Literature Reporter Siyu Peng Supervisor Prof Yong Huang


- Slides: 41

Literature Reporter: Siyu Peng( 彭思宇 ) Supervisor: Prof. Yong Huang 2021/5/19

Acc. Chem. Res. 2015, 48, 986− 997 2

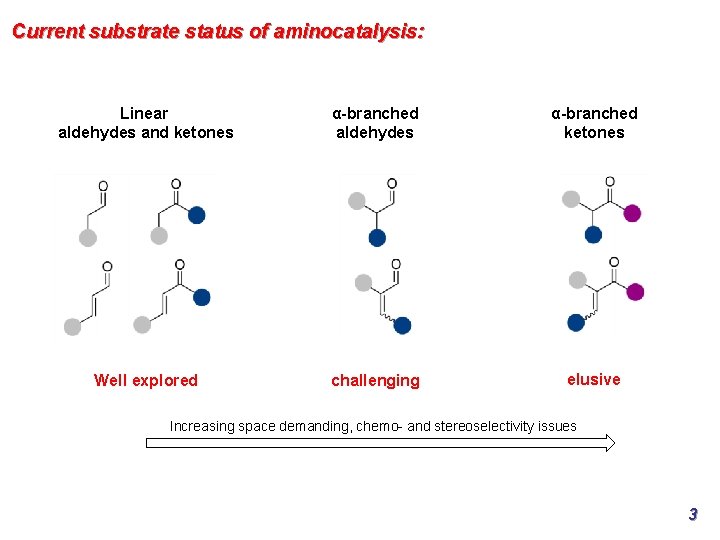
Current substrate status of aminocatalysis: Linear aldehydes and ketones α-branched aldehydes α-branched ketones Well explored challenging elusive Increasing space demanding, chemo- and stereoselectivity issues 3

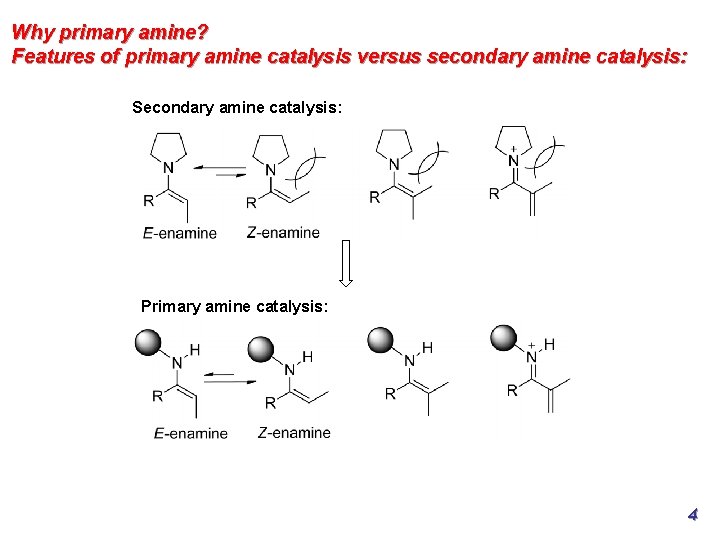
Why primary amine? Features of primary amine catalysis versus secondary amine catalysis: Secondary amine catalysis: Primary amine catalysis: 4

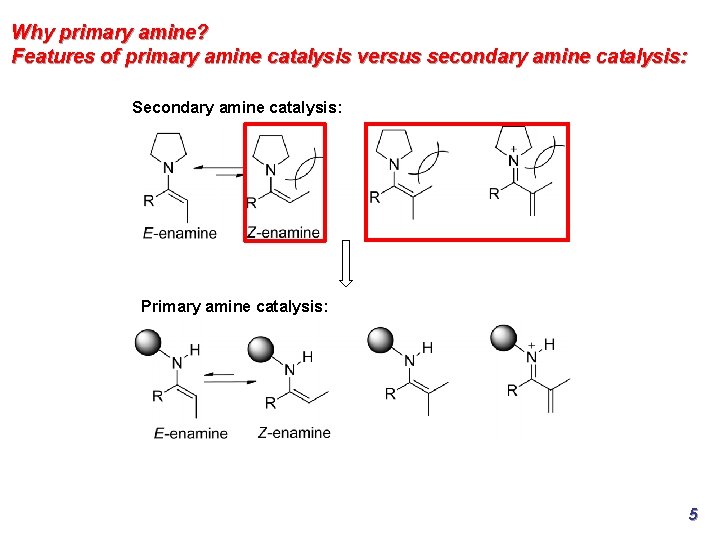
Why primary amine? Features of primary amine catalysis versus secondary amine catalysis: Secondary amine catalysis: Primary amine catalysis: 5

