Clinical Outcomes of the Attain Performa Lead and
















- Slides: 63

Clinical Outcomes of the Attain® Performa™ Lead ® and Adaptiv. CRT Algorithm Viva™ Quad CRT-D and Attain Performa LV Lead System George H. Crossley, MD, FHRS, FACC Vanderbilt

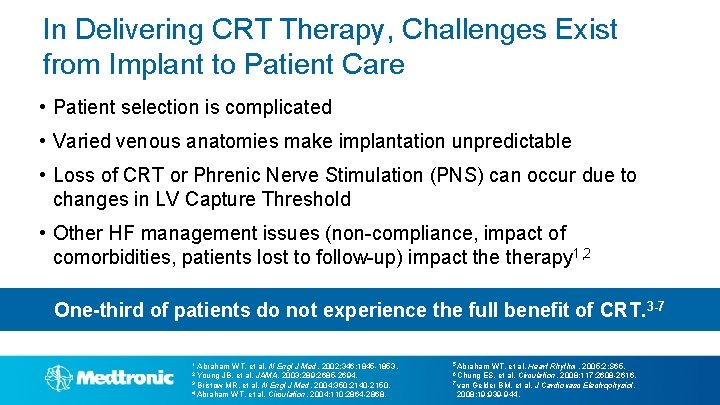
In Delivering CRT Therapy, Challenges Exist from Implant to Patient Care • Patient selection is complicated • Varied venous anatomies make implantation unpredictable • Loss of CRT or Phrenic Nerve Stimulation (PNS) can occur due to changes in LV Capture Threshold • Other HF management issues (non-compliance, impact of comorbidities, patients lost to follow-up) impact therapy 1, 2 One-third of patients do not experience the full benefit of CRT. 3 -7 1 Abraham WT, et al. N Engl J Med. 2002; 346: 1845 -1853. 5 Abraham WT, et al. Heart Rhythm. 2005; 2: S 65. 2 Young JB, et al. JAMA. 2003; 289: 2685 -2694. 6 Chung ES, et al. Circulation. 2008; 117: 2608 -2616. 3 Bristow MR, et al. N 7 van Gelder BM, et al. J Engl J Med. 2004; 350: 2140 -2150. 4 Abraham WT, et al. Circulation. 2004; 110: 2864 -2868. 2008; 19: 939 -944. Cardiovasc Electrophysiol.

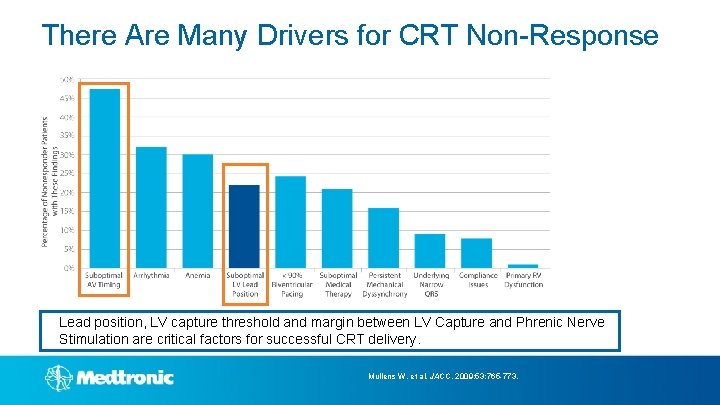
There Are Many Drivers for CRT Non-Response Lead position, LV capture threshold and margin between LV Capture and Phrenic Nerve Stimulation are critical factors for successful CRT delivery. Mullens W, et al. JACC. 2009; 53: 765 -773.

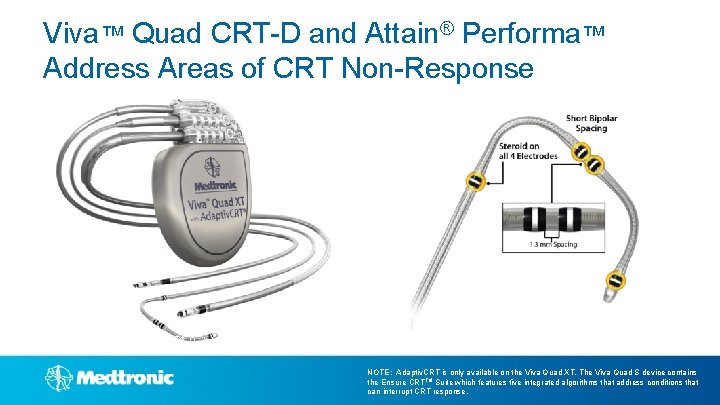
Viva™ Quad CRT-D and Attain® Performa™ Address Areas of CRT Non-Response LV 3 LV 2 LV 4 LV 1 With Adaptiv. CRT®* & Vector. Express™ 5. 3 Fr, double cant shape NOTE: Adaptiv. CRT is only available on the Viva Quad XT. The Viva Quad S device contains the Ensure CRTTM Suite which features five integrated algorithms that address conditions that can interrupt CRT response.

Implant and Follow-Up Benefits of Viva™ Quad CRT-D and Attain® Performa™ System • Advanced Quadripolar Lead Design provides more viable options for delivering CRT • Vector. Express® eliminates timeconsuming manual testing 1 • Adaptiv. CRT® improves CRT Response 2 1 Demmer W. Vector. Express Performance Results. Medtronic Data on File. January 2013. 2 Birnie D, et al. Heart Rhythm. 2013; 9: 1368 -1374.

Viva™ Quad CRT-D and Attain® Performa™ System Advanced Quadripolar Lead Vector. Express® Adaptiv. CRT®

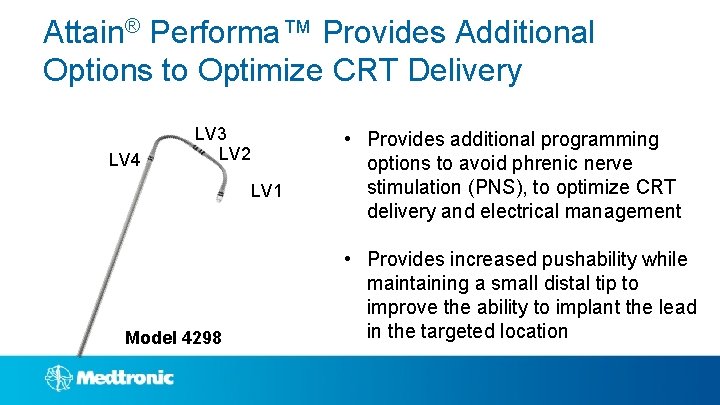
Attain® Performa™ Provides Additional Options to Optimize CRT Delivery LV 4 LV 3 LV 2 LV 1 Model 4298 • Provides additional programming options to avoid phrenic nerve stimulation (PNS), to optimize CRT delivery and electrical management • Provides increased pushability while maintaining a small distal tip to improve the ability to implant the lead in the targeted location

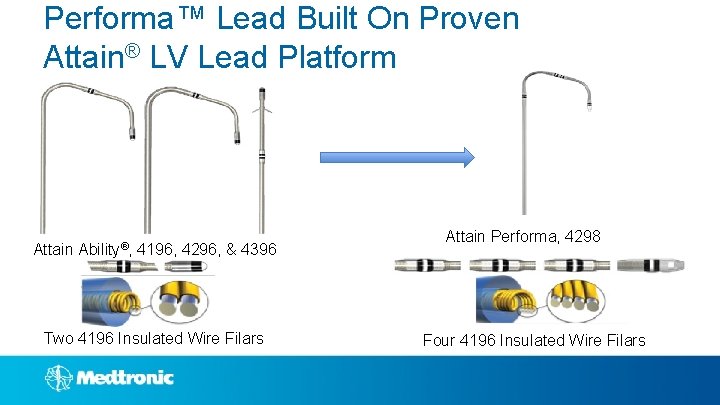
Performa™ Lead Built On Proven Attain® LV Lead Platform Attain Ability®, 4196, 4296, & 4396 Two 4196 Insulated Wire Filars Attain Performa, 4298 Four 4196 Insulated Wire Filars

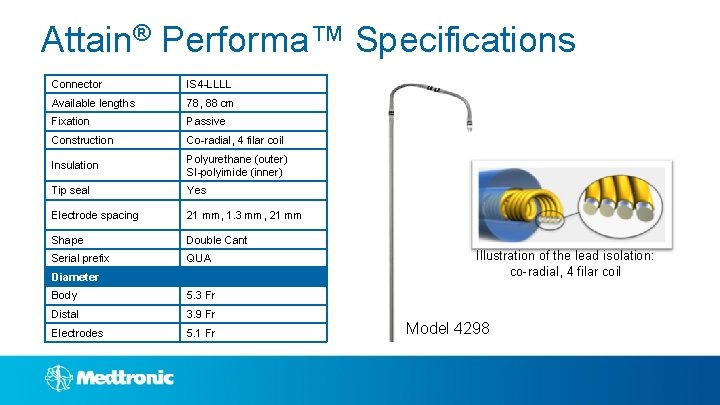
Attain® Performa™ Specifications Connector IS 4 -LLLL Available lengths 78, 88 cm Fixation Passive Construction Co-radial, 4 filar coil Insulation Polyurethane (outer) SI-polyimide (inner) Tip seal Yes Electrode spacing 21 mm, 1. 3 mm, 21 mm Shape Double Cant Serial prefix QUA Diameter Body 5. 3 Fr Distal 3. 9 Fr Electrodes 5. 1 Fr Illustration of the lead isolation: co-radial, 4 filar coil Model 4298


