Medtronic nonpolymeric DES development from nanoporous coatings to






- Slides: 29

Medtronic non-polymeric DES development: from nanoporous coatings to drug filled tubes Josiah N. Wilcox, Ph. D. Vice President and Resident Scholar Science & Technology Medtronic Cardio. Vascular CRT 2010

Disclosures • Cy Wilcox is a full time employee of Medtronic Cardio. Vascular, Inc. • Resolute™ using the Bio. Linx™ polymer is not approved for sale or use in the US • Integrity™ is not approved for sale or use in the US • Additional new technologies and product concepts discussed in this presentation are not approved for sale or commercial use in the US

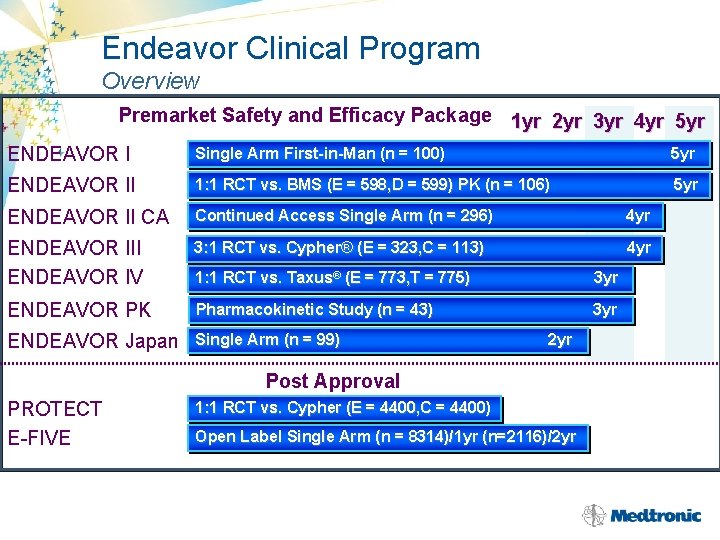
Endeavor Clinical Program Overview Premarket Safety and Efficacy Package 1 yr 2 yr 3 yr 4 yr 5 yr ENDEAVOR I Single Arm First-in-Man (n = 100) 5 yr ENDEAVOR II 1: 1 RCT vs. BMS (E = 598, D = 599) PK (n = 106) 5 yr ENDEAVOR II CA Continued Access Single Arm (n = 296) 4 yr ENDEAVOR III ENDEAVOR IV 3: 1 RCT vs. Cypher® (E = 323, C = 113) 4 yr 1: 1 RCT vs. Taxus® (E = 773, T = 775) 3 yr ENDEAVOR PK Pharmacokinetic Study (n = 43) 3 yr ENDEAVOR Japan Single Arm (n = 99) 2 yr Post Approval PROTECT E-FIVE 1: 1 RCT vs. Cypher (E = 4400, C = 4400) Open Label Single Arm (n = 8314)/1 yr (n=2116)/2 yr

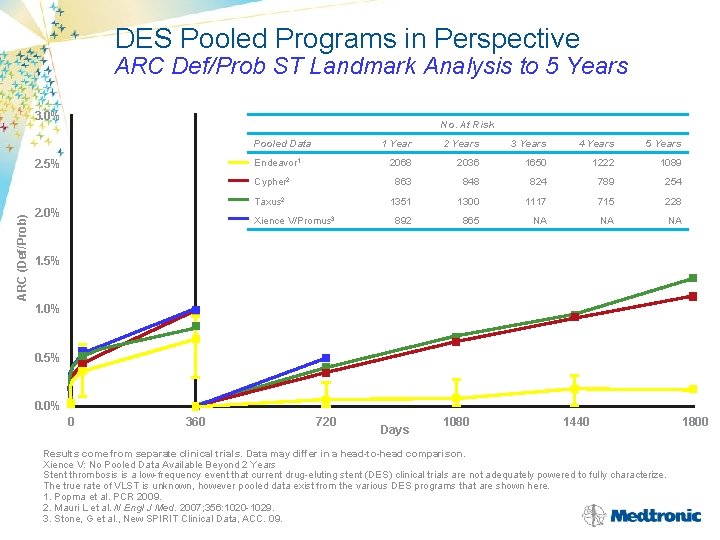
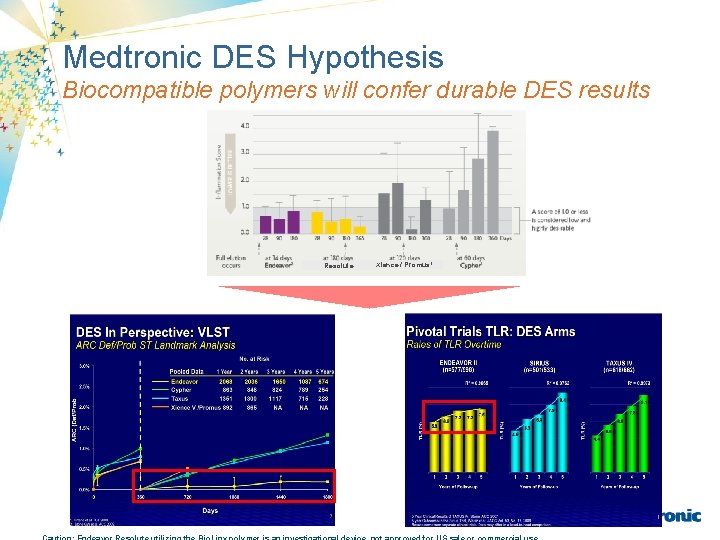
DES Pooled Programs in Perspective ARC Def/Prob ST Landmark Analysis to 5 Years 3. 0% No. At Risk Pooled Data ARC (Def/Prob) 2. 5% 1 Year 2 Years 3 Years 4 Years 5 Years 2068 2036 1650 1222 1089 Cypher 2 863 848 824 789 254 Taxus 2 1351 1300 1117 715 228 892 865 NA NA NA Endeavor 1 2. 0% Xience V/Promus 3 1. 5% 1. 0% 0. 5% 0. 0% 0 360 720 Days 1080 1440 Results come from separate clinical trials. Data may differ in a head-to-head comparison. Xience V: No Pooled Data Available Beyond 2 Years Stent thrombosis is a low-frequency event that current drug-eluting stent (DES) clinical trials are not adequately powered to fully characterize. The true rate of VLST is unknown, however pooled data exist from the various DES programs that are shown here. 1. Popma et al. PCR 2009. 2. Mauri L et al. N Engl J Med. 2007; 356: 1020 -1029. 3. Stone, G et al. , New SPIRIT Clinical Data, ACC. 09. 1800