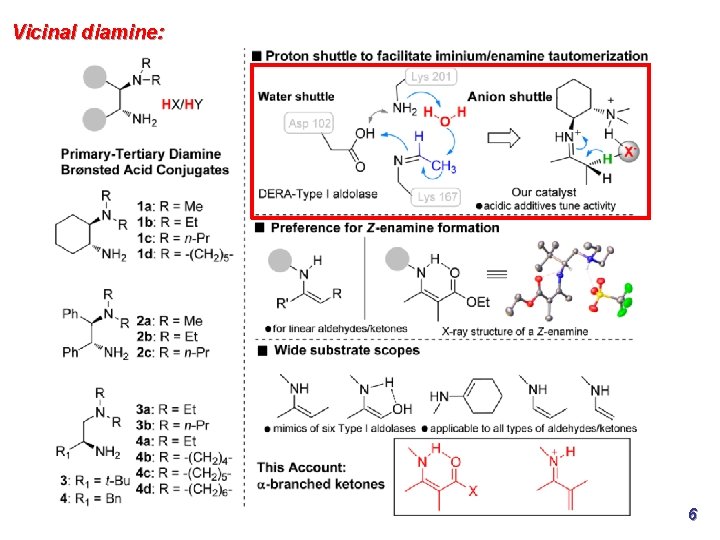
Vicinal diamine: 6

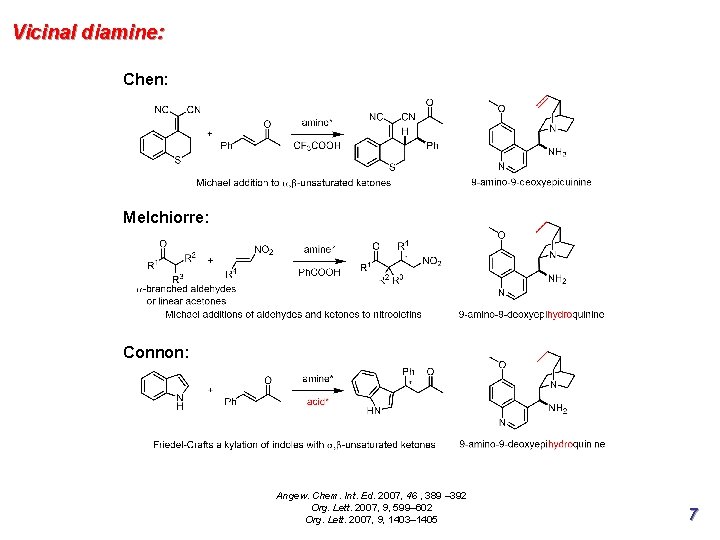
Vicinal diamine: Chen: Melchiorre: Connon: Angew. Chem. Int. Ed. 2007, 46 , 389 – 392 Org. Lett. 2007, 9, 599– 602 Org. Lett. 2007, 9, 1403– 1405 7

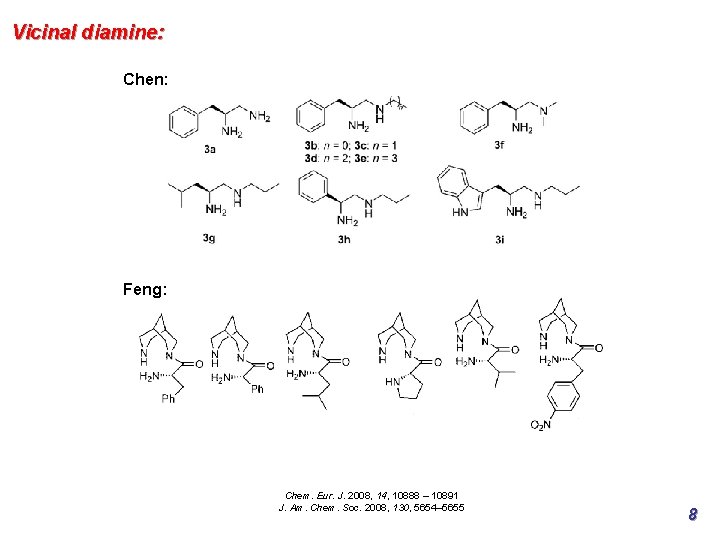
Vicinal diamine: Chen: Feng: Chem. Eur. J. 2008, 14, 10888 – 10891 J. Am. Chem. Soc. 2008, 130, 5654– 5655 8

