Medtronic NonPolymeric DES Development Update on the Drug

















- Slides: 26

Medtronic Non-Polymeric DES Development: Update on the Drug Filled Stent Josiah N. Wilcox, Ph. D. Vice President and Resident Scholar Science and Technology Medtronic Cardio. Vascular

Disclosures • I am a full time employee of Medtronic Cardio. Vascular • I will be talking about products and product concepts that are currently not approved for use in the USA – Resolute, DFS, S 10/Core. Wire

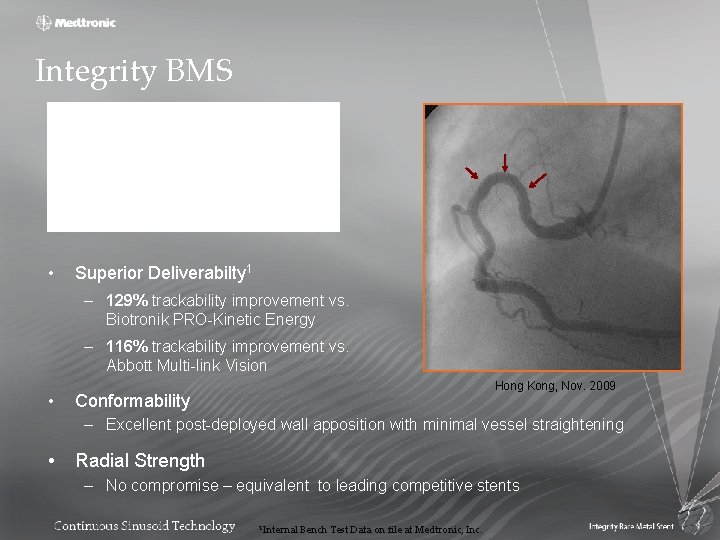
Integrity BMS • Superior Deliverabilty 1 – 129% trackability improvement vs. Biotronik PRO-Kinetic Energy – 116% trackability improvement vs. Abbott Multi-link Vision • Hong Kong, Nov. 2009 Conformability – Excellent post-deployed wall apposition with minimal vessel straightening • Radial Strength – No compromise – equivalent to leading competitive stents 1 Internal Bench Test Data on file at Medtronic, Inc.

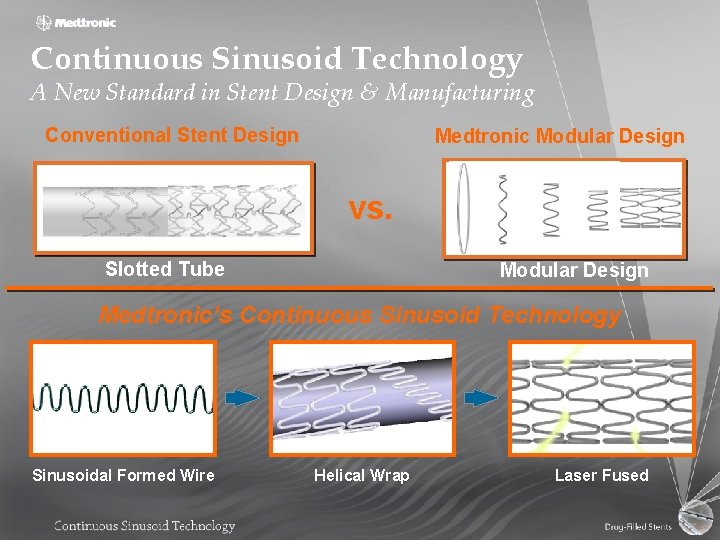
Continuous Sinusoid Technology A New Standard in Stent Design & Manufacturing Conventional Stent Design Medtronic Modular Design vs. Slotted Tube Modular Design Medtronic’s Continuous Sinusoid Technology Sinusoidal Formed Wire Helical Wrap Laser Fused


Continuous Sinusoid Technology Continuous sinusoid technology allow greater stent flexibility Flex Stiff Separate stiff and flexible segments limit range of motion Continuous sinusoid technology will flex continually

Continuous Sinusoid Technology The Next Revolution in Stent Technology Enabling designs that could not be attempted in the past Bare Metal Stents Integrity Drug-Eluting Stents Drug-Filled Stent Resolute Integrity Core Wire New Alloys Platforms for next gen DES coatings • • Bio. Linx (Resolute) PC Technology (Endeavor) Bioabsorbable polymer Nanoporous Product concepts not currently approved for us in the USA

Continuous Sinusoidal Technology Core Wire and New Alloy Stents Program Targets • Maintain / extend Medtronic lead in stent technology • Enhance performance w/o compromising strength & opacity • Create a backbone for DES coating technologies 0. 0038" Cobalt Chromium 0. 0034" Core Wire 0. 0030" 0. 0025" 0. 0020" Product concepts not currently approved for us in the USA New alloys