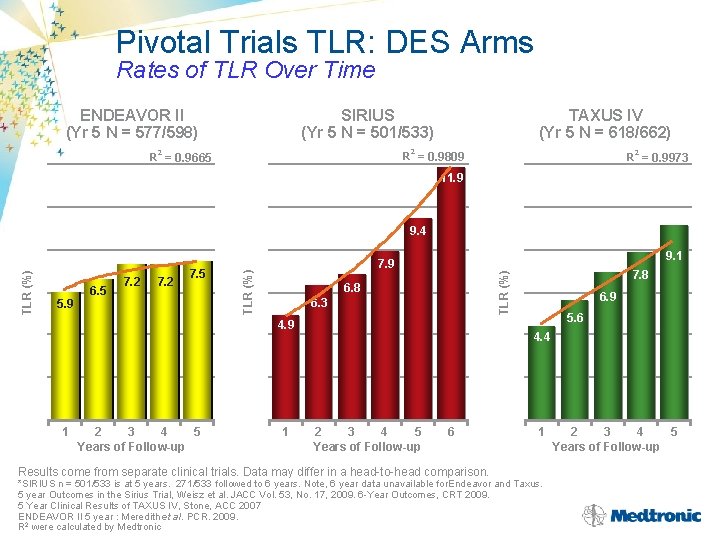
Pivotal Trials TLR: DES Arms Rates of TLR Over Time ENDEAVOR II (Yr 5 N = 577/598) SIRIUS (Yr 5 N = 501/533) TAXUS IV (Yr 5 N = 618/662) 2 2 2 R = 0. 9809 R = 0. 9665 R = 0. 9973 11. 9 7. 2 7. 5 6. 8 6. 3 4. 9 1 2 3 4 5 Years of Follow-up 1 7. 8 TLR (%) 5. 9 6. 5 7. 2 9. 1 7. 9 TLR (%) 9. 4 6. 9 5. 6 4. 4 2 3 4 5 Years of Follow-up 6 Results come from separate clinical trials. Data may differ in a head-to-head comparison. 1 *SIRIUS n = 501/533 is at 5 years. 271/533 followed to 6 years. Note, 6 year data unavailable for. Endeavor and Taxus. 5 year Outcomes in the Sirius Trial, Weisz et al. JACC Vol. 53, No. 17, 2009. 6 -Year Outcomes, CRT 2009. 5 Year Clinical Results of TAXUS IV, Stone, ACC 2007 ENDEAVOR II 5 year : Meredith et al. PCR. 2009. R 2 were calculated by Medtronic 2 3 4 5 Years of Follow-up

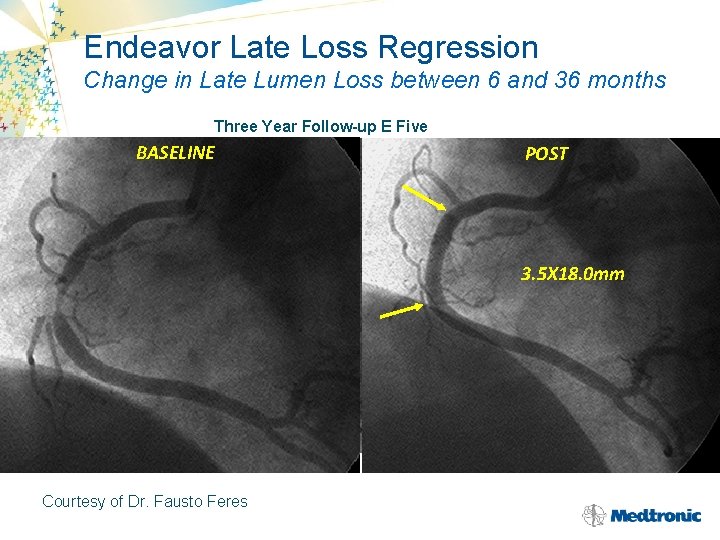
Endeavor Late Loss Regression Change in Late Lumen Loss between 6 and 36 months Three Year Follow-up E Five BASELINE 6 MONTHS Courtesy of Dr. Fausto Feres POST 3. 5 X 18. 0 mm 3 YEARS

