Medtronic Harmony Transcatheter pulmonary Valve System The First

















- Slides: 25

Medtronic Harmony™ Transcatheter pulmonary Valve System The First Global Pediatric Trial Hiroko Ookubo – CVG Clinical, Medtronic Japan

<First Name> <Last Name>, <Degree(s)> I have no relevant financial relationships

Speaker Name: Hiroko Ookubo I am a Medtronic Japan Employee.

OBJECTIVE • Share an overview of the Harmony Trial which has been accepted as part of the Harmonization by Doing (HBD) for Children program • Provide insights and learnings on the clinical strategy and experience for Harmony™ TPV System utilizing the HBD for Children Medtronic Confidential | CRT Feb 2019

AGENDA • Intended Patient Population • Harmony ™ TPV System Product Overview • Non-clinical Testing • Early Feasibility Study • Regulatory and Clinical Strategy • Pivotal Study Protocol Overview • Japan –US HBD Medtronic Confidential | CRT Feb 2019

Driven By Our Mission Written in 1960, our mission dictates everything we do. To contribute to human welfare by application of biomedical engineering in the research, design, manufacture, and sale of instruments or appliances that alleviate pain, restore health, and extend life. To strive without reserve for the greatest possible reliability and quality in our products.


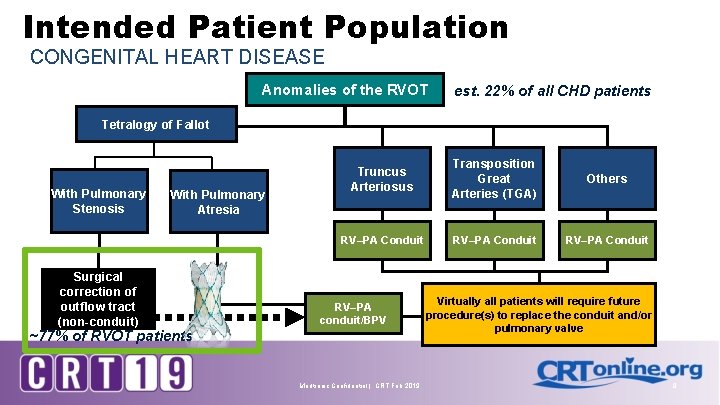
Intended Patient Population CONGENITAL HEART DISEASE Anomalies of the RVOT est. 22% of all CHD patients Tetralogy of Fallot With Pulmonary Stenosis Surgical correction of outflow tract (non-conduit) With Pulmonary Atresia Truncus Arteriosus Transposition Great Arteries (TGA) Others RV–PA Conduit RV–PA conduit/BPV ~77% of RVOT patients Medtronic Confidential | CRT Feb 2019 Virtually all patients will require future procedure(s) to replace the conduit and/or pulmonary valve 8


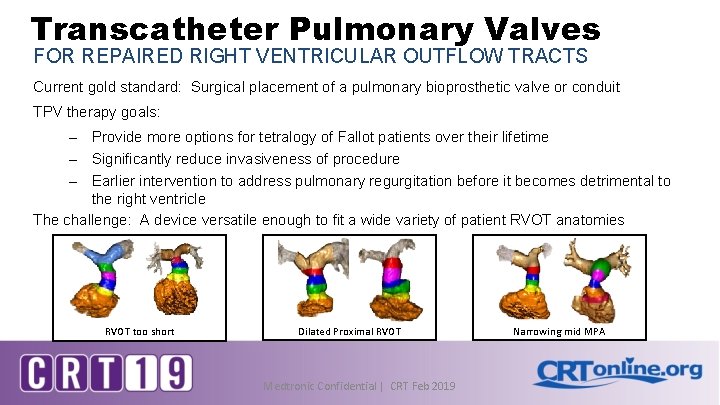
Transcatheter Pulmonary Valves FOR REPAIRED RIGHT VENTRICULAR OUTFLOW TRACTS Current gold standard: Surgical placement of a pulmonary bioprosthetic valve or conduit TPV therapy goals: – Provide more options for tetralogy of Fallot patients over their lifetime – Significantly reduce invasiveness of procedure – Earlier intervention to address pulmonary regurgitation before it becomes detrimental to the right ventricle The challenge: A device versatile enough to fit a wide variety of patient RVOT anatomies RVOT too short Dilated Proximal RVOT Medtronic Confidential | CRT Feb 2019 Narrowing mid MPA