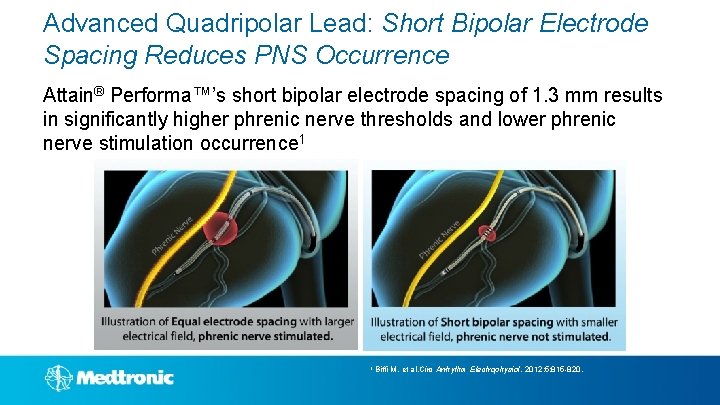
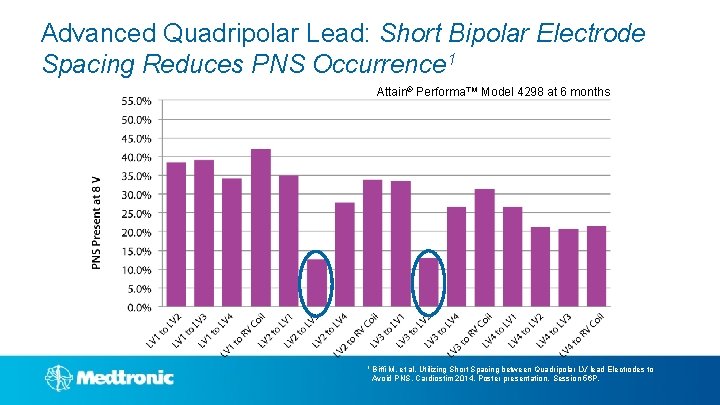
Advanced Quadripolar Lead: Short Bipolar Electrode Spacing Reduces PNS Occurrence Attain® Performa™’s short bipolar electrode spacing of 1. 3 mm results in significantly higher phrenic nerve thresholds and lower phrenic nerve stimulation occurrence 1 1 Biffi M, et al. Circ Arrhythm Electrophysiol. 2012; 5: 815 -820.

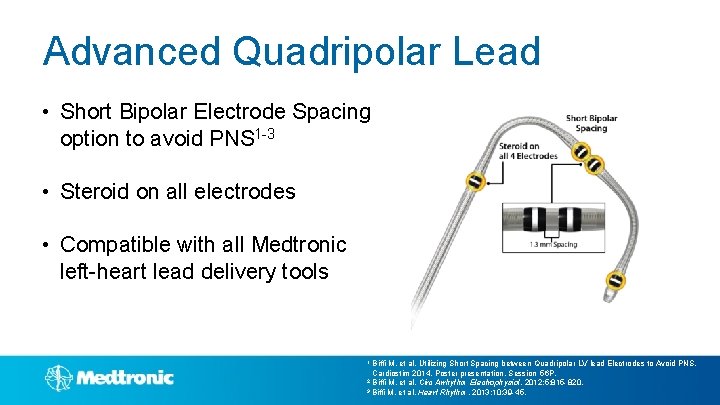
Advanced Quadripolar Lead • Short Bipolar Electrode Spacing option to avoid PNS 1 -3 • Steroid on all electrodes • Compatible with all Medtronic left-heart lead delivery tools 1 Biffi M, et al, Utilizing Short Spacing between Quadripolar LV lead Electrodes to Avoid PNS. Cardiostim 2014, Poster presentation, Session 56 P. 2 Biffi M, et al. Circ Arrhythm Electrophysiol. 2012; 5: 815 -820. 3 Biffi M, et al. Heart Rhythm. 2013; 10: 39 -45.

Managing PNS at Implant • When PNS is detected at implant, several approaches are used: – Move the lead to another site – Program the LVP cathode other than the lead tip in bipolar/multipolar leads featuring cathode/anode programmability 1 -8 – Lower the LV output so as to avoid PNS when other options have failed 1 -6, 9 • Abandoning the target site may cause failure to deliver effective CRT and accompanying clinical improvements 10 -13 1 Biffi M, et al. Circ Arrhythm Electrophysiol. 2009; 2: 402 -410. 8 Klein N, et al. Europace. 2012; 14: 826 -832. 2 Seifert M, et al. Europace. 2010; 12: 961 -967. 9 Biffi M, et al. Pacing 3 Gurevitz O, et al. Pacing 10 Dekker AL, et al. J Clin Electrophysiol. 2005; 28: 1255 -1259. 4 Champagne J, et al. Europace. 2011; 13: 409 -415. 5 Biffi M, et al. Pacing Clin Electrophysiol. 2011; 34: 1201 -1208. 6 Jastrzebski M, et al. Europace. 2011; 13: 520 -525. 7 Sperzel J, et al. Europace. 2012; 14: 365 -372. Clin Electrophysiol. 2009; 32: 346 -353. Thorac Cardiovasc Surg. 2004; 127: 1641 -1647. 11 Ypenburg C, et al. J Am Coll Cardiol. 2008; 52: 1402 -1409. 12 Ypenburg C, et al. J Am Coll Cardiol. 2009; 53: 483 -490. 13 Khan FZ, et al. J Am Coll Cardiol. 2012; 59: 1509 -1518.

Managing PNS at Long-Term Follow-Up Reprogramming the pacing vector or changing the pacing output are the two non-surgical means to manage PNS at pre-discharge or during longterm follow-up.

Advanced Quadripolar Lead: Short Bipolar Electrode Spacing Reduces PNS Occurrence 1 Attain® Performa™ Model 4298 at 6 months 1 Biffi M, et al, Utilizing Short Spacing between Quadripolar LV lead Electrodes to Avoid PNS. Cardiostim 2014, Poster presentation, Session 56 P.

Summary: Short Bipolar Spacing • Attain Performa’s short bipolar spacing provides more options to help avoid PNS: – Reduces PNS occurrence at implant and follow-up – Reduces the need for lead repositioning – Reduces PNS-related complications Attain® Performa™ offers two bipolar vectors with dedicated short bipolar spacing resulting in significantly higher phrenic nerve thresholds and lower PNS occurrence 1 1 Biffi M, et al. Circ Arrhythm Electrophysiol. 2012; 5: 815 -820.

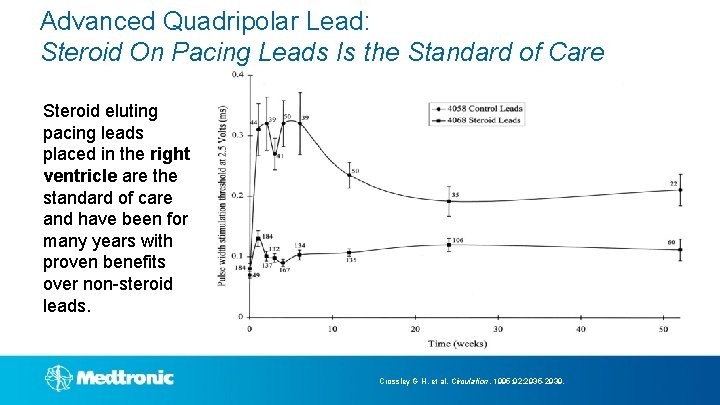
Advanced Quadripolar Lead: Steroid On Pacing Leads Is the Standard of Care Steroid eluting pacing leads placed in the right ventricle are the standard of care and have been for many years with proven benefits over non-steroid leads. Crossley G H, et al. Circulation. 1995; 92: 2935 -2939.

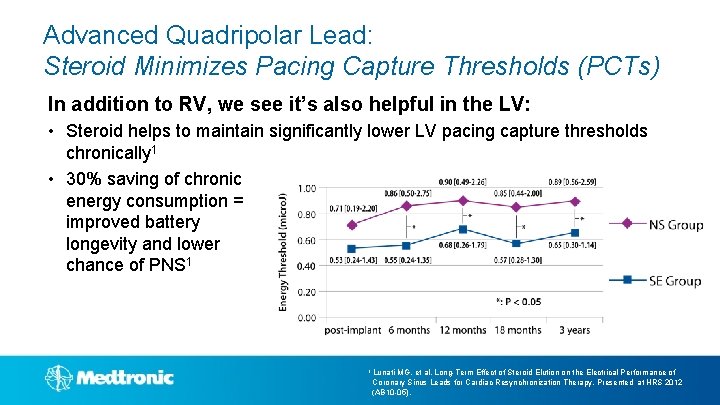
Advanced Quadripolar Lead: Steroid Minimizes Pacing Capture Thresholds (PCTs) In addition to RV, we see it’s also helpful in the LV: • Steroid helps to maintain significantly lower LV pacing capture thresholds chronically 1 • 30% saving of chronic energy consumption = improved battery longevity and lower chance of PNS 1 1 Lunati MG, et al. Long-Term Effect of Steroid Elution on the Electrical Performance of Coronary Sinus Leads for Cardiac Resynchronization Therapy. Presented at HRS 2012 (AB 10 -05).

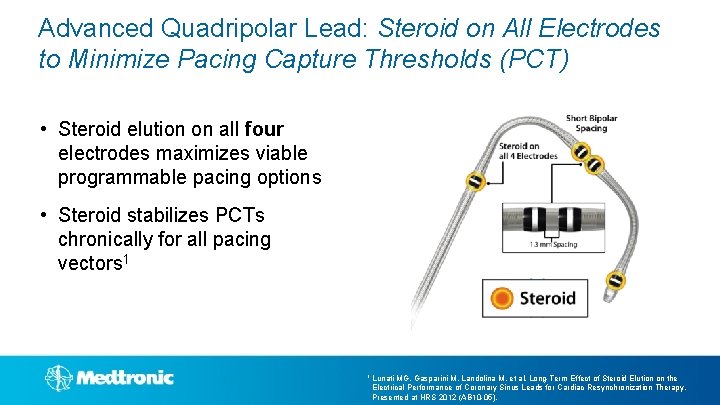
Advanced Quadripolar Lead: Steroid on All Electrodes to Minimize Pacing Capture Thresholds (PCT) • Steroid elution on all four electrodes maximizes viable programmable pacing options • Steroid stabilizes PCTs chronically for all pacing vectors 1 1 Lunati MG, Gasparini M, Landolina M, et al. Long-Term Effect of Steroid Elution on the Electrical Performance of Coronary Sinus Leads for Cardiac Resynchronization Therapy. Presented at HRS 2012 (AB 10 -05).

