Drug discovery and development Drug discovery and development




- Slides: 8

Drug discovery and development

Drug discovery and development The discovery phase The development phase Pre-discovery Gather as much information as possible about the disease and try to understand its nature. Pre-clinical testing In vitro and in vivo testing to determine if the drug is safe enough for human testing. Target identification Choose a molecule in the body to target with a drug; often a protein. Clinical trial exceptions (CTX) applications File CTX with appropriate authorities before clinical testing can begin. Target validation Test the target and confirm its role in the disease. Phase 1 clinical trial Initial human testing in a small group of healthy volunteers. Drug discovery Find a promising molecule (a ‘lead compound’) that could become a drug. Phase 2 clinical trial Test in a small group of patients. Phase 3 clinical trial Test in a large group of patients to show safety and efficacy. Early safety tests Initial tests on lead compounds, including pharmacokinetics, by experiment and/or computer modelling. Marketing authorisation application Apply to appropriate authorities for approval. Lead optimisation Alter the structure of lead candidates to improve properties; this may include formulation, delivery mechanism and scale-up. On-going studies and Phase 4 trials Continuing monitoring and checking of the drug in use. Manufacturing Begin full-scale production.

Drug discovery and development Drug targets Drug design A target is a molecule that the drug needs to find act upon. Often it is a protein molecule such as an enzyme. Modern technologies allow chemists to work out the molecular structure of a target molecule and represent it using physical or computer-generated models. This enables them to investigate the interaction of potential drug molecules with the target. Increasingly, the starting point for the design of new drug is an understanding at the molecular level of the disease to be treated.

Drug discovery and development Looking for a lead compound Lead optimisation and scale up With the target identified, thousands of compounds are made using a technique called combinatorial chemistry. These are narrowed down to one compound to be studied further. This is the lead compound. The lead compound chemically modified to produce a number of structurally similar compounds. The is done by parallel synthesis. From these the most likely one is chosen for pre-clinical testing. This is followed by scaling up, through pilot scale to manufacture.

Drug discovery and development Clinical trials A case study in drug discovery and development Any new medicinal drug must undergo a series of rigorous clinical trials to show its effectiveness and safety. … the discovery of zanamivir In 2011, the Ekjut Trial in Jharkhand Orissa was awarded Trial of the Year by the Society for Clinical Trials. The NHS has produced a video in which Dr Ben Goldacre explains why clinical trials are important, what they involve and who can take part in one. He also describes common concerns patients might have and gives tips on what questions to ask before taking part in any research. http: //www. nhs. uk/Conditions/Clinicaltrials/Pages/Introduction. aspx? url=Pages%2 FWhat-is -it. aspx Zanamivir is a treatment for influenza caused by influenza A virus and influenza B virus. It was discovered in 1989 and is marketed by GSK under the trade name Relenza.

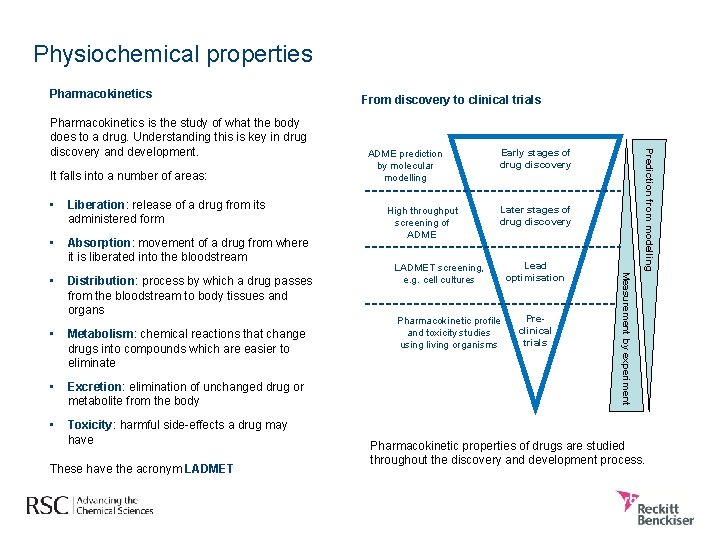
Physiochemical properties Pharmacokinetics It falls into a number of areas: • Liberation: release of a drug from its administered form • Absorption: movement of a drug from where it is liberated into the bloodstream Distribution: process by which a drug passes from the bloodstream to body tissues and organs • Metabolism: chemical reactions that change drugs into compounds which are easier to eliminate • Excretion: elimination of unchanged drug or metabolite from the body • Toxicity: harmful side-effects a drug may have These have the acronym LADMET High throughput screening of ADME Early stages of drug discovery Later stages of drug discovery LADMET screening, e. g. cell cultures Pharmacokinetic profile and toxicity studies using living organisms Lead optimisation Preclinical trials Measurement by experiment • ADME prediction by molecular modelling Prediction from modelling Pharmacokinetics is the study of what the body does to a drug. Understanding this is key in drug discovery and development. From discovery to clinical trials Pharmacokinetic properties of drugs are studied throughout the discovery and development process.

