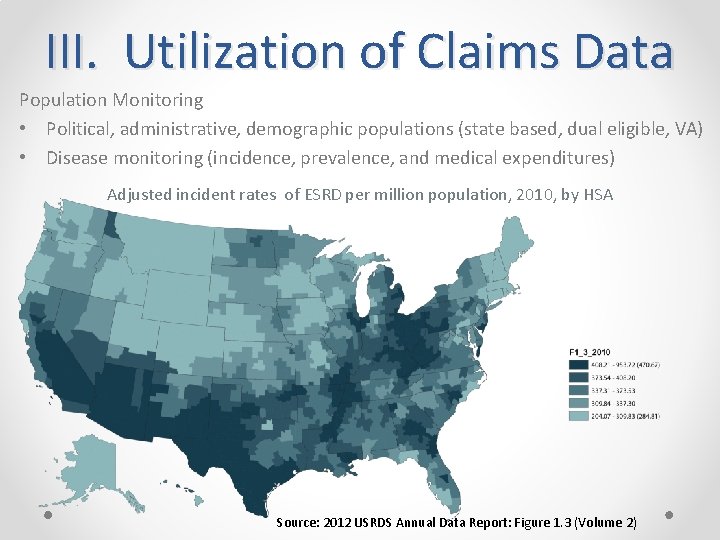
III. Utilization of Claims Data Population Monitoring • Political, administrative, demographic populations (state based, dual eligible, VA) • Disease monitoring (incidence, prevalence, and medical expenditures) Adjusted incident rates of ESRD per million population, 2010, by HSA Source: 2012 USRDS Annual Data Report: Figure 1. 3 (Volume 2)

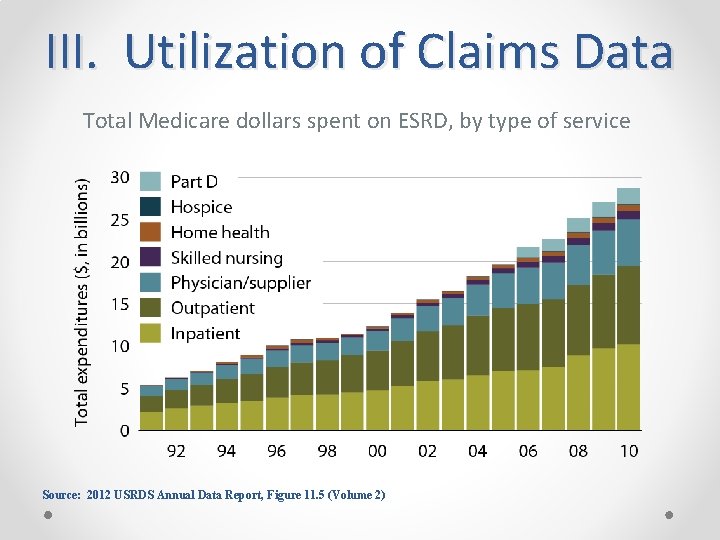
III. Utilization of Claims Data Total Medicare dollars spent on ESRD, by type of service Source: 2012 USRDS Annual Data Report, Figure 11. 5 (Volume 2)

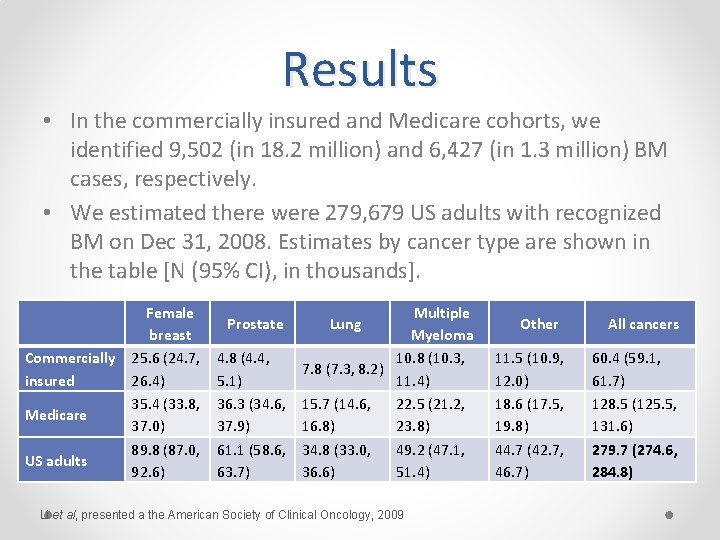
III. Utilization of Claims Prevalence of Recognized Bone Metastases in the US Adult Population Methods: o All available claims from 2004 -2008 were studied in 2 point-prevalent cohorts with insurance coverage on Dec 31, 2008: • 1) persons aged 18 -64 years enrolled in commercial plans (Market. Scan) and • 2) persons aged ≥ 65 years enrolled in traditional Medicare (Medicare 5% sample). o Presence of BM was defined by 1 inpatient or 2 outpatient claims in any 1 -year interval with a diagnosis of BM or 1 claim for zoledronic acid or pamidronate with a qualifying diagnosis for cancer. o BM prevalence was extrapolated to the national commercially insured population aged 18 -64 years and to the traditional Medicare population aged ≥ 65 years. o Applying age/sex-specific rates to the 2008 US census population, we estimated BM prevalence in the US adult population overall and for select cancers. Li et al, presented a the American Society of Clinical Oncology, 2009