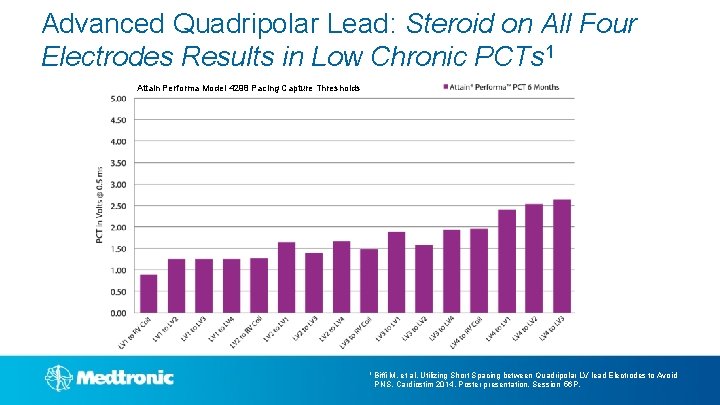
Advanced Quadripolar Lead: Steroid on All Four Electrodes Results in Low Chronic PCTs 1 Attain Performa Model 4298 Pacing Capture Thresholds 1 Biffi M, et al, Utilizing Short Spacing between Quadripolar LV lead Electrodes to Avoid PNS. Cardiostim 2014, Poster presentation, Session 56 P.

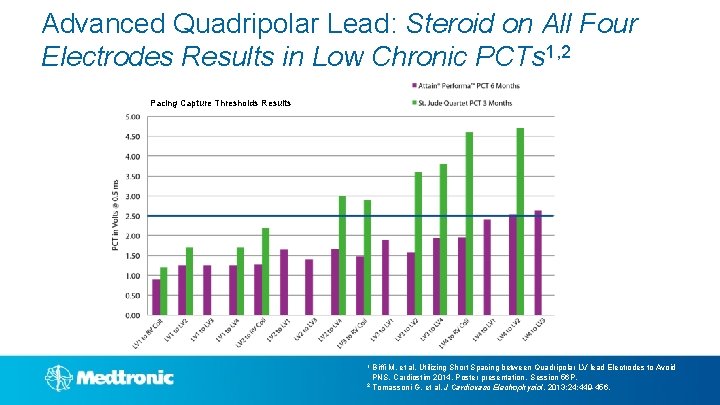
Advanced Quadripolar Lead: Steroid on All Four Electrodes Results in Low Chronic PCTs 1, 2 Pacing Capture Thresholds Results 1 Biffi M, et al, Utilizing Short Spacing between Quadripolar LV lead Electrodes to Avoid PNS. Cardiostim 2014, Poster presentation, Session 56 P. 2 Tomassoni G, et al. J Cardiovasc Electrophysiol. 2013; 24: 449 -456.

Advanced Quadripolar Lead: Performa™ Canted Implant Considerations (4298) • Moderate vessel curvature • Medium vessel diameter

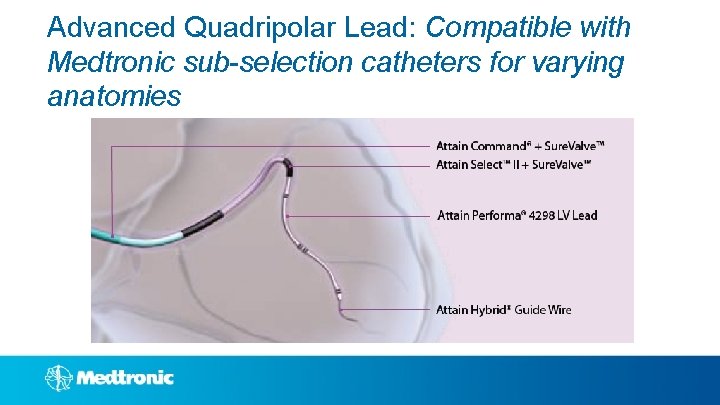
Advanced Quadripolar Lead: Compatible with Medtronic sub-selection catheters for varying anatomies

Attain® Performa™ Clinical Study Highlights 1 • Largest Medtronic LV Lead Clinical Study in History • For Model 4298: • Met Primary Safety End Point – 499 subjects enrolled – 487 successful implants (97. 6%) – United States, Canada, Europe, Australia, Asia, Middle East, Africa, India, and Latin America • Fastest Enrolling Lead Study at Medtronic – 9 months – Freedom from LV lead-related complications • Met Primary Efficacy End Points – Permanently programmed polarity pacing threshold ≤ 2. 5 V – Non-programmed polarity pacing threshold ≤ 4. 0 V • Longest Medtronic LV Lead Study – 6 months for primary end point 1 Crossley, GH, et. al. A Novel Quadripolar Lead with a Narrow-Spaced Bipole Allows for Effective LV Pacing While Avoiding Phrenic Nerve Stimulation - Attain Performa LV Lead Study Primary Results. Heart Rhythm. in press.

Viva™ Quad CRT-D and Attain® Performa™ System Advanced Quadripolar Lead Vector. Express® Adaptiv. CRT®

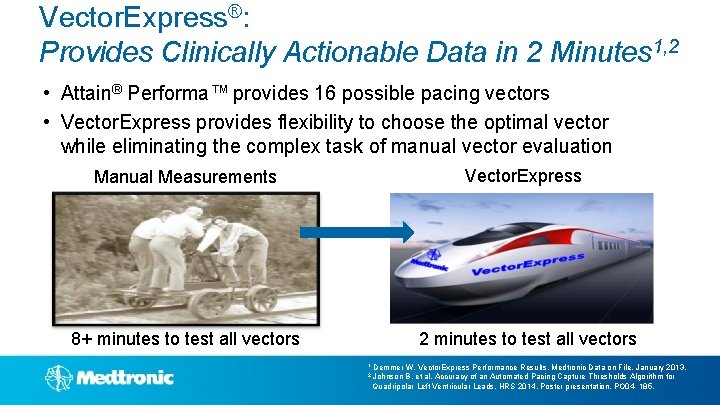
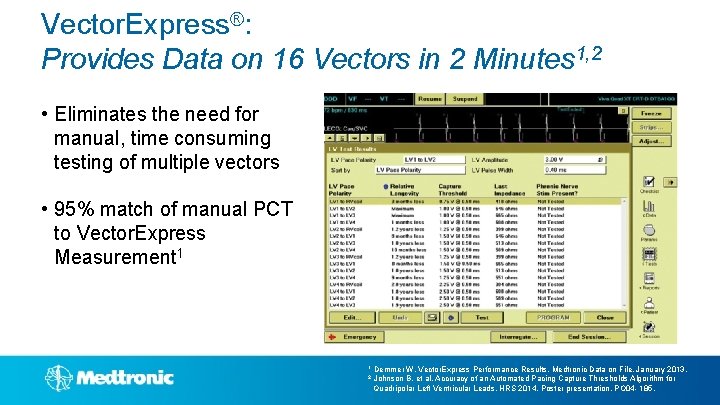
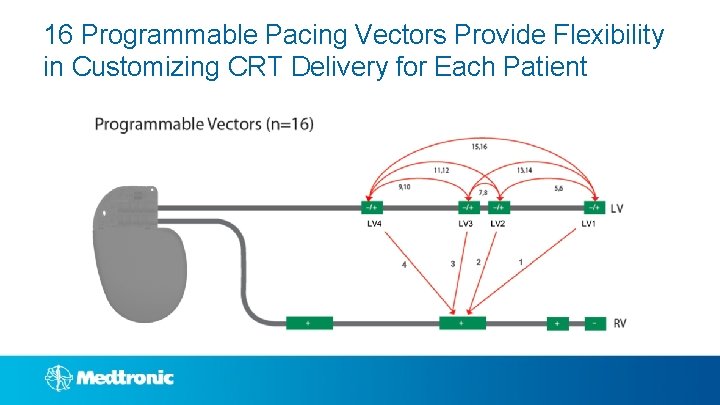
Vector. Express®: Provides Clinically Actionable Data in 2 Minutes 1, 2 • Attain® Performa™ provides 16 possible pacing vectors • Vector. Express provides flexibility to choose the optimal vector while eliminating the complex task of manual vector evaluation Manual Measurements Vector. Express 8+ minutes to test all vectors 2 minutes to test all vectors 1 Demmer W. Vector. Express Performance Results. Medtronic Data on File. January 2013. 2 Johnson B, et al, Accuracy of an Automated Pacing Capture Thresholds Algorithm for Quadripolar Left Ventricular Leads. HRS 2014, Poster presentation, PO 04 - 185.

Vector. Express®: Provides Data on 16 Vectors in 2 Minutes 1, 2 • Eliminates the need for manual, time consuming testing of multiple vectors • 95% match of manual PCT to Vector. Express Measurement 1 1 Demmer W. Vector. Express Performance Results. Medtronic Data on File. January 2013. 2 Johnson B, et al, Accuracy of an Automated Pacing Capture Thresholds Algorithm for Quadripolar Left Ventricular Leads. HRS 2014, Poster presentation, PO 04 - 185.

16 Programmable Pacing Vectors Provide Flexibility in Customizing CRT Delivery for Each Patient

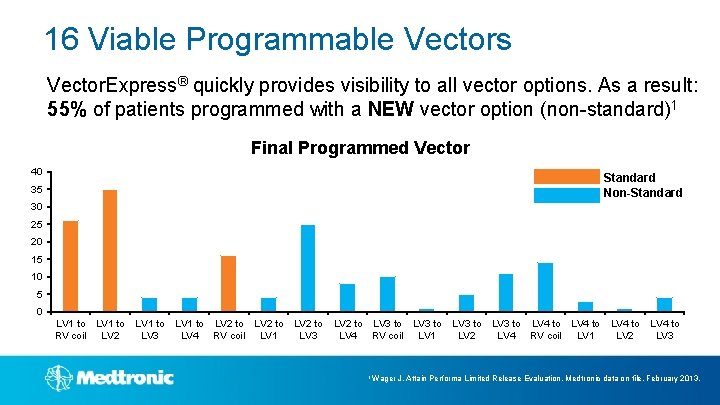
16 Viable Programmable Vectors Vector. Express® quickly provides visibility to all vector options. As a result: 55% of patients programmed with a NEW vector option (non-standard)1 Final Programmed Vector 40 35 Standard Non-Standard 30 25 20 15 10 5 0 LV 1 to LV 2 to LV 3 to LV 4 to RV coil LV 2 LV 3 LV 4 RV coil LV 1 LV 2 LV 3 1 Wager J. Attain Performa Limited Release Evaluation. Medtronic data on file. February 2013.