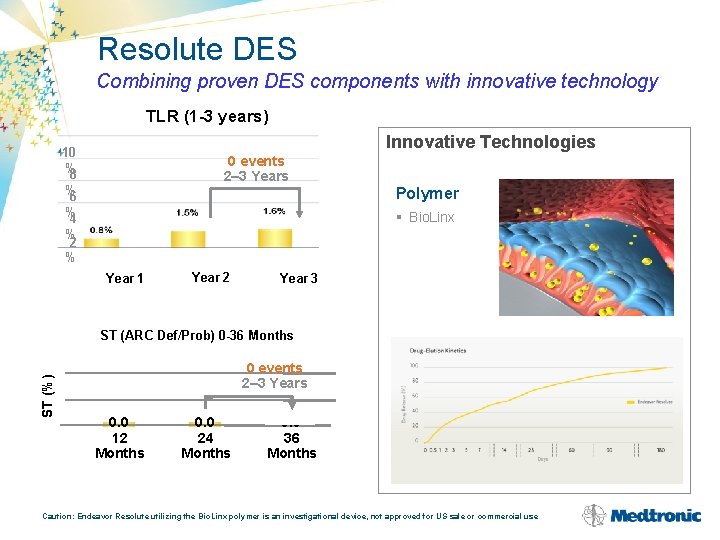
Resolute DES Combining proven DES components with innovative technology TLR (1 -3 years) Innovative Technologies 10 % 8 0 events 2– 3 Years % 6 % 4 % 2 % Polymer § Bio. Linx Year 1 Year 2 Year 3 ST (%) ST (ARC Def/Prob) 0 -36 Months 0 events 2– 3 Years 0. 0 12 Months 0. 0 24 Months 0. 0 36 Months Caution: Endeavor Resolute utilizing the Bio. Linx polymer is an investigational device, not approved for US sale or commercial use

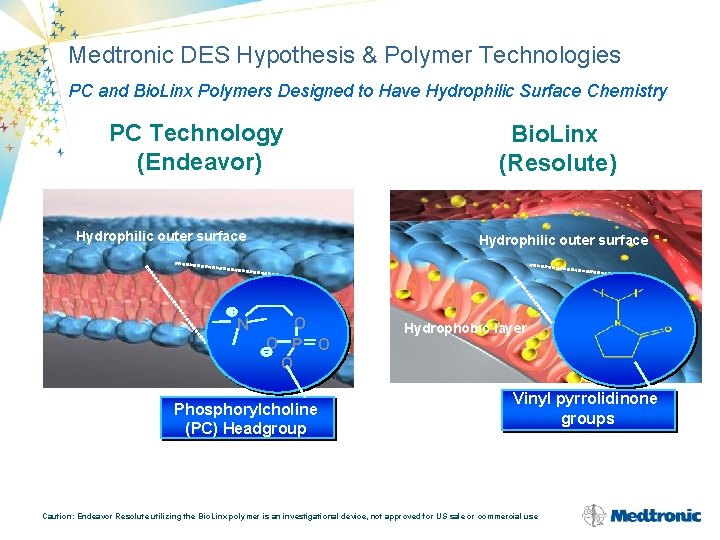
Medtronic DES Hypothesis & Polymer Technologies PC and Bio. Linx Polymers Designed to Have Hydrophilic Surface Chemistry PC Technology (Endeavor) Hydrophilic outer surface N Bio. Linx (Resolute) Hydrophilic outer surface O O Phosphorylcholine (PC) Headgroup Hydrophobic layer Vinyl pyrrolidinone groups Caution: Endeavor Resolute utilizing the Bio. Linx polymer is an investigational device, not approved for US sale or commercial use

Medtronic DES Hypothesis Biocompatible polymers will confer durable DES results Resolute Xience / Promus 1

Next Generation DES Needs 1. Better efficacy? 2. Increased deliverability? 3. Better long-term safety? 4. DES with ability to prescribe shorter DAPT 5. Confidence to interrupt DAPT?