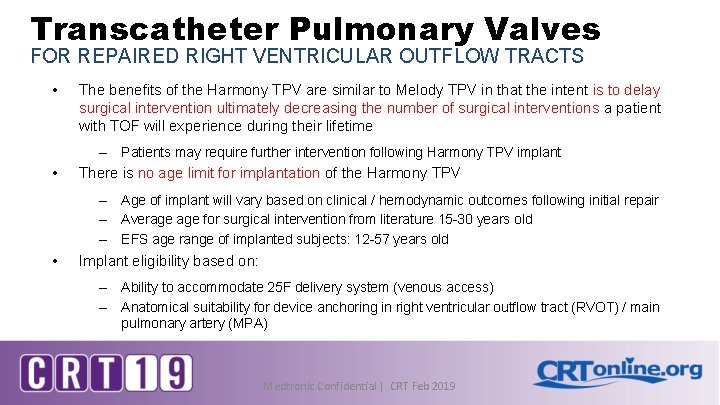
Transcatheter Pulmonary Valves FOR REPAIRED RIGHT VENTRICULAR OUTFLOW TRACTS • The benefits of the Harmony TPV are similar to Melody TPV in that the intent is to delay surgical intervention ultimately decreasing the number of surgical interventions a patient with TOF will experience during their lifetime – • There is no age limit for implantation of the Harmony TPV – – – • Patients may require further intervention following Harmony TPV implant Age of implant will vary based on clinical / hemodynamic outcomes following initial repair Average for surgical intervention from literature 15 -30 years old EFS age range of implanted subjects: 12 -57 years old Implant eligibility based on: – – Ability to accommodate 25 F delivery system (venous access) Anatomical suitability for device anchoring in right ventricular outflow tract (RVOT) / main pulmonary artery (MPA) Medtronic Confidential | CRT Feb 2019

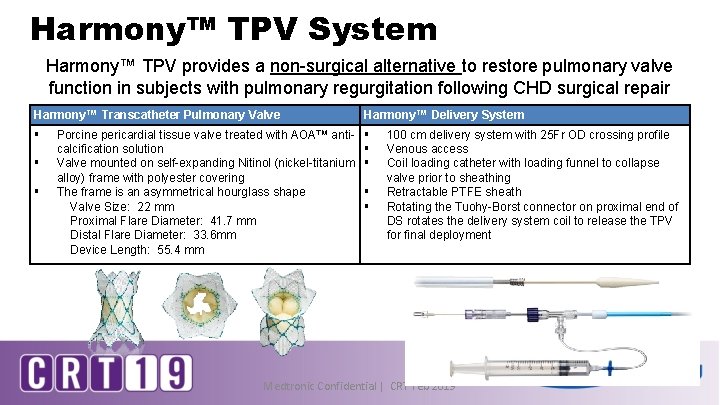
Harmony™ TPV System Harmony™ TPV provides a non-surgical alternative to restore pulmonary valve function in subjects with pulmonary regurgitation following CHD surgical repair Harmony™ Transcatheter Pulmonary Valve Harmony™ Delivery System § § § Porcine pericardial tissue valve treated with AOA™ anticalcification solution Valve mounted on self-expanding Nitinol (nickel-titanium alloy) frame with polyester covering The frame is an asymmetrical hourglass shape Valve Size: 22 mm Proximal Flare Diameter: 41. 7 mm Distal Flare Diameter: 33. 6 mm Device Length: 55. 4 mm § § 100 cm delivery system with 25 Fr OD crossing profile Venous access Coil loading catheter with loading funnel to collapse valve prior to sheathing Retractable PTFE sheath Rotating the Tuohy-Borst connector on proximal end of DS rotates the delivery system coil to release the TPV for final deployment Medtronic Confidential | CRT Feb 2019