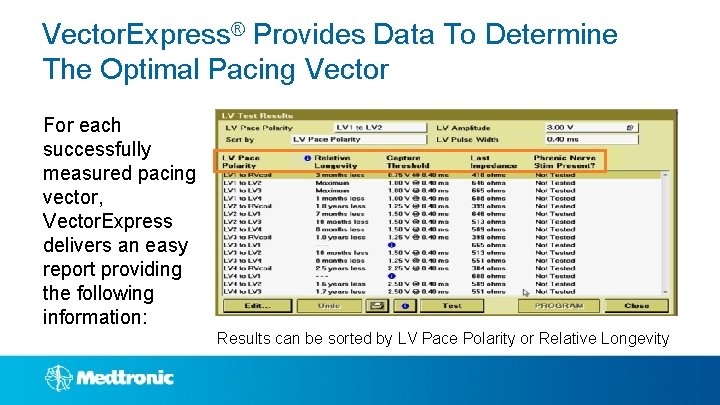
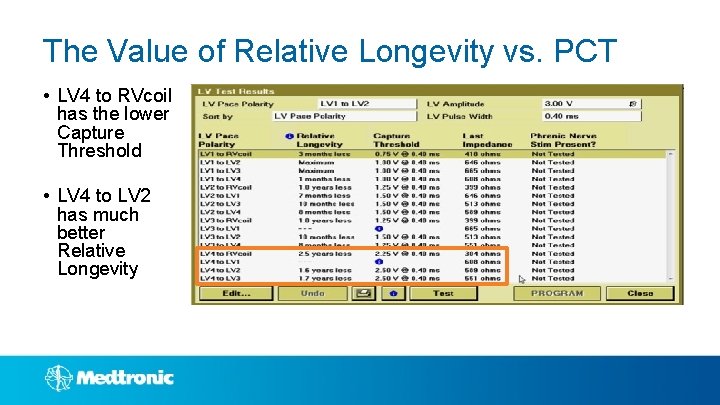
Vector. Express® Provides Data To Determine The Optimal Pacing Vector For each successfully measured pacing vector, Vector. Express delivers an easy report providing the following information: Results can be sorted by LV Pace Polarity or Relative Longevity

The Value of Relative Longevity vs. PCT • LV 4 to RVcoil has the lower Capture Threshold • LV 4 to LV 2 has much better Relative Longevity

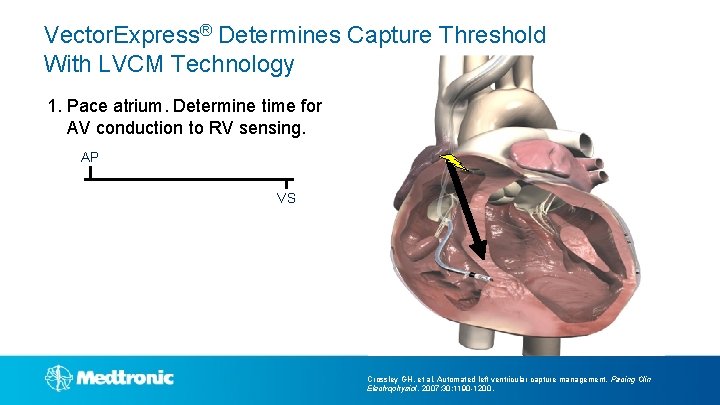
Vector. Express® Determines Capture Threshold With LVCM Technology 1. Pace atrium. Determine time for AV conduction to RV sensing. AP VS Crossley GH, et al. Automated left ventricular capture management. Pacing Clin Electrophysiol. 2007; 30: 1190 -1200.

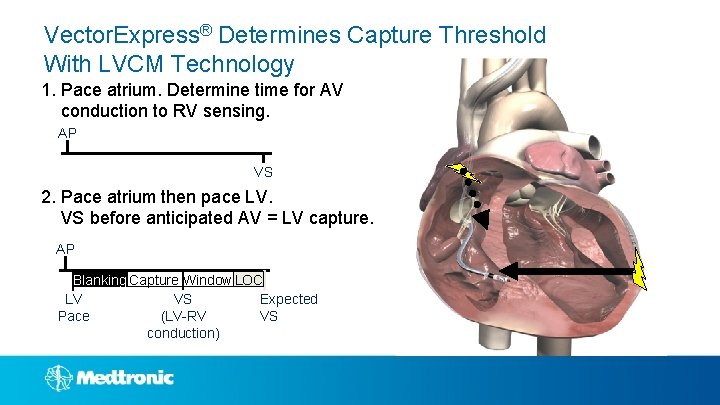
Vector. Express® Determines Capture Threshold With LVCM Technology 1. Pace atrium. Determine time for AV conduction to RV sensing. AP VS 2. Pace atrium then pace LV. VS before anticipated AV = LV capture. AP Blanking Capture Window LOC LV Expected VS Pace VS (LV-RV conduction)

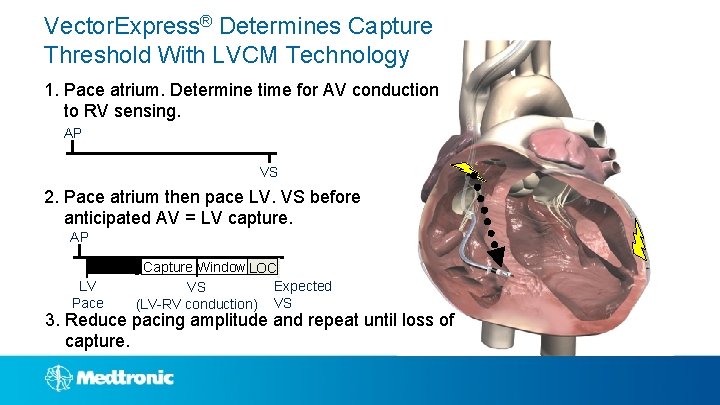
Vector. Express® Determines Capture Threshold With LVCM Technology 1. Pace atrium. Determine time for AV conduction to RV sensing. AP VS 2. Pace atrium then pace LV. VS before anticipated AV = LV capture. AP Blanking Capture Window LOC LV Expected VS Pace (LV-RV conduction) VS 3. Reduce pacing amplitude and repeat until loss of capture.

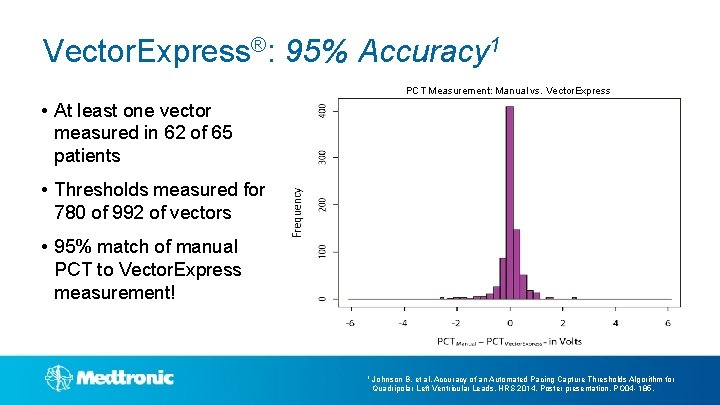
Vector. Express®: 95% Accuracy 1 PCT Measurement: Manual vs. Vector. Express • At least one vector measured in 62 of 65 patients • Thresholds measured for 780 of 992 of vectors • 95% match of manual PCT to Vector. Express measurement! 1 Johnson B, et al, Accuracy of an Automated Pacing Capture Thresholds Algorithm for Quadripolar Left Ventricular Leads. HRS 2014, Poster presentation, PO 04 - 185.

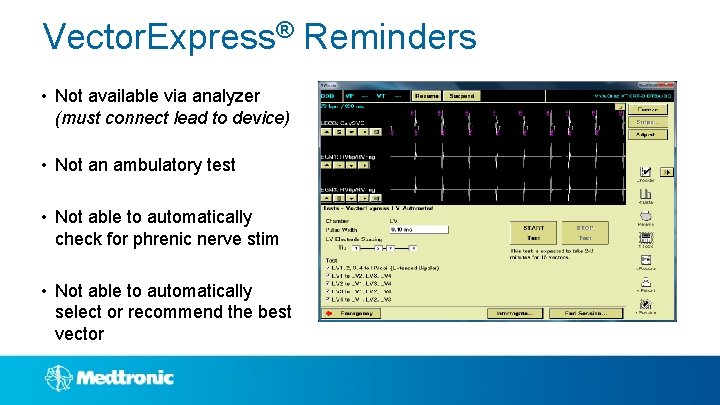
Vector. Express® Reminders • Not available via analyzer (must connect lead to device) • Not an ambulatory test • Not able to automatically check for phrenic nerve stim • Not able to automatically select or recommend the best vector

Viva™ Quad CRT-D and Attain® Performa™ System Advanced Quadripolar Lead Vector. Express® Adaptiv. CRT®

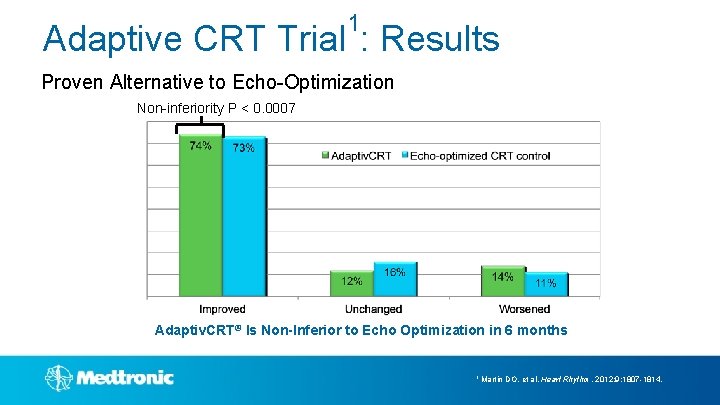
1 Adaptive CRT Trial : Results Proven Alternative to Echo-Optimization Non-inferiority P < 0. 0007 Adaptiv. CRT® Is Non-Inferior to Echo Optimization in 6 months 1 Martin DO, et al. Heart Rhythm. 2012; 9: 1807 -1814.