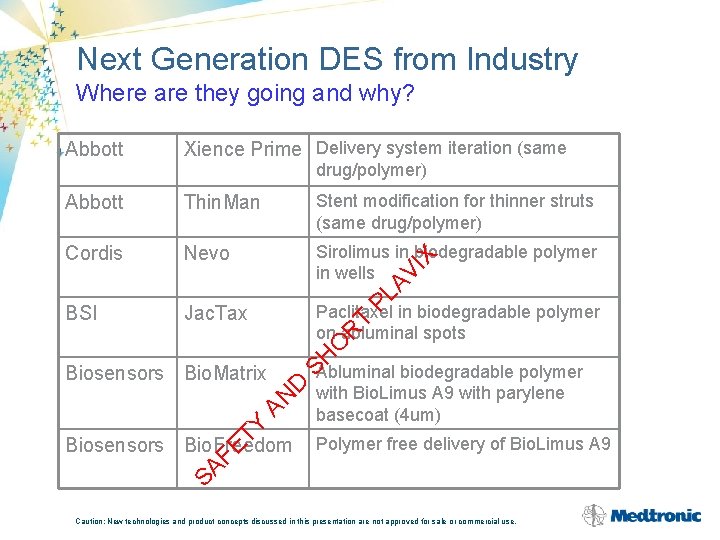
Next Generation DES from Industry Where are they going and why? Abbott Xience Prime Delivery system iteration (same Abbott Thin. Man Stent modification for thinner struts (same drug/polymer) Cordis Nevo Sirolimus in biodegradable polymer in wells BSI Jac. Tax Paclitaxel in biodegradable polymer on abluminal spots Y D AN Biosensors Bio. Matrix SH O R T PL AV I X drug/polymer) Polymer free delivery of Bio. Limus A 9 SA FE T Biosensors Bio. Freedom Abluminal biodegradable polymer with Bio. Limus A 9 with parylene basecoat (4 um) Caution: New technologies and product concepts discussed in this presentation are not approved for sale or commercial use.

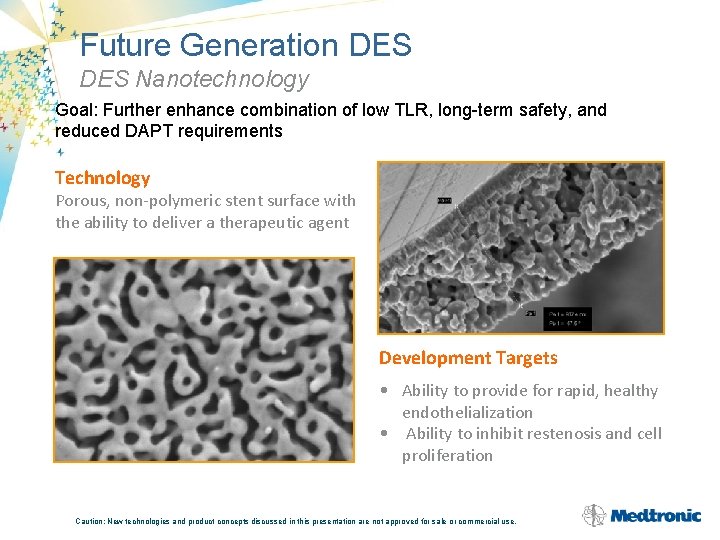
Future Generation DES Nanotechnology Goal: Further enhance combination of low TLR, long-term safety, and reduced DAPT requirements Technology Porous, non-polymeric stent surface with the ability to deliver a therapeutic agent Development Targets • Ability to provide for rapid, healthy endothelialization • Ability to inhibit restenosis and cell proliferation Caution: New technologies and product concepts discussed in this presentation are not approved for sale or commercial use.

Nanoporous Concept Drug Eluting Stent without Polymer • Polymer Coating replaced by Metal Coating • Metal Coating is porous to hold the drug • Metal Coating composition is the same as the bulk stent Expectations • Achieve a BMS surface at time of implant • Expect similar safety as BMS due to same composition • Expect control of elution due to pores • Easily scaled manufacturing that is cost effective Caution: New technologies and product concepts discussed in this presentation are not approved for sale or commercial use.

Nanopores - What do they look like? Stent Porous Metal Coating Metal Adhesion Layers Porous Metal Coating 1 μm Coating Surface Caution: New technologies and product concepts discussed in this presentation are not approved for sale or commercial use.

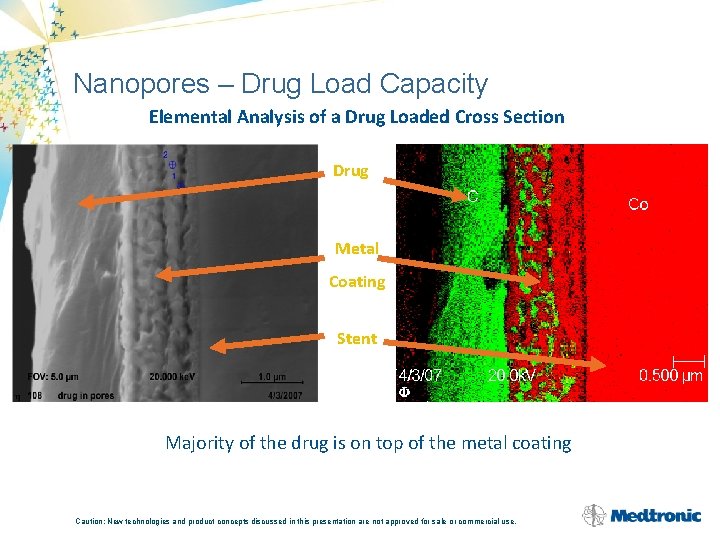
Nanopores – Drug Load Capacity Elemental Analysis of a Drug Loaded Cross Section Drug Metal Coating Stent Majority of the drug is on top of the metal coating Caution: New technologies and product concepts discussed in this presentation are not approved for sale or commercial use.