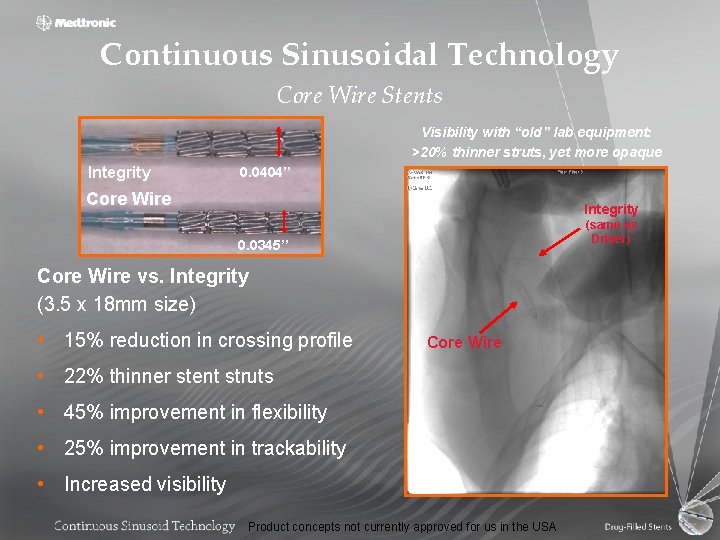
Continuous Sinusoidal Technology Core Wire Stents Visibility with “old” lab equipment: >20% thinner struts, yet more opaque Integrity 0. 0404’’ Core Wire Integrity (same as Driver) 0. 0345’’ Core Wire vs. Integrity (3. 5 x 18 mm size) • 15% reduction in crossing profile Core Wire • 22% thinner stent struts • 45% improvement in flexibility • 25% improvement in trackability • Increased visibility Product concepts not currently approved for us in the USA

Drug Filled Stent (DFS) Technology Polymer Free Drug Delivery Innovative DES design • Essentially a BMS surface • No drug carrier issues such as § Polymer biocompatibility § Inflammation upon polymer degradation § Surface coating durability • Allows for controlled, prolonged, and tailored elution profiles § Has not been achievable with other nonpolymeric approaches Product concepts not currently approved for us in the USA

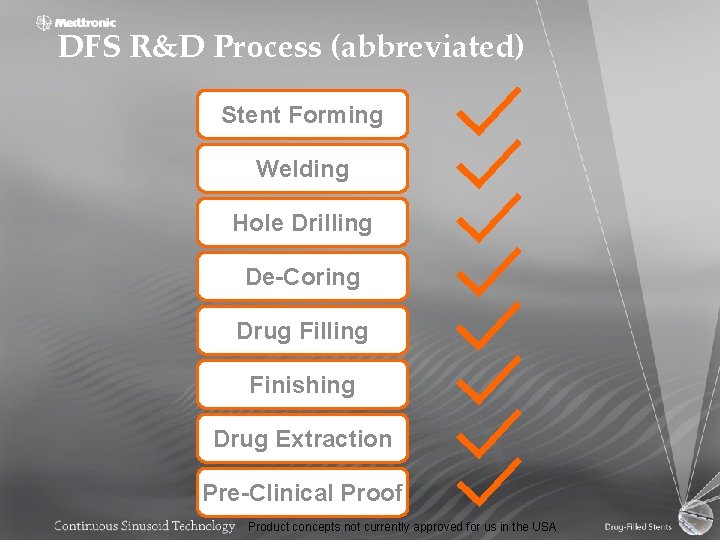
DFS R&D Process (abbreviated) Stent Forming Welding Hole Drilling De-Coring Drug Filling Finishing Drug Extraction Pre-Clinical Proof Product concepts not currently approved for us in the USA

Drug Filled Stent (DFS) Technology Core Material Core Wire Construction Co Alloy Shell Product concepts not currently approved for us in the USA