Early Feasibility Study OVERVIEW • Prospective, Non-randomized • First FDA approved Early Feasibility Study • Primary Objective: Obtain in vivo data to confirm assumptions on loading conditions for future in vitro frame evaluations • Secondary Objectives: Characterize procedural feasibility, safety & TPV performance • 20 patients implanted for 5 year follow-up at 3 centers (May 2013 – May 2015) – The Hospital for Sick Children, Toronto Canada – Dr. Lee Benson – Nationwide Children’s Hospital, Columbus Ohio – Dr. John Cheatham – Boston Children’s Hospital, Boston, MA – Dr. Lisa Bergersen • Screening Committee to review all potential candidates • Data Safety Monitoring Board oversight of study Medtronic Confidential | CRT Feb 2019

Early Feasibility Study BASELINE DEMOGRAPHICS AND SUBJECT FOLLOW-UP Demographics – Catheterized Cohort Male Age (yr) Weight (kg) Height (cm) Original Diagnosis (%) Tetralogy of Fallot Dysplastic Pulmonary Valve Subjects (n=21) 52. 4 (11/21) 27. 8± 13. 8 (12, 57) 72. 1± 23. 5 (34. 1, 116. 7) 166. 3± 12. 2 (131. 0, 185. 0) 95. 2 (20/21) 4. 8 (1/21) RVOT/PA Sizing – Catheterized Cohort Narrowest dimension at implant site (mm) Subjects (n=21) 22. 3± 3. 3 (18. 0, 28. 6) Narrowest dimension at distal PA (mm) 24. 03± 3. 3 (18. 0, 28. 6) Narrowest dimension at proximal PA (mm) 28. 7± 4. 5 (22. 0, 36. 5) Length of Main PA (mm) 48. 0± 9. 0 (26. 5, 63. 7) Pulmonic annular diameter (mm) 24. 9± 4. 4 (17. 5, 31. 9) Medtronic Confidential | CRT Feb 2019

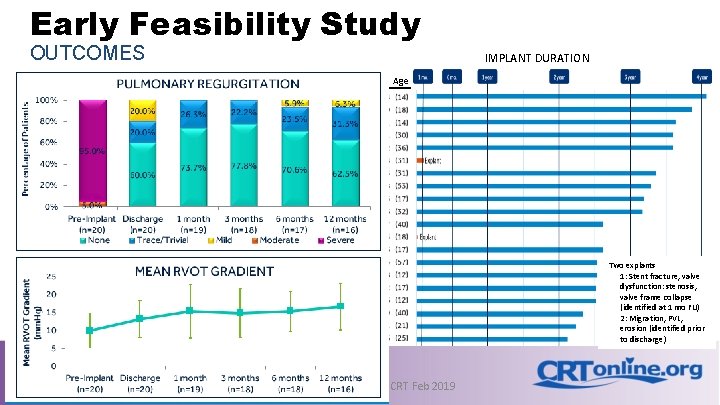
Early Feasibility Study OUTCOMES IMPLANT DURATION ID Age Two explants 1: Stent fracture, valve dysfunction: stenosis, valve frame collapse (identified at 1 mo FU) 2: Migration, PVL, erosion (identified prior to discharge) Medtronic Confidential | CRT Feb 2019


Global Trial Opportunity HOW TO MAKE A DECISION TO JOIN HBD PROGRAM FOR CHILDREN • Advantages of HBD • Considerations – Awareness of the HBD initiative is key to partnership and collaboration – Device challenges and to meet patient needs – Engaging stakeholders as early as possible • Physicians / Investigators • Regulators (FDA, PMDA etc. ) • Investigational centers – Enrollment and patient variability (ages, size, anatomical characteristics, etc) – Sponsorship and commitment to this therapeutic area and patient population – Scalability for Global Trials • Training & proctoring • Trial administration and oversight Medtronic Confidential | CRT Feb 2019