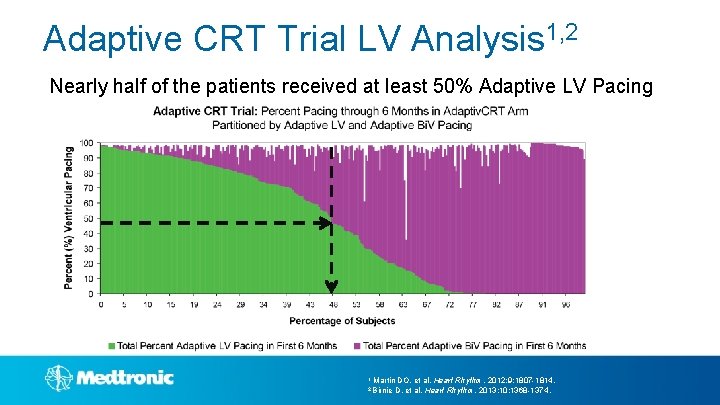
Adaptive CRT Trial LV Analysis 1, 2 Nearly half of the patients received at least 50% Adaptive LV Pacing 1 Martin DO, et al. Heart 2 Birnie D, et al. Heart Rhythm. 2012; 9: 1807 -1814. Rhythm. 2013; 10: 1368 -1374.

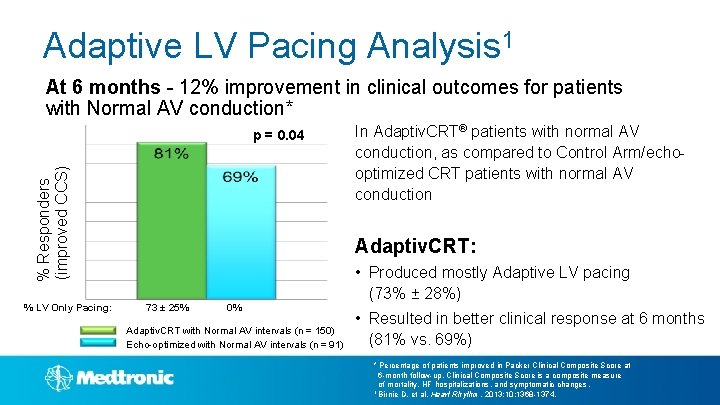
Adaptive LV Pacing Analysis 1 At 6 months - 12% improvement in clinical outcomes for patients with Normal AV conduction* % Responders (improved CCS) p = 0. 04 % LV Only Pacing: In Adaptiv. CRT® patients with normal AV conduction, as compared to Control Arm/echooptimized CRT patients with normal AV conduction Adaptiv. CRT: 73 ± 25% 0% Adaptiv. CRT with Normal AV intervals (n = 150) Echo-optimized with Normal AV intervals (n = 91) • Produced mostly Adaptive LV pacing (73% ± 28%) • Resulted in better clinical response at 6 months (81% vs. 69%) * Percentage of patients improved in Packer Clinical Composite Score at 6 -month follow-up. Clinical Composite Score is a composite measure of mortality, HF hospitalizations, and symptomatic changes. 1 Birnie D, et al. Heart Rhythm. 2013; 10: 1368 -1374.

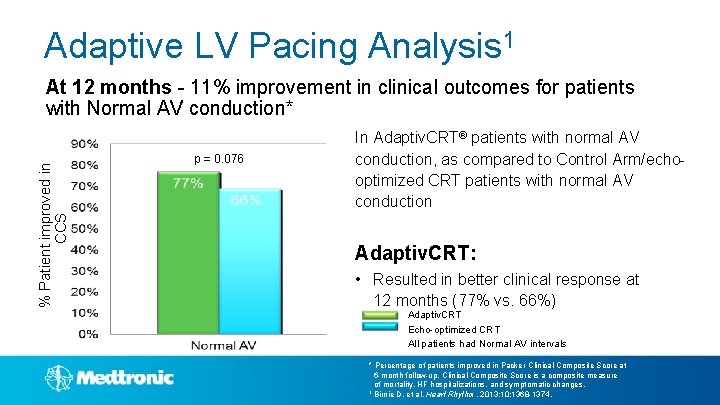
Adaptive LV Pacing Analysis 1 % Patient improved in CCS At 12 months - 11% improvement in clinical outcomes for patients with Normal AV conduction* p = 0. 076 In Adaptiv. CRT® patients with normal AV conduction, as compared to Control Arm/echooptimized CRT patients with normal AV conduction Adaptiv. CRT: • Resulted in better clinical response at 12 months (77% vs. 66%) Adaptiv. CRT Echo-optimized CRT All patients had Normal AV intervals * Percentage of patients improved in Packer Clinical Composite Score at 6 -month follow-up. Clinical Composite Score is a composite measure of mortality, HF hospitalizations, and symptomatic changes. 1 Birnie D, et al. Heart Rhythm. 2013; 10: 1368 -1374.

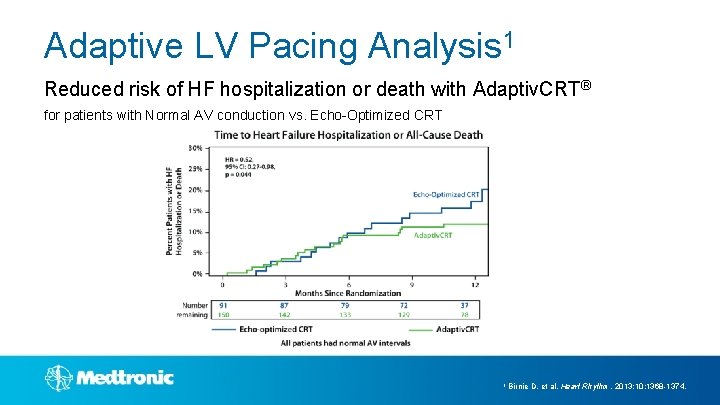
Adaptive LV Pacing Analysis 1 Reduced risk of HF hospitalization or death with Adaptiv. CRT® for patients with Normal AV conduction vs. Echo-Optimized CRT 1 Birnie D, et al. Heart Rhythm. 2013; 10: 1368 -1374.

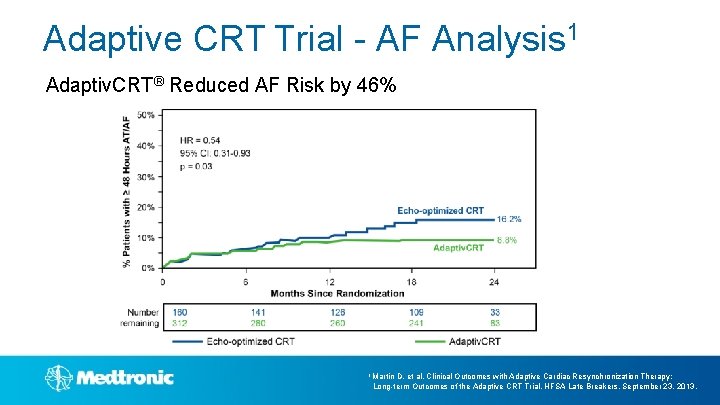
Adaptive CRT Trial - AF Analysis 1 Adaptiv. CRT® Reduced AF Risk by 46% 1 Martin D, et al. Clinical Outcomes with Adaptive Cardiac Resynchronization Therapy: Long-term Outcomes of the Adaptive CRT Trial. HFSA Late Breakers. September 23, 2013.

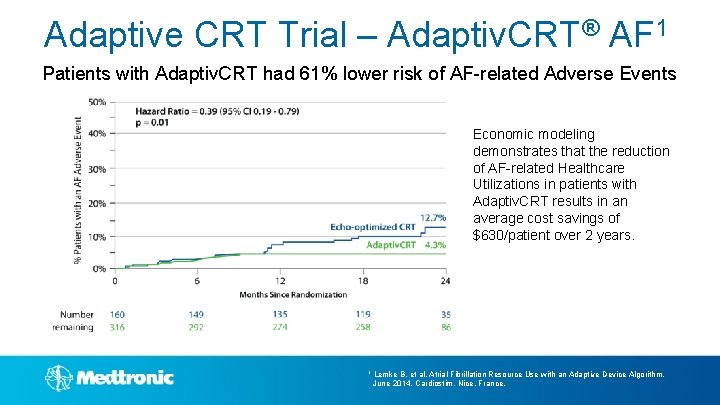
Adaptive CRT Trial – Adaptiv. CRT® AF 1 Patients with Adaptiv. CRT had 61% lower risk of AF-related Adverse Events Economic modeling demonstrates that the reduction of AF-related Healthcare Utilizations in patients with Adaptiv. CRT results in an average cost savings of $630/patient over 2 years. 1 Lemke B, et al. Atrial Fibrillation Resource Use with an Adaptive Device Algorithm. June 2014, Cardiostim, Nice, France.