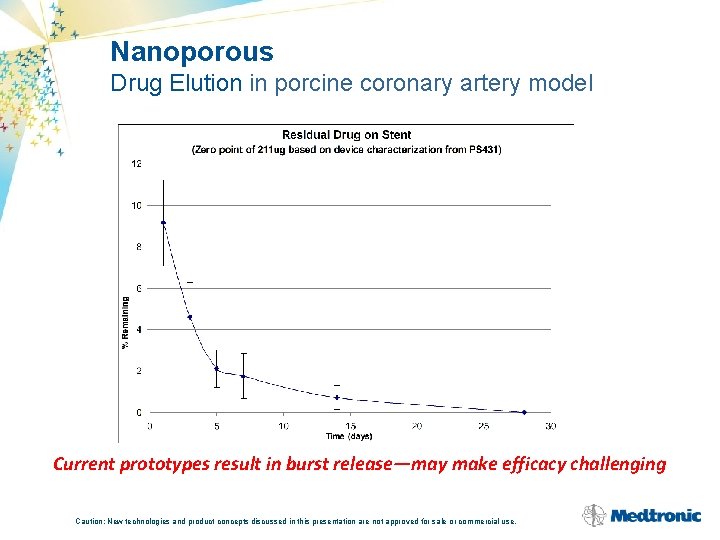
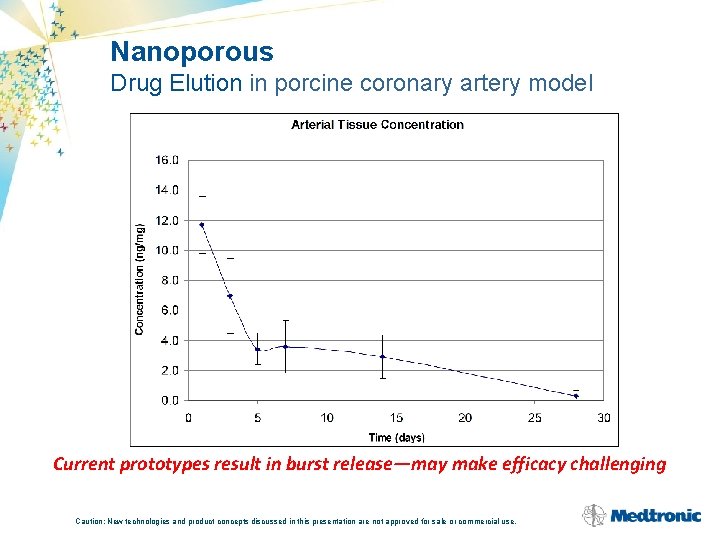
Nanoporous Drug Elution in porcine coronary artery model Current prototypes result in burst release—may make efficacy challenging Caution: New technologies and product concepts discussed in this presentation are not approved for sale or commercial use.

Nanoporous Drug Elution in porcine coronary artery model Current prototypes result in burst release—may make efficacy challenging Caution: New technologies and product concepts discussed in this presentation are not approved for sale or commercial use.

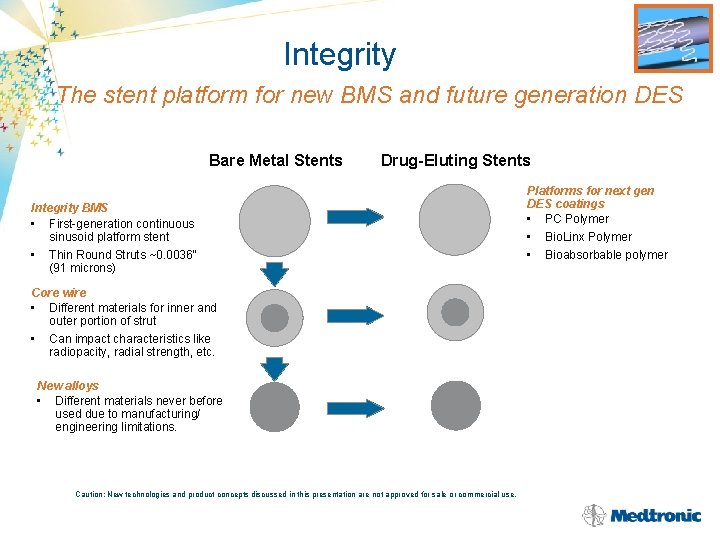
INTEGRITY™ Innovative Stent Platform Next Generation BMS and DES Clinical Rationale Develop a new standard in coronary stent deliverability and conformability for clinical needs in small, long, and tortuous vessels Development Targets • Stent platform for new BMS and future generation DES • Continuous, sinusoidal design • Thin round struts (0. 0036” = Driver) without compromising radiopacity • Enhanced vessel scaffolding in tortuous vessels without compromising flexibility • Increase range of available sizes CE Mark January Caution: New technologies and product concepts discussed in this presentation are not approved for sale or commercial use.