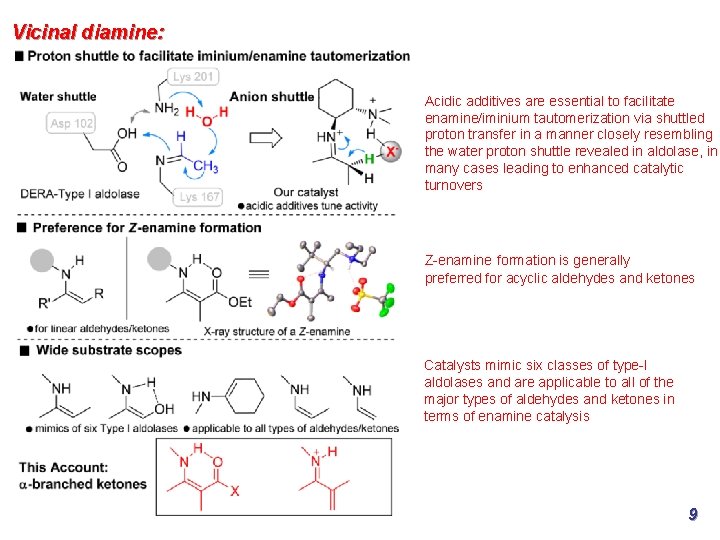
Vicinal diamine: Acidic additives are essential to facilitate enamine/iminium tautomerization via shuttled proton transfer in a manner closely resembling the water proton shuttle revealed in aldolase, in many cases leading to enhanced catalytic turnovers Z-enamine formation is generally preferred for acyclic aldehydes and ketones Catalysts mimic six classes of type-I aldolases and are applicable to all of the major types of aldehydes and ketones in terms of enamine catalysis 9

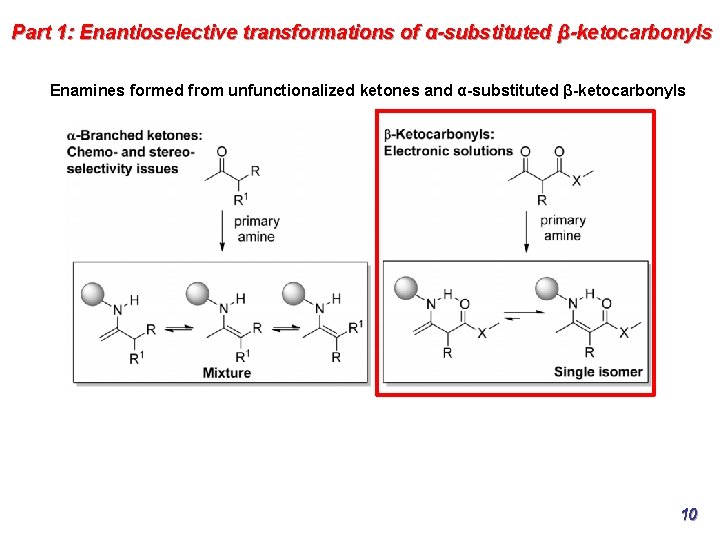
Part 1: Enantioselective transformations of α-substituted β-ketocarbonyls Enamines formed from unfunctionalized ketones and α-substituted β-ketocarbonyls 10

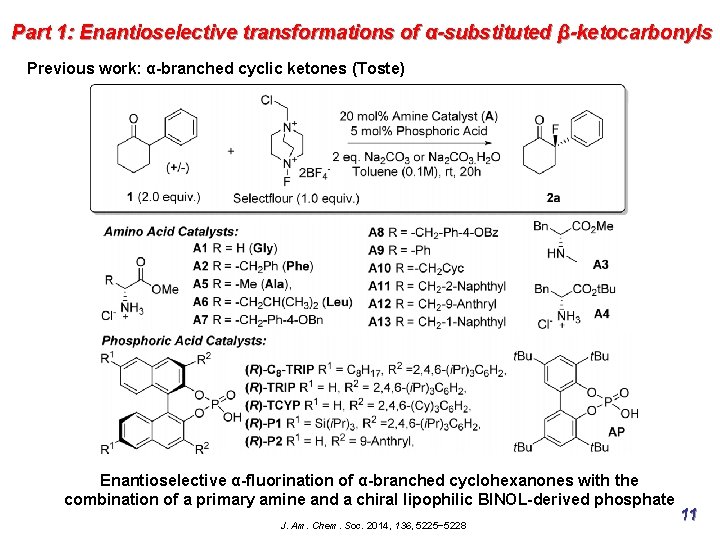
Part 1: Enantioselective transformations of α-substituted β-ketocarbonyls Previous work: α-branched cyclic ketones (Toste) Enantioselective α-fluorination of α-branched cyclohexanones with the combination of a primary amine and a chiral lipophilic BINOL-derived phosphate J. Am. Chem. Soc. 2014, 136, 5225− 5228 11