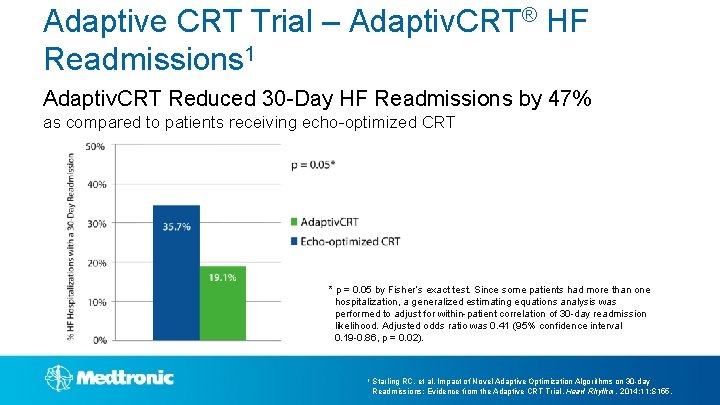
Adaptive CRT Trial – Adaptiv. CRT® HF Readmissions 1 Adaptiv. CRT Reduced 30 -Day HF Readmissions by 47% as compared to patients receiving echo-optimized CRT * p = 0. 05 by Fisher’s exact test. Since some patients had more than one hospitalization, a generalized estimating equations analysis was performed to adjust for within-patient correlation of 30 -day readmission likelihood. Adjusted odds ratio was 0. 41 (95% confidence interval 0. 19 -0. 86, p = 0. 02). 1 Starling RC, et al. Impact of Novel Adaptive Optimization Algorithms on 30 -day Readmissions: Evidence from the Adaptive CRT Trial. Heart Rhythm. 2014; 11: S 155.

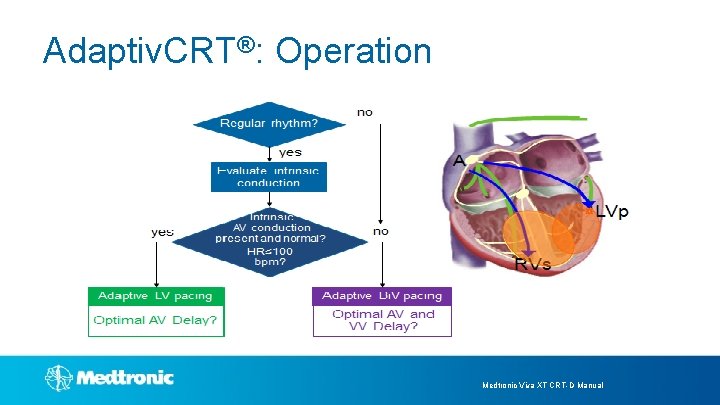
Adaptiv. CRT® Operation

Adaptiv. CRT®: Operation Medtronic Viva XT CRT-D Manual

Physiology of CRT: LV Pacing

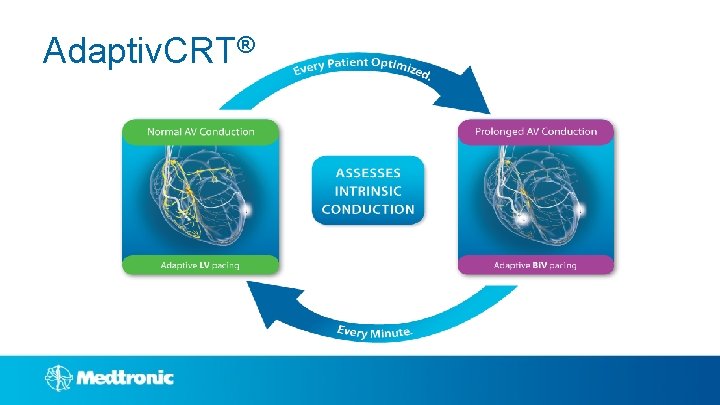
Adaptiv. CRT®

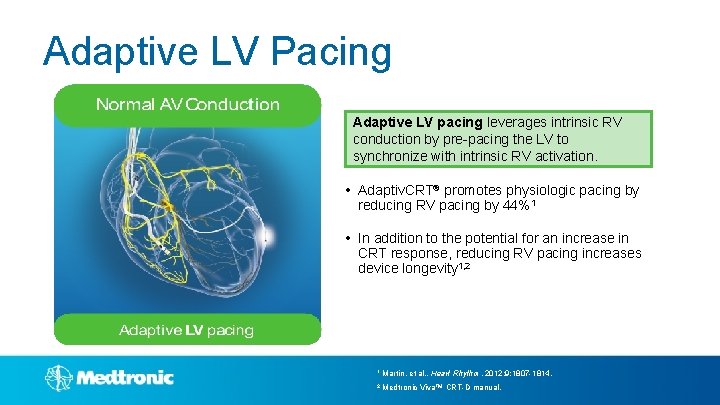
Adaptive LV Pacing Adaptive LV pacing leverages intrinsic RV conduction by pre-pacing the LV to synchronize with intrinsic RV activation. • Adaptiv. CRT® promotes physiologic pacing by reducing RV pacing by 44%1 • In addition to the potential for an increase in CRT response, reducing RV pacing increases device longevity 1, 2 1 Martin, et al. , Heart Rhythm. 2012; 9: 1807 -1814. 2 Medtronic Viva™ CRT-D manual.

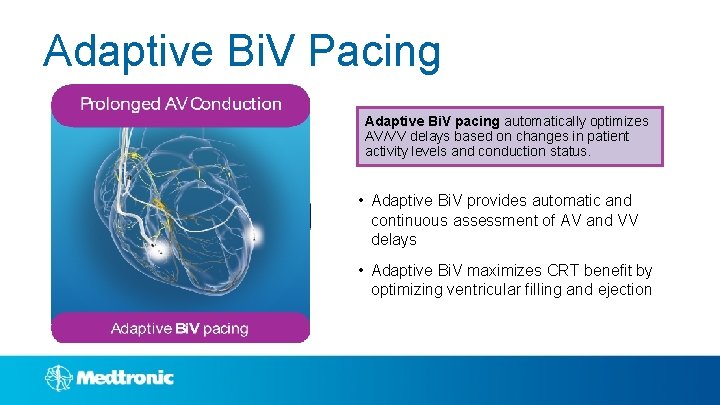
Adaptive Bi. V Pacing Adaptive Bi. V pacing automatically optimizes AV/VV delays based on changes in patient activity levels and conduction status. • Adaptive Bi. V provides automatic and continuous assessment of AV and VV delays • Adaptive Bi. V maximizes CRT benefit by optimizing ventricular filling and ejection

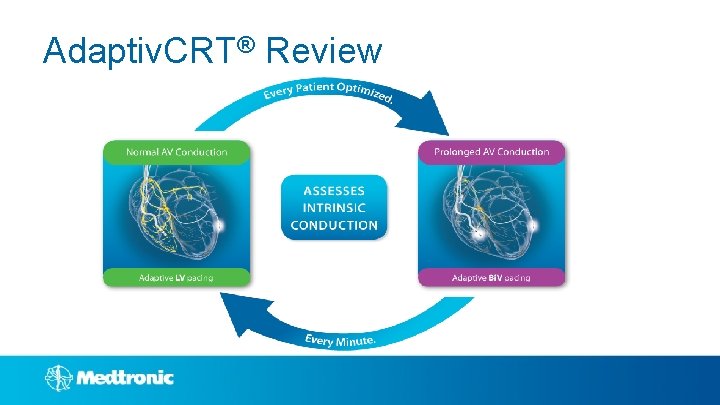
Adaptiv. CRT® Review

Viva™ Quad CRT-D and Attain® Performa™ System Advanced Quadripolar Lead Vector. Express® Adaptiv. CRT®

Viva™ Quad CRT-D and Attain® Performa™ Summary • Advanced Quadripolar Lead – The only quadripolar LV lead with a short bipolar electrode spacing to achieve desired pacing location and avoid phrenic nerve stimulation – The only quadripolar LV lead with steroid on every electrode to increase longevity – Designed for compatibility with all Medtronic left-heart delivery tools • Vector. Express® – Provides clinically actionable information in 2 minutes to manage capture thresholds, longevity, and CRT response 1 – Eliminates time-consuming manual testing of multiple vectors to help you identify the best option for your patient • Adaptiv. CRT® – The Medtronic Viva Quad XT CRT-D system combines the benefits at implant of an advanced quadripolar lead with the demonstrated clinical benefits of Adaptiv. CRT 2 1 Demmer W. Vector. Express Performance Results. Medtronic Data on File. January 2013. 2 Birnie D, et al. Heart Rhythm. September 2013; 9: 1368 -1374.

References Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. June 13, 2002; 346(24): 1845 -1853. Biffi M, Moschini C, Bertini M, et al. Phrenic stimulation: a challenge for cardiac resynchronization therapy. Circ Arrhythm Electrophysiol. August 2009; 2(4): 402 -410. Abraham WT, Gras D, Yu CM, et al. Late-Breaking Clinical Trials. HRS 2010. Denver, CO. Biffi M, Zanon F, Bertaglia E, et al. Short-spaced dipole for managing phrenic nerve stimulation in patients with CRT: The "phrenic nerve mapping and stimulation EP" catheter study. Heart Rhythm. January 2013; 10(1): 39 -45. Abraham WT, Leon AR, Hannon C, et al. Results of the In. Sync III Marquis clinical trial (Conference Abstract). Heart Rhythm. May 2005; 2(5, Suppl 1): S 65. Abraham WT, Young JB, León AR, et al. Effects of cardiac resynchronization on disease progression in patients with left ventricular systolic dysfunction, an indication for an implantable cardioverter-defibrillator, and mildly symptomatic chronic heart failure. Circulation. November 2, 2004; 110(18): 2864 -2868. Attain Performa Lead Manuals. Attain Performa Model 4298 Quadripolar Left Ventricular Lead Clinical Study Summary, 2014, M 957312 A 001 A. Biffi M, Bertini M, Saporito D, et al. Automatic management of left ventricular stimulation: hints for technologic improvement. Pacing Clin Electrophysiol. March 2009; 32(3): 346 -353. Biffi M, Bertini M, Ziacchi M, et al. Management of phrenic stimulation in CRT patients over the long term: still an unmet need? Pacing Clin Electrophysiol. October 2011; 34(10): 12011208. Biffi M, Exner DV, Crossley GH, et al. Occurrence of phrenic nerve stimulation in cardiac resynchronization therapy patients: the role of left ventricular lead type and placement site. Europace. January 2013; 15(1): 77 -82. Biffi M, Foerster L, Eastman W, et al. Effect of bipolar electrode spacing on phrenic nerve stimulation and left ventricular pacing thresholds: an acute canine study. Circ Arrhythm Electrophysiol. August 1, 2012; 5(4): 815 -820. Dekker AL, Phelps B, Dijkman B, et al. Epicardial left ventricular lead placement for cardiac resynchronization therapy: optimal pace site selection with pressure-volume loops. J Thorac Cardiovasc Surg. June 2004; 127(6): 1641 -1647. Demmer W. Vector. Express Performance Results. Medtronic Data on file. January 2013. Biffi M, et al, Utilizing Short Spacing between Quadripolar LV lead Electrodes to Avoid PNS. Cardiostim 2014, Poster presentation, Session 56 P. Gras D, et al. Left ventricular lead adverse events observed at 1 year follow up in the multicenter randomized FREEDOM trial. Eur Heart J. 2011; 32(Abstract Suppl): 178. Birnie D, Lemke B, Aonuma K, et al. Clinical outcome es with synchronized left ventricular pacing: Analysis of the adaptive CRT trial. Heart Rhythm. September 2013; 9(10): 1368 -1374. Gurevitz O, Nof E, Carasso S, et al. Programmable multiple pacing configurations help to overcome high left ventricular pacing thresholds and avoid phrenic nerve stimulation. Pacing Clin Electrophysiol. December 2005; 28(12): 1255 -1259. Bristow MR, Saxon LA, Boehmer J, et al. Cardiac resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. New Engl J Med. May 20, 2004; 350(21): 2140 -2150. Champagne J, Healey JS, Krahn AD, et al. The effect of electronic repositioning on left ventricular pacing and phrenic nerve stimulation. Europace. March 2011; 13(3): 409 -415. Chung ES, Leon AR, Tavazzi L, et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. May 20, 2008; 117(20): 2608 -2616. Crossley GH, Brinker JA, Reynolds D, et al. Steroid elution improves the stimulation threshold in an active-fixation atrial permanent pacing lead. A randomized, controlled study. Model 4068 Investigators. Circulation. November 15, 1995; 92(10): 2935 -2939. Jastrzebski M, Bacior B, Wojciechowska W, Czarnecka D. Left ventricular lead implantation at a phrenic stimulation site is safe and effective. Europace. April 2011; 13(4): 520 -525. Khan FZ, Virdee MS, Palmer CR, et al. Targeted left ventricular lead placement to guide cardiac resynchronization therapy: the TARGET study: a randomized, controlled trial. J Am Coll Cardiol. April 24, 2012; 59(17): 1509 -1518. Kirubakaran S, Rinaldi CA. Phrenic nerve stimulation with the quadripolar left ventricular lead not overcome by 'electronic repositioning'. Europace. April 2012; 14(4): 608 -609. Klein N, Klein M, Weglage H, et al. Clinical efficacy of left ventricular pacing vector programmability in cardiac resynchronization therapy defibrillator patients for management of phrenic nerve stimulation and/or elevated left ventricular pacing thresholds: insights from the Efface Phrenic Stim study. Europace. June 2012; 14(6): 826 -832.

References Lunati MG, Gasparini M, Landolina M, et al. Long-Term Effect of Steroid Elution on the Electrical Performance of Coronary Sinus Leads for Cardiac Resynchronization Therapy. Presented at HRS 2012 (AB 10 -05). Martin DO, Lemke B, Birnie D, et al. Investigation of a novel algorithm for synchronized left-ventricular pacing and ambulatory optimization of cardiac resynchronization therapy: results of the adaptive CRT trial. Heart Rhythm. November 2012; 9(11): 1807 -1814. Martin D, et al. Clinical Outcomes with Adaptive Cardiac Resynchronization Therapy: Long-Term Outcomes of the Adaptive CRT Trial. HFSA Late Breakers. September 23, 2013. Seifert M, Schau T, Moeller V, Neuss M, Meyhoefer J, Butter C. Influence of pacing configurations, body mass index, and position of coronary sinus lead on frequency of phrenic nerve stimulation and pacing thresholds under cardiac resynchronization therapy. Europace. July 2010; 12(7): 961 -967. Sperzel J. Initial Clinical Experience with Novel Left Ventricular Quadripolar Lead. Presented at ESC Congress August 2010. Sperzel J, Dänschel W, Gutleben KJ, et al. First prospective, multi-centre clinical experience with a novel left ventricular quadripolar lead. Europace. March 2012; 14(3): 365 -372. MDT Attain Ability Model 4196 LV Lead Final Clinical Report (V 1, February 25, 2009). Starling RC, Krum H, Bril S, et al. Impact of Novel Adaptive Optimization Algorithms on 30 -Day Readmissions. Evidence from the Adaptive CRT Trial. Heart Rhythm. 2014; 11(5)(Suppl. ): S 155. Medtronic Attain Performa 4298 Lead Manual. Tarab AD, et al. Value Health. 2012; 15: A 349. Medtronic Viva Quad XT CRT-D Manual. Tomassoni G, Baker J, Corbisiero R, et al. Postoperative performance of the Quartet ® left ventricular heart lead. J Cardiovasc Electrophysiol. April 2013; 24(4): 449 -456. Medtronic Viva Quad S CRT-D Manual. Mond HG, Stokes KB. The electrode-tissue interface: the revolutionary role of steroid elution. Pacing Clin Electrophysiol. January 1992; 15(1): 95 -107. Mond HG, Stokes KB. The steroid-eluting electrode: a 10 -year experience. Pacing Clin Electrophysiol. July 1996; 19(7): 10161020. Mullens W, Grimm RA, Verga T, et al. Insights from a cardiac resynchronization optimization clinic as part of a heart failure disease management program. J Am Coll Cardiol. March 3, 2009; 53(9): 765 -773. Parahuleva MS, Chasan R, Soydan N, et al. Quadripolar Left Ventricular Lead in a Patient with CRT-D Does Not Overcome Phrenic Nerve Stimulation. Clin Med Insights Cardiol. April 27, 2011; 5: 45 -47. van Gelder BM, Meijer A, Bracke FA. The optimized V-V interval determined by interventricular conduction times versus invasive measurement by LVd. P/dt. MAX. J Cardiovasc Electrophysiol. September 2008; 19(9): 939 -944. Wager J. Attain Performa Limited Release Evaluation. Medtronic data on file. February 2013. Young JB, Abraham WT, Smith AL, et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA. May 28, 2003; 289(20): 2685 -2694. Ypenburg C, van Bommel RJ, Borleffs CJ, et al. Long-term prognosis after cardiac resynchronization therapy is related to the extent of left ventricular reverse remodeling at midterm follow-up. J Am Coll Cardiol. February 10, 2009; 53(6): 483 -490. Ypenburg C, van Bommel RJ, Delgado V, et al. Optimal left ventricular lead position predicts reverse remodeling and survival after cardiac resynchronization therapy. J Am Coll Cardiol. October 21, 2008; 52(17): 1402 -1409.

Brief Statement Attain® Performa™ 4298 Lead Indications: The Attain Performa 4298 steroid eluting, quadripolar electrode, IS 4 transvenous leads are indicated for chronic pacing and sensing in the left ventricle via the cardiac vein, when used with a compatible Medtronic Cardiac Resynchronization Therapy (CRT) system. Extended bipolar pacing is available using these leads in combination with a compatible CRT-D system and RV defibrillation lead. Contraindications Coronary vasculature – These leads are contraindicated for patients with coronary venous vasculature that is inadequate for lead placement, as indicated by venogram. Steroid use – Do not use in patients for whom a single dose of 288 μg of dexamethasone acetate maybe contraindicated. Warnings and Precautions Chronic repositioning or removal of leads may be difficult because of fibrotic tissue development. accompanying leads should not receive diathermy treatment. The interaction between the implant and diathermy can cause tissue damage, fibrillation, or damage to the device components, which could result in serious injury, loss of therapy, and/or the need to reprogram or replace the device. Leads should be handled with great care at all times. Use an anchoring sleeve with all leads. Ensure the anchoring sleeve is positioned close to the lead connector pin, to prevent inadvertent passage of the sleeve into the vein. Use care when handling stylets. Any severe bending kinking, stretching, handling with surgical instruments, or excessive force when inserting a stylet may cause permanent damage to the lead. Rust stylets are not recommended with this lead due to the risk of conductor coil or insulation perforation. Use care when handling guide wires. Damage to the guide wire may prevent the guide wire from performing with accurate torque response and may cause vessel damage. Do not use excessive force to retract the guide wire from the lead. Refer to the product documentation packaged with the Output pulses, especially from unipolar devices, may guide wire for additional information. adversely affect device sensing capabilities. If a patient Do not use magnetic resonance imaging (MRI) on patients requires a separate stimulation device, either permanent or who have this device implanted. MRI can induce currents on temporary, allow enough space between the leads of the implanted leads, potentially causing tissue damage and the separate systems to avoid interference in the sensing induction of tachyarrhythmias. capabilities of the devices. Previously implanted pulse generators and implantable cardioverter defibrillators should For Model 4298 lead, total patient exposure to dexamethasone acetate should be considered. Drug generally be explanted. interactions of dexamethasone acetate with this lead have not been studied. It has not been determined whether the warnings, precautions, or complications usually associated People with metal implants such as pacemakers, with injectable dexamethasone acetate apply to the use of implantable cardioverter defibrillators (ICDs) and this highly localized, controlled-release device. For a list of potential adverse effects, refer to the Physicians’ Desk Reference. Do not force the guide catheter or leads if significant resistance is encountered. Use of guide catheters and/or leads may cause trauma to the heart. Keep external defibrillation equipment nearby for immediate use during acute lead system testing, the implant procedure, or whenever arrhythmias are possible or intentionally induced during the postimplant testing. Backup pacing should be readily available during implant. Use of the delivery system or leads may cause heart block. To minimize the likelihood of trauma to the vein and to maintain lead flexibility while advancing the lead through the vein, keep the stylet withdrawn 1 to 2 cm or select a more flexible stylet. Do not insert the proximal end of the guide wire through the lead tip seal without using the guide wire insertion tool. Inserting the guide wire without the guide wire insertion tool may damage the lead. During lead implant and testing, use only battery- powered equipment or line-powered equipment specifically designed for this purpose to protect against fibrillation that may be caused by alternating currents.