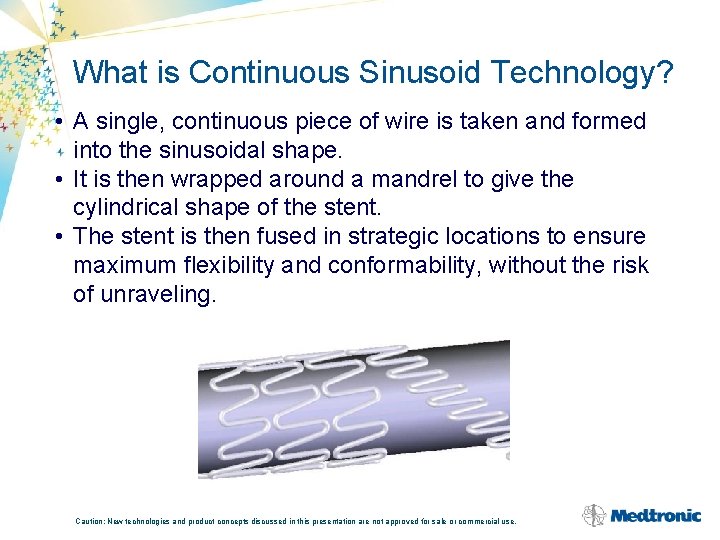
What is Continuous Sinusoid Technology? • A single, continuous piece of wire is taken and formed into the sinusoidal shape. • It is then wrapped around a mandrel to give the cylindrical shape of the stent. • The stent is then fused in strategic locations to ensure maximum flexibility and conformability, without the risk of unraveling. Caution: New technologies and product concepts discussed in this presentation are not approved for sale or commercial use.

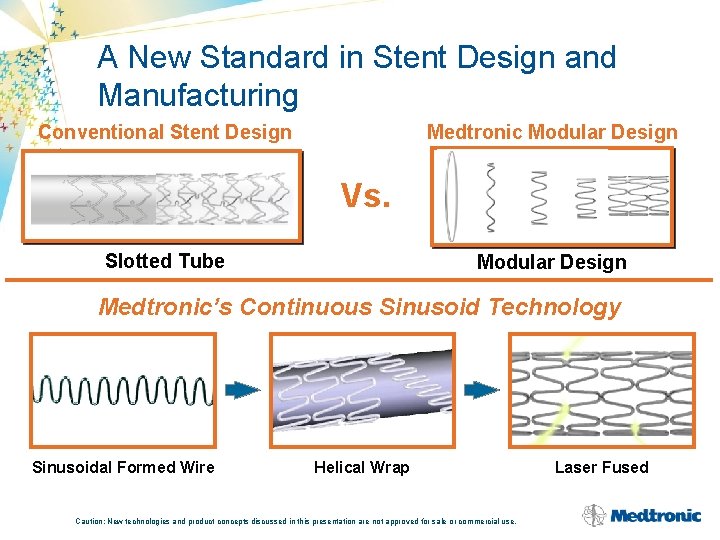
A New Standard in Stent Design and Manufacturing Conventional Stent Design Medtronic Modular Design Vs. Slotted Tube Modular Design Medtronic’s Continuous Sinusoid Technology Sinusoidal Formed Wire Helical Wrap Caution: New technologies and product concepts discussed in this presentation are not approved for sale or commercial use. Laser Fused

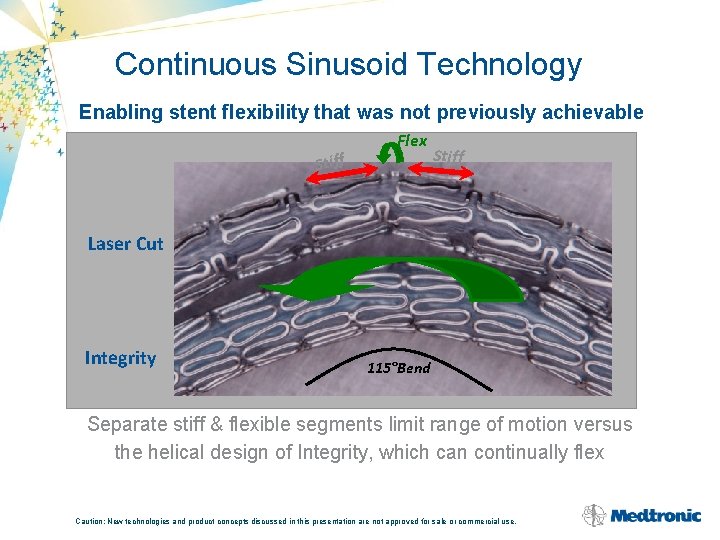
Continuous Sinusoid Technology Enabling stent flexibility that was not previously achievable Stiff Flex Stiff Laser Cut Integrity 115°Bend Separate stiff & flexible segments limit range of motion versus the helical design of Integrity, which can continually flex Caution: New technologies and product concepts discussed in this presentation are not approved for sale or commercial use.