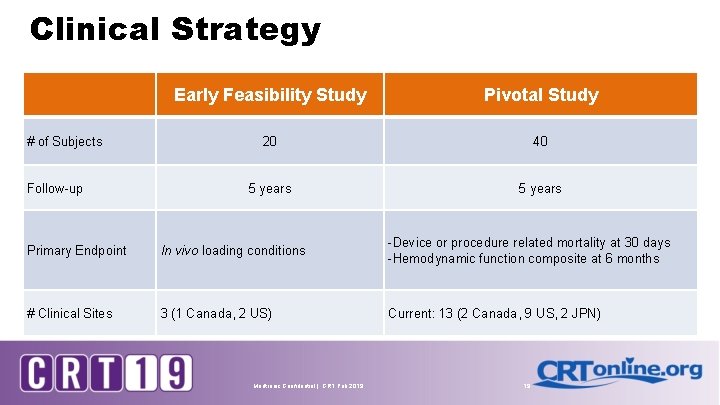
Clinical Strategy # of Subjects Follow-up Early Feasibility Study Pivotal Study 20 40 5 years Primary Endpoint In vivo loading conditions -Device or procedure related mortality at 30 days -Hemodynamic function composite at 6 months # Clinical Sites 3 (1 Canada, 2 US) Current: 13 (2 Canada, 9 US, 2 JPN) Medtronic Confidential | CRT Feb 2019 19

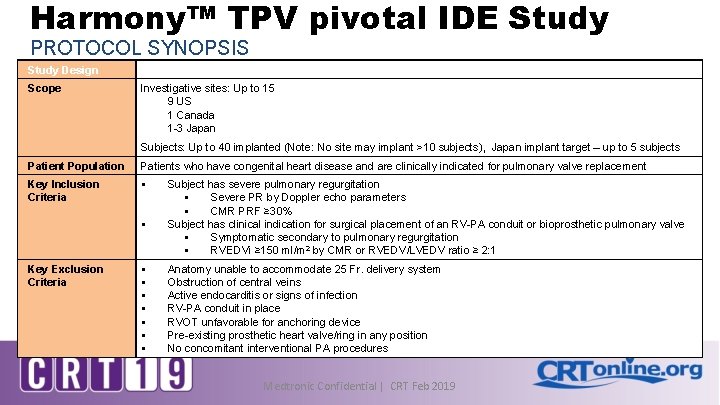
Harmony™ TPV pivotal IDE Study PROTOCOL SYNOPSIS Study Design Multi-center, prospective, non-randomized, interventional, pre-market Scope Investigative sites: Up to 15 9 US 1 Canada 1 -3 Japan Subjects: Up to 40 implanted (Note: No site may implant >10 subjects), Japan implant target – up to 5 subjects Patient Population Patients who have congenital heart disease and are clinically indicated for pulmonary valve replacement Key Inclusion Criteria § § Key Exclusion Criteria § § § § Subject has severe pulmonary regurgitation § Severe PR by Doppler echo parameters § CMR PRF ≥ 30% Subject has clinical indication for surgical placement of an RV-PA conduit or bioprosthetic pulmonary valve § Symptomatic secondary to pulmonary regurgitation § RVEDVi ≥ 150 ml/m 2 by CMR or RVEDV/LVEDV ratio ≥ 2: 1 Anatomy unable to accommodate 25 Fr. delivery system Obstruction of central veins Active endocarditis or signs of infection RV-PA conduit in place RVOT unfavorable for anchoring device Pre-existing prosthetic heart valve/ring in any position No concomitant interventional PA procedures Medtronic Confidential | CRT Feb 2019