Brief Statement (cont. ) Attain® Performa™ 4298 Lead, continued Potential Complications: Potential complications related to the use of transvenous leads include, but are not limited to the following patient-related conditions: cardiac dissection, cardiac perforation, cardiac tamponade, coronary sinus dissection, death, endocarditis, erosion through the skin, extracardiac muscle or nerve stimulation, fibrillation or other arrhythmias, heart block, heart wall or vein wall rupture, hematoma/seroma, infection, myocardial irritability, myopotential sensing, pericardial effusion, pericardial rub, pneumothorax, rejection phenomena, threshold elevation, thrombosis, thrombotic or air embolism, and valve damage. See the device manual for detailed information regarding the implant procedure, indications, contraindications, warnings, precautions, and potential complications/adverse events. For further information, please call Medtronic at 1 (800) 723 -4636 and/or consult Medtronic’s website at www. medtronic. com. Caution: Federal law (USA) restricts these devices to sale by or on the order of a physician.

Brief Statement (cont. ) Viva™ Quad S CRT-D Model DTBB 1 QQ and Viva Quad S CRT-D Model DTBB 1 Q 1 Indications: The Viva Quad S CRT-D system is indicated for ventricular antitachycardia pacing and ventricular defibrillation for automated treatment of life-threatening ventricular arrhythmias, for use in patients with atrial tachyarrhythmias, or those patients who are at significant risk for developing atrial tachyarrhythmias and for providing cardiac resynchronization therapy in heart failure patients on stable, optimal heart failure medical therapy if indicated, and meet any of the following classifications: New York Heart Association (NYHA) Functional Class III or IV and who have a left ventricular ejection fraction ≤ 35% and a prolonged QRS duration. Left bundle branch block (LBBB) with a QRS duration ≥ 130 ms, left ventricular ejection fraction ≤ 30%, and NYHA Functional Class II. NYHA Functional Class I, II, or III and who have left ventricular ejection fraction ≤ 50% and atrioventricular block (AV block) that are expected to require a high percentage of ventricular pacing that cannot be managed with algorithms to minimize right ventricular pacing. Optimization of heart failure medical therapy that is limited due to AV block or the urgent need for pacing should be done post-implant. Lead Integrity Alert The RV Lead Integrity Alert feature is intended primarily for patients who have a Medtronic ICD or CRT-D device and a Sprint Fidelis lead (Models 6949, 6948, 6931, and 6930) based on performance data. The RV LIA feature may not perform as well with a St. Jude Medical Riata™/Durata ® lead or a Boston Scientific Endotak lead as it does when used with a Medtronic Sprint Fidelis lead. This is because different lead designs may have different failure signatures and conditions that may or may not be detected early by the RV LIA feature. Contraindications: The Viva S CRT-D system is contraindicated for patients experiencing tachyarrhythmias with transient or reversible causes including, but not limited to, the following: acute myocardial infarction, drug intoxication, drowning, electric shock, electrolyte imbalance, hypoxia, or sepsis. The device is contraindicated for patients who have a unipolar pacemaker implanted. The device is contraindicated for patients with incessant VT or VF. The device is contraindicated for patients whose primary disorder is chronic atrial tachyarrhythmia with no concomitant VT or VF. Warnings and Precautions: Changes in a patient’s disease and/or medications may alter the efficacy of the device’s programmed parameters. Patients should avoid sources of magnetic and electromagnetic radiation to avoid possible underdetection, inappropriate sensing and/or therapy delivery, tissue damage, induction of an arrhythmia, device electrical reset, or device damage. Do not place transthoracic defibrillation paddles directly over the device. Certain programming and device operations may not provide cardiac resynchronization. Potential Complications: Potential complications include, but are not limited to, rejection phenomena, erosion through the skin, muscle or nerve stimulation, oversensing, failure to detect and/or terminate tachyarrhythmia episodes, acceleration of ventricular tachycardia, and surgical complications such as hematoma, infection, inflammation, and thrombosis. See the device manual for detailed information regarding the implant procedure, indications, contraindications, warnings, precautions, and potential complications/adverse events. For further information, please call Medtronic at 1 (800) 328 -2518 and/or consult Medtronic’s website at www. medtronic. com. Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.

Brief Statement (cont. ) Viva™ Quad XT CRT-D Model DTBA 1 QQ, Viva Quad XT CRT-D Model DTBA 1 Q 1 Indications: The Viva Quad XT CRT-D system is indicated for ventricular antitachycardia pacing and ventricular defibrillation for automated treatment of life-threatening ventricular arrhythmias, for use in patients with atrial tachyarrhythmias, or those patients who are at significant risk for developing atrial tachyarrhythmias and for providing cardiac resynchronization therapy in heart failure patients on stable, optimal heart failure medical therapy if indicated, and meet any of the following classifications: New York Heart Association (NYHA) Functional Class III or IV and who have a left ventricular ejection fraction ≤ 35% and a prolonged QRS duration. Left bundle branch block (LBBB) with a QRS duration ≥ 130 ms, left ventricular ejection fraction ≤ 30%, and NYHA Functional Class II. NYHA Functional Class I, II, or III and who have left ventricular ejection fraction ≤ 50% and atrioventricular block (AV block) that are expected to require a high percentage of ventricular pacing that cannot be managed with algorithms to minimize right ventricular pacing. Optimization of heart failure medical therapy that is limited due to AV block or the urgent need for pacing should be done post-implant. Lead Integrity Alert The RV Lead Integrity Alert feature is intended primarily for patients who have a Medtronic ICD or CRT-D device and a Sprint Fidelis lead (Models 6949, 6948, 6931, and 6930) based on performance data. The RV LIA feature may not perform as well with a St. Jude Medical Riata™/Durata ® lead or a Boston Scientific Endotak lead as it does when used with a Medtronic Sprint Fidelis lead. This is because different lead designs may have different failure signatures and conditions that may or may not be detected early by the RV LIA feature. Contraindications: The Viva XT CRT-D system is contraindicated for patients experiencing tachyarrhythmias with transient or reversible causes including, but not limited to, the following: acute myocardial infarction, drug intoxication, drowning, electric shock, electrolyte imbalance, hypoxia, or sepsis. The device is contraindicated for patients who have a unipolar pacemaker implanted. The device is contraindicated for patients with incessant VT or VF. The device is contraindicated for patients whose primary disorder is chronic atrial tachyarrhythmia with no concomitant VT or VF. Warnings and Precautions: Changes in a patient’s disease and/or medications may alter the efficacy of the device’s programmed parameters. Patients should avoid sources of magnetic and electromagnetic radiation to avoid possible underdetection, inappropriate sensing and/or therapy delivery, tissue damage, induction of an arrhythmia, device electrical reset, or device damage. Do not place transthoracic defibrillation paddles directly over the device. Certain programming and device operations may not provide cardiac resynchronization. Potential Complications: Potential complications include, but are not limited to, rejection phenomena, erosion through the skin, muscle or nerve stimulation, oversensing, failure to detect and/or terminate tachyarrhythmia episodes, acceleration of ventricular tachycardia, and surgical complications such as hematoma, infection, inflammation, and thrombosis. See the device manual for detailed information regarding the implant procedure, indications, contraindications, warnings, precautions, and potential complications/adverse events. For further information, please call Medtronic at 1 (800) 328 -2518 and/or consult Medtronic’s website at www. medtronic. com. Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.

Off-Label Use Disclaimer Medtronic’s Medical Education programs are offered to provide attendees education on the FDA approved indications and use of our products. In the event a question is asked during the program that is not consistent with FDA approved product labeling, the Faculty has been provided guidance to inform the attendee that the question is an off-label use of the Medtronic product. A conversation may take place between the attendee and Faculty to address the question, but should not necessarily be considered endorsed by Medtronic. At any time, a question regarding a Medtronic product may be directed to Medtronic’s Office of Medical Affairs: CRHF: Jason Sims, Sr. Medical Affairs Manager Office: 877 -359 -6415 Email: RS. CRDMOMA@Medtronic. com

Compensation Disclosure This faculty is being paid as a consultant for the services being provided in accordance with the Sunshine Act.

Thank You UC 201504796 EN January 2015
 Lvcm medtronic
Lvcm medtronic Tuliskan fungsi kemasan menurut mutu performa
Tuliskan fungsi kemasan menurut mutu performa Mid day meal annual performa haryana
Mid day meal annual performa haryana Benchmarks are baseline values the system seeks to attain.
Benchmarks are baseline values the system seeks to attain. Dcb clinical outcomes
Dcb clinical outcomes Frequency dependence of dielectric constant
Frequency dependence of dielectric constant Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Slidetodoc
Slidetodoc Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Tư thế worm breton là gì
Tư thế worm breton là gì Alleluia hat len nguoi oi
Alleluia hat len nguoi oi Môn thể thao bắt đầu bằng chữ đua
Môn thể thao bắt đầu bằng chữ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 101012 bằng
101012 bằng độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thế nào là giọng cùng tên
Thế nào là giọng cùng tên Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Fecboak
Fecboak Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Số nguyên tố là số gì
Số nguyên tố là số gì Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Hổ sinh sản vào mùa nào
Hổ sinh sản vào mùa nào Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Hệ hô hấp
Hệ hô hấp Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Third step of nursing process
Third step of nursing process Example of learning objectives
Example of learning objectives Collaborative interventions nursing
Collaborative interventions nursing Direct and indirect speech objectives
Direct and indirect speech objectives Objectives of input output management
Objectives of input output management Examples of ifsp outcomes and strategies
Examples of ifsp outcomes and strategies Sound energy definition
Sound energy definition Learning objectives of profit and loss
Learning objectives of profit and loss Learning objectives of work and energy
Learning objectives of work and energy Chapter 13 section 4 the power of the church
Chapter 13 section 4 the power of the church Indian and international number system
Indian and international number system Aims objectives and outcomes
Aims objectives and outcomes Chapter 13 section 4 the power of the church
Chapter 13 section 4 the power of the church Ancient rome outcomes geography and early republic
Ancient rome outcomes geography and early republic In the afylin framework learning outcomes are arranged
In the afylin framework learning outcomes are arranged Water cycle learning outcomes
Water cycle learning outcomes