Physiochemical properties LADMET and drug discovery and development Measurement of the physiochemical properties of drugs has always been important, but high throughput and fast ADME profiling has become increasingly important as scientists seek ways of shortening the drug discovery and development process. The following extract is taken from an article by Dr Jianling Wang and Dr Laszlo Urban in Drug Discovery World (2004). The increased costs in the discovery and development of new drugs, due in part to the high attrition rate of drug candidates in development, has led to a new strategy to introduce early, parallel evaluation of efficacy and biopharmaceutical properties of drug candidates. Investigation of terminated projects revealed that the primary cause for drug failure in the development phase was the poor pharmacokinetic and ADMET (Absorption, Distribution, Metabolism, Discretion and Toxicity) properties rather than unsatisfactory efficacy. In addition, the applications of parallel synthesis and combinatory chemistry to expedite lead finding and lead optimisation processes has shifted the chemical libraries towards poorer biopharmaceutical properties. Establishments of high throughput and fast ADMET profiling assays allow for the prioritisation of leads or drug candidates by their biopharmaceutical properties in parallel with optimisation of their efficacy at early discovery phases. This is expected to not only improve the overall quality of drug candidates and therefore the probability of their success, but also shorten the drug discovery and development process.

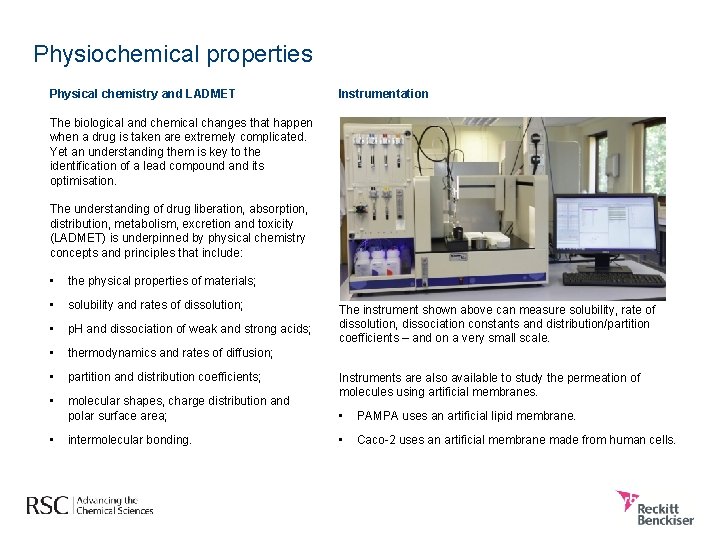
Physiochemical properties Physical chemistry and LADMET Instrumentation The biological and chemical changes that happen when a drug is taken are extremely complicated. Yet an understanding them is key to the identification of a lead compound and its optimisation. The understanding of drug liberation, absorption, distribution, metabolism, excretion and toxicity (LADMET) is underpinned by physical chemistry concepts and principles that include: • the physical properties of materials; • solubility and rates of dissolution; • p. H and dissociation of weak and strong acids; • thermodynamics and rates of diffusion; • partition and distribution coefficients; • molecular shapes, charge distribution and polar surface area; • intermolecular bonding. The instrument shown above can measure solubility, rate of dissolution, dissociation constants and distribution/partition coefficients – and on a very small scale. Instruments are also available to study the permeation of molecules using artificial membranes. • PAMPA uses an artificial lipid membrane. • Caco-2 uses an artificial membrane made from human cells.