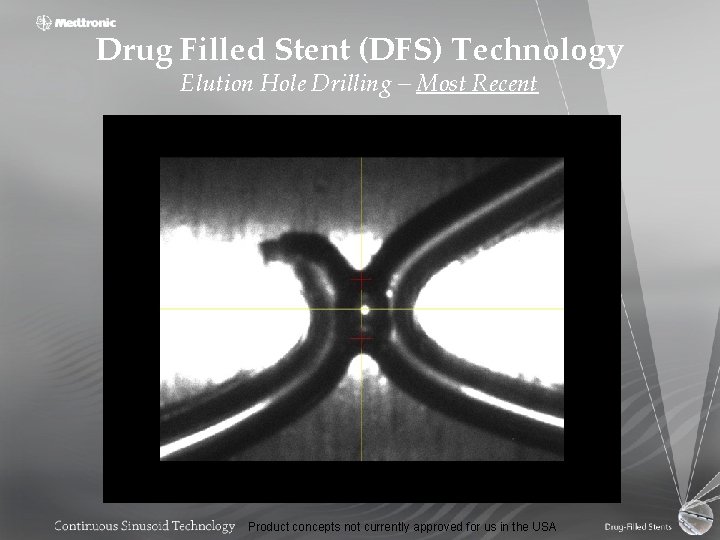
Drug Filled Stent (DFS) Technology Elution Hole Drilling Product concepts not currently approved for us in the USA

Laser Hole Drilling • • Facilitate drug filling and elution 500 – 5000 holes per stent (18 mm) Variable hole diameter and shape Automation – Throughput – Reliability Product concepts not currently approved for us in the USA

Drug Filled Stent (DFS) Technology Elution Hole Drilling – Most Recent Product concepts not currently approved for us in the USA

Drug Filled Stent (DFS) Technology Elution Hole Drilling – The Future Product concepts not currently approved for us in the USA

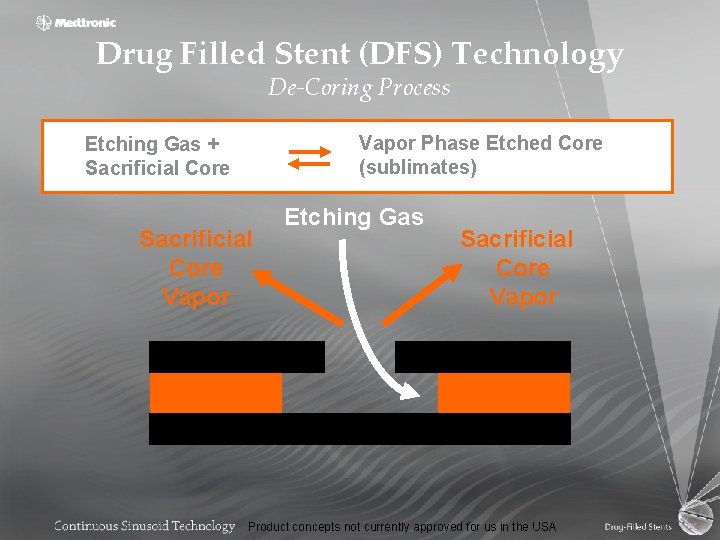
Drug Filled Stent (DFS) Technology De-Coring Process Vapor Phase Etched Core (sublimates) Etching Gas + Sacrificial Core Vapor Etching Gas Sacrificial Core Vapor Product concepts not currently approved for us in the USA

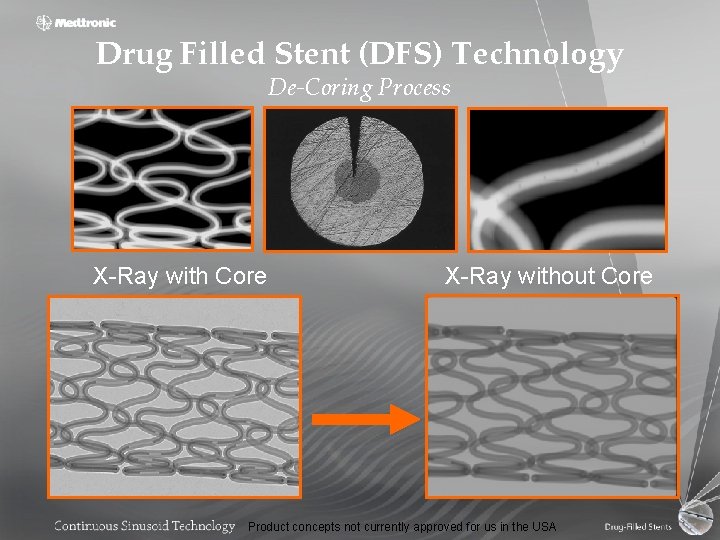
Drug Filled Stent (DFS) Technology De-Coring Process X-Ray with Core X-Ray without Core Product concepts not currently approved for us in the USA