Results • In the commercially insured and Medicare cohorts, we identified 9, 502 (in 18. 2 million) and 6, 427 (in 1. 3 million) BM cases, respectively. • We estimated there were 279, 679 US adults with recognized BM on Dec 31, 2008. Estimates by cancer type are shown in the table [N (95% CI), in thousands]. Female breast Commercially 25. 6 (24. 7, insured 26. 4) 35. 4 (33. 8, Medicare 37. 0) 89. 8 (87. 0, US adults 92. 6) Multiple Myeloma 4. 8 (4. 4, 10. 8 (10. 3, 7. 8 (7. 3, 8. 2) 5. 1) 11. 4) 36. 3 (34. 6, 15. 7 (14. 6, 22. 5 (21. 2, 37. 9) 16. 8) 23. 8) 61. 1 (58. 6, 34. 8 (33. 0, 49. 2 (47. 1, 63. 7) 36. 6) 51. 4) Prostate Lung Li et al, presented a the American Society of Clinical Oncology, 2009 Other 11. 5 (10. 9, 12. 0) 18. 6 (17. 5, 19. 8) 44. 7 (42. 7, 46. 7) All cancers 60. 4 (59. 1, 61. 7) 128. 5 (125. 5, 131. 6) 279. 7 (274. 6, 284. 8)

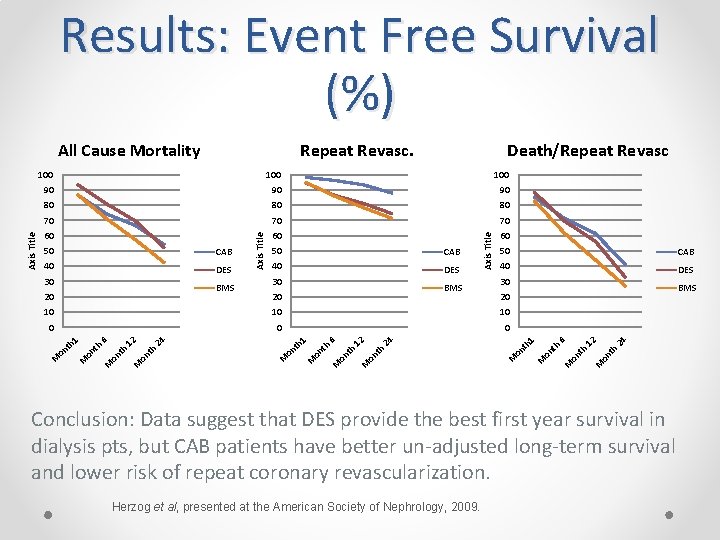
III. Utilization of Claims Long-Term Survival and Repeat Revascularization in US Dialysis Patients after Surgical versus Percutaneous Coronary Intervention (ASN Renal Week 2009) Methods • Searched United States Renal Data System claims database to identify 4, 351 dialysis pts having coronary artery bypass surgery, (CAB), bare metal stents (BMS), or drug-eluting stents (DES) in 2005. • Outcomes of Long-term event-free survival for all-cause mortality, repeat revascularization (CAB or PCI), and the combined event of death or repeat revascularization was estimated by Kaplan-Meier method.

Results: Event Free Survival (%) Death/Repeat Revasc 100 90 90 90 80 80 80 70 70 70 60 60 60 4 th 2 2 on 1 on th M 2 on th 1 M th on M on th 1 M on th 4 0 2 0 6 0 4 10 2 10 6 10 M BMS 20 1 BMS 20 DES 30 M 30 40 th BMS 20 DES CAB 6 30 40 50 on DES CAB th 40 50 M CAB on 50 Axis Title 100 M Repeat Revasc. Axis Title All Cause Mortality Conclusion: Data suggest that DES provide the best first year survival in dialysis pts, but CAB patients have better un-adjusted long-term survival and lower risk of repeat coronary revascularization. Herzog et al, presented at the American Society of Nephrology, 2009.