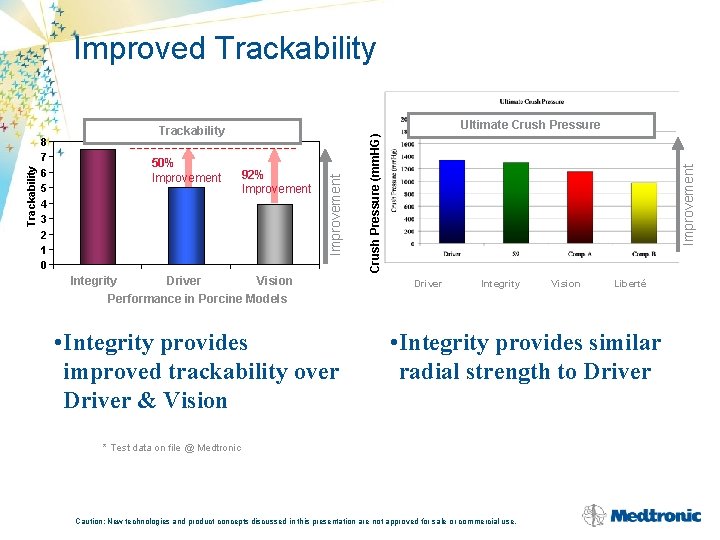
92% Improvement Integrity Driver Vision S 9 Driver Vision Performance in Porcine Models • Integrity provides improved trackability over Driver & Vision Improvement 50% Improvement Crush Pressure (mm. HG) 8 7 6 5 4 3 2 1 0 Ultimate Crush Pressure Trackability Improvement Trackability Improved Trackability Driver Integrity Vision Liberté • Integrity provides similar radial strength to Driver * Test data on file @ Medtronic Caution: New technologies and product concepts discussed in this presentation are not approved for sale or commercial use.

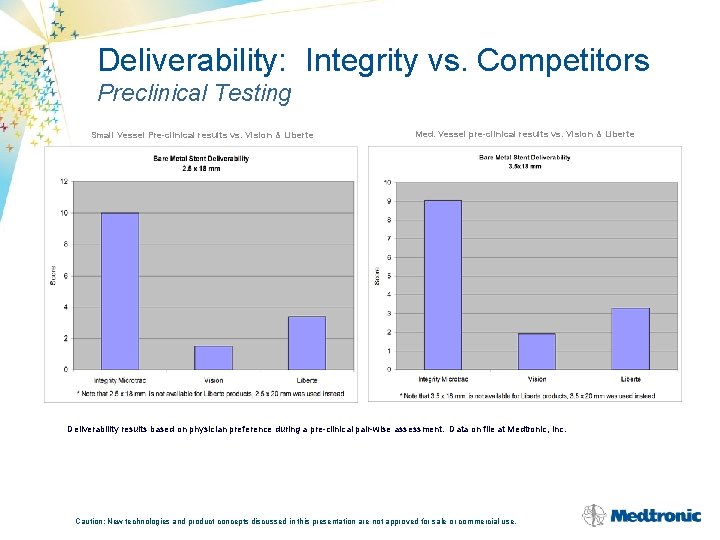
Deliverability: Integrity vs. Competitors Preclinical Testing Small Vessel Pre-clinical results vs. Vision & Liberte Med. Vessel pre-clinical results vs. Vision & Liberte Deliverability results based on physician preference during a pre-clinical pair-wise assessment. Data on file at Medtronic, Inc. Caution: New technologies and product concepts discussed in this presentation are not approved for sale or commercial use.

Integrity The stent platform for new BMS and future generation DES Bare Metal Stents Drug-Eluting Stents Integrity BMS • First-generation continuous sinusoid platform stent • Thin Round Struts ~0. 0036“ (91 microns) Core wire • Different materials for inner and outer portion of strut • Can impact characteristics like radiopacity, radial strength, etc. New alloys • Different materials never before used due to manufacturing/ engineering limitations. Caution: New technologies and product concepts discussed in this presentation are not approved for sale or commercial use. Platforms for next gen DES coatings • PC Polymer • Bio. Linx Polymer • Bioabsorbable polymer

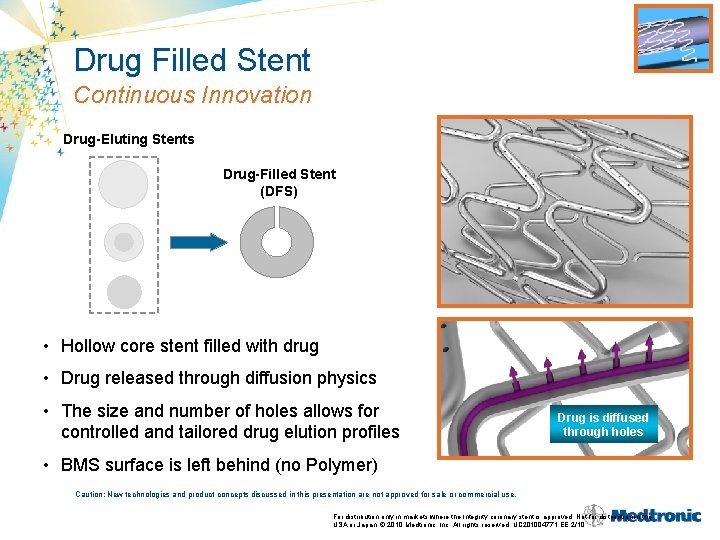
Drug Filled Stent Continuous Innovation Drug-Eluting Stents Drug-Filled Stent (DFS) • Hollow core stent filled with drug • Drug released through diffusion physics • The size and number of holes allows for controlled and tailored drug elution profiles Drug is diffused through holes • BMS surface is left behind (no Polymer) Caution: New technologies and product concepts discussed in this presentation are not approved for sale or commercial use. For distribution only in markets where the Integrity coronary stent is approved. Not for distribution in the USA or Japan. © 2010 Medtronic, Inc. All rights reserved. UC 201004771 EE 2/10

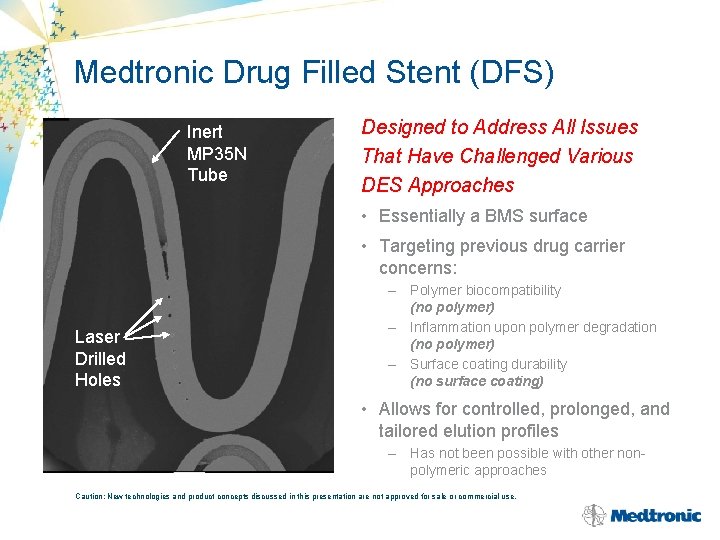
Medtronic Drug Filled Stent (DFS) Inert MP 35 N Tube Designed to Address All Issues That Have Challenged Various DES Approaches • Essentially a BMS surface • Targeting previous drug carrier concerns: Laser Drilled Holes – Polymer biocompatibility (no polymer) – Inflammation upon polymer degradation (no polymer) – Surface coating durability (no surface coating) • Allows for controlled, prolonged, and tailored elution profiles – Has not been possible with other nonpolymeric approaches Caution: New technologies and product concepts discussed in this presentation are not approved for sale or commercial use.

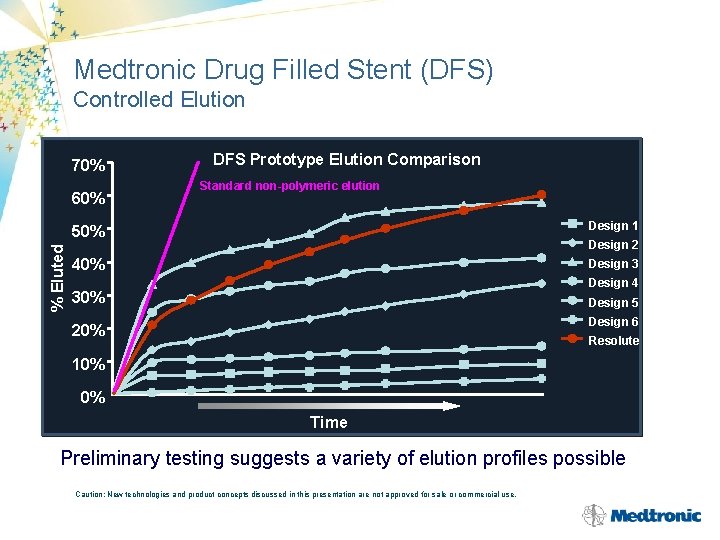
Medtronic Drug Filled Stent (DFS) Controlled Elution 70% % Eluted 60% DFS Prototype Elution Comparison Standard non-polymeric elution 50% Design 1 40% Design 3 Design 2 Design 4 30% Design 5 Design 6 20% Resolute 10% 0% Time Preliminary testing suggests a variety of elution profiles possible Caution: New technologies and product concepts discussed in this presentation are not approved for sale or commercial use.

Development Progress Major advancements in key milestones Initial Prototypes Stent Forming Drilling Filling January 2010 Prototypes Eluting Testing Caution: New technologies and product concepts discussed in this presentation are not approved for sale or commercial use.

THANK YOU!
 Des des des
Des des des Markskill llc
Markskill llc Plural component spray
Plural component spray Hospital wall coatings
Hospital wall coatings Hts coatings
Hts coatings Pinnacle coatings group
Pinnacle coatings group St powder coatings
St powder coatings Kale group
Kale group St powder coatings
St powder coatings Sherwin williams chemical coatings store
Sherwin williams chemical coatings store Medtronic supplier change portal
Medtronic supplier change portal Mpxr
Mpxr Vector express medtronic
Vector express medtronic Mpxr medtronic
Mpxr medtronic Correction factor insulin
Correction factor insulin Medtronic sofamor danek usa
Medtronic sofamor danek usa Branded icon
Branded icon Laura mauri
Laura mauri Medtronic structural heart
Medtronic structural heart Sextant medtronic
Sextant medtronic Medtronic sofamor danek usa
Medtronic sofamor danek usa Medtronic
Medtronic Nerven beckenbereich
Nerven beckenbereich Reveal linq app
Reveal linq app Medtronic organizational chart
Medtronic organizational chart John wainwright medtronic
John wainwright medtronic Rick kuntz
Rick kuntz Sean salmon medtronic salary
Sean salmon medtronic salary Facectomy
Facectomy Medtronic symplicity
Medtronic symplicity