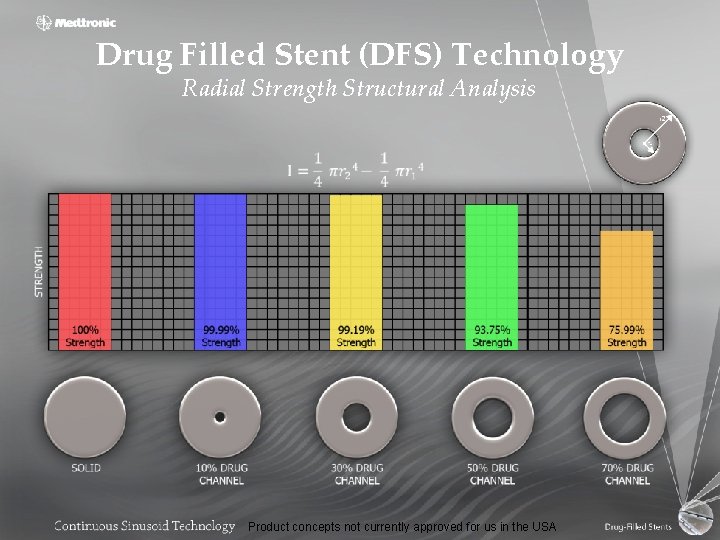
Drug Filled Stent (DFS) Technology Radial Strength Structural Analysis Product concepts not currently approved for us in the USA

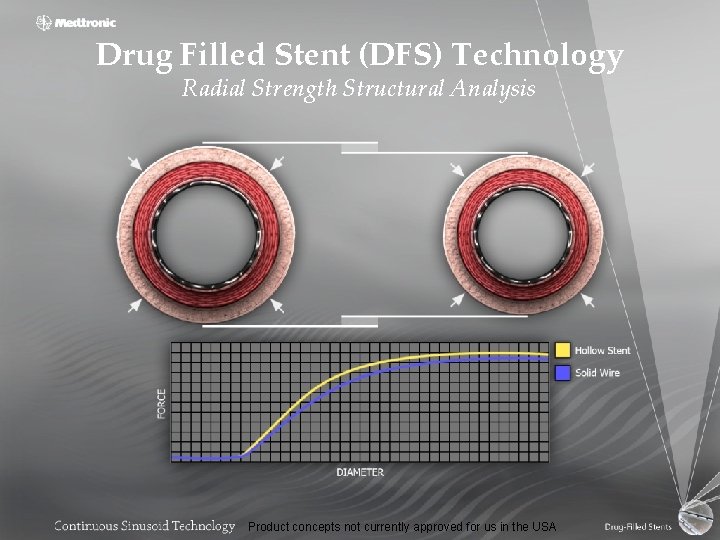
Drug Filled Stent (DFS) Technology Radial Strength Structural Analysis Product concepts not currently approved for us in the USA

Drug Filled Stent (DFS) Technology Possible Elution Mechanism Product concepts not currently approved for us in the USA

Drug Filled Stent (DFS) Technology Controllable Elution 60% 50% DFS Prototype Elution Comparison % Eluted 70% Standard non-polymeric elution Design 1 Design 2 40% Design 3 Design 4 30% Design 5 20% Design 6 Resolute 10% 0% Time Testing suggests a variety of elution profiles possible Product concepts not currently approved for us in the USA

Drug Filled Stent (DFS) Technology In Vitro Elution into Agar Gel Product concepts not currently approved for us in the USA

Design Comparisons Elution Product concepts not currently approved for us in the USA

Percent Drug In Tissue – DFS vs Resolute Product concepts not currently approved for us in the USA

THANK YOU!
 Immediate update and deferred update in dbms
Immediate update and deferred update in dbms Des des des
Des des des Example of crude drug adulterated with exhausted drug
Example of crude drug adulterated with exhausted drug Medtronic purchasing portal
Medtronic purchasing portal Quality begins with me
Quality begins with me Vector express medtronic
Vector express medtronic Mpxr medtronic
Mpxr medtronic Medtronic minimed 530g
Medtronic minimed 530g Medtronic sofamor danek usa
Medtronic sofamor danek usa Medtronic brand central
Medtronic brand central Laura mauri medtronic
Laura mauri medtronic Medtronic structural heart
Medtronic structural heart Sextant medtronic
Sextant medtronic Medtronic pyramid
Medtronic pyramid Medtronic
Medtronic Sakrale neuromodulation medtronic
Sakrale neuromodulation medtronic Medtronic loop recorder lnq11
Medtronic loop recorder lnq11 Medtronic organizational chart
Medtronic organizational chart John wainwright medtronic
John wainwright medtronic Rick kuntz
Rick kuntz Sean salmon medtronic salary
Sean salmon medtronic salary Sextant medtronic
Sextant medtronic Medtronic symplicity
Medtronic symplicity Mpxr medtronic
Mpxr medtronic Medtronic harmony
Medtronic harmony Richard kuntz
Richard kuntz Medtronic dbm
Medtronic dbm