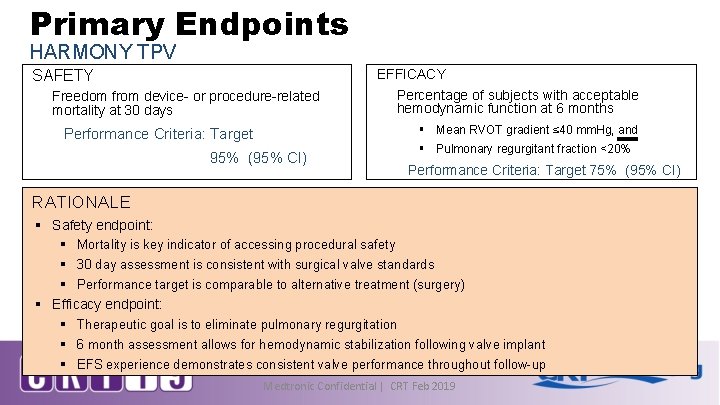
Primary Endpoints HARMONY TPV SAFETY Freedom from device- or procedure-related mortality at 30 days EFFICACY Percentage of subjects with acceptable hemodynamic function at 6 months § Mean RVOT gradient ≤ 40 mm. Hg, and Performance Criteria: Target 95% (95% CI) § Pulmonary regurgitant fraction <20% Performance Criteria: Target 75% (95% CI) RATIONALE § Safety endpoint: § Mortality is key indicator of accessing procedural safety § 30 day assessment is consistent with surgical valve standards § Performance target is comparable to alternative treatment (surgery) § Efficacy endpoint: § Therapeutic goal is to eliminate pulmonary regurgitation § 6 month assessment allows for hemodynamic stabilization following valve implant § EFS experience demonstrates consistent valve performance throughout follow-up Medtronic Confidential | CRT Feb 2019


Opportunities & learning for Medtronic Japan ADVANTAGES TO JOIN THE HBD AT JAPAN END Frequent Communications on regular basis (face to face meetings and conference calls) At the Initial Phase n To start a clinical trail (pre-market IDE) among both geographies ü Early alignment between FDA and PMDA ü Smooth study protocol development to meet both regulator requests. Accommodating local procedural approach. n To select appropriate investigational sites for this kind of orphan disease ü Advice and collaborate between industry and academia from the HBD for Children ü Help to evaluate potential sites for this study recruiting activities During the Enrollment Phase n To keep consistent screening process between US and Japan ü Global screening committee available to review eligibilities at both geographies. n To share safety concerns and discuss the next step at the both regulatory bodies concurrently Medtronic Confidential | CRT Feb 2019

Opportunities & learning for Medtronic Japan CONCLUSION Especially under different medical environments and options, the HBD Program is considered to be useful and of value in establishing the global study to the regulatory bodies, industries, academia and even to patients. Medtronic Confidential | CRT Feb 2019

 Medtronic harmony
Medtronic harmony Auricle of heart
Auricle of heart Papillary muscles sheep heart
Papillary muscles sheep heart Coronary sulcus
Coronary sulcus Pulmonary semilunar valve
Pulmonary semilunar valve Pulmonary valve
Pulmonary valve Pulmonary semilunar valve
Pulmonary semilunar valve Where is the pulmonary semilunar valve located
Where is the pulmonary semilunar valve located Servo valve types
Servo valve types Systemic artery
Systemic artery Medtronic supplier change portal
Medtronic supplier change portal Medtronic mpxr
Medtronic mpxr Viva quad xt
Viva quad xt Mpxr medtronic
Mpxr medtronic Correction factor insulin
Correction factor insulin Medtronic sofamor danek usa
Medtronic sofamor danek usa Brandcentral medtronic
Brandcentral medtronic Laura mauri medtronic
Laura mauri medtronic Structural heart medtronic
Structural heart medtronic Sextant medtronic
Sextant medtronic Medtronic pyramid
Medtronic pyramid Medtronic
Medtronic Sakrale neuromodulation medtronic
Sakrale neuromodulation medtronic Medtronic loop recorder lnq11
Medtronic loop recorder lnq11 Medtronic organizational chart
Medtronic organizational chart John wainwright medtronic
John wainwright medtronic