Zzzzzz? !

III. Utilization of Claims Data Benchmarking • Quality of care: ESRD Quality Incentive Program (QIP), Hospital Readmission Penalty • Performance measurement: State-specific, Agency-specific, Facility-specific measures (Transplant Program-specific Reports, Dialysis Facility Compare, etc) • Accountable Care Organization – performance monitoring and payment/penalty system Evaluating Policy • CBO, GAO – Cost assessment of ESRD Bundle o Differing findings on including Oral Drugs in Bundle

IV. Market Opportunities • Data Linkages: o o o o US Census Cancer Registries (SEER) Other Providers (VA, Medicaid) National death index/vital statistics Surveys (MCBS, NHANES, Health and Retirement Study) Provider Information EHR Clinical Trial Data

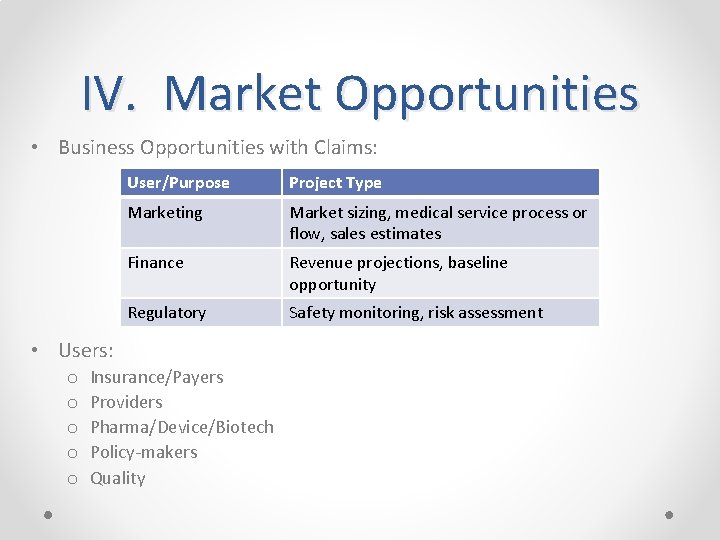
IV. Market Opportunities • Business Opportunities with Claims: User/Purpose Project Type Marketing Market sizing, medical service process or flow, sales estimates Finance Revenue projections, baseline opportunity Regulatory Safety monitoring, risk assessment • Users: o o o Insurance/Payers Providers Pharma/Device/Biotech Policy-makers Quality

V. MILI Students and Affiliates • • MILISA MILI Specialization MILI Affiliates/Alumni MILI Valuation Lab

Tying It Together: MILI DC Field Trip March 17 -19 Sign up today! http: //bit. ly/YDS 8 j. K
 Unlike routine claims, persuasive claims:
Unlike routine claims, persuasive claims: Memorandrum
Memorandrum Mili data
Mili data Elements of fire insurance contract
Elements of fire insurance contract Valores de mili micro nano pico
Valores de mili micro nano pico Pesma djaka prvaka
Pesma djaka prvaka Co ns
Co ns Jednotky kilo mega giga
Jednotky kilo mega giga Freze malafası
Freze malafası Kilo mili
Kilo mili Power system topology
Power system topology Sesuatu yang dapat diukur dan memiliki nilai adalah
Sesuatu yang dapat diukur dan memiliki nilai adalah Apostrofa stilska figura primeri
Apostrofa stilska figura primeri Deci centi mili
Deci centi mili Mili centi deci chart
Mili centi deci chart Mili mikro nano piko značky
Mili mikro nano piko značky Osnovne mjerne jedinice
Osnovne mjerne jedinice Notacion cientifica kilo mega giga tera
Notacion cientifica kilo mega giga tera Prefiksi fizika
Prefiksi fizika Mili e micro
Mili e micro Cjelivat ću gospodine
Cjelivat ću gospodine Medicaid claims data
Medicaid claims data Ttuhsc counseling center
Ttuhsc counseling center Fau employee benefits
Fau employee benefits Umms benefits
Umms benefits Blue cross tonik plan
Blue cross tonik plan Ouhsc health club
Ouhsc health club Raksha tpa lucknow office
Raksha tpa lucknow office Susorp valic
Susorp valic Health insurance market segmentation
Health insurance market segmentation Deloitte health insurance
Deloitte health insurance What is deductible in health insurance
What is deductible in health insurance Health insurance premium payment program ny
Health insurance premium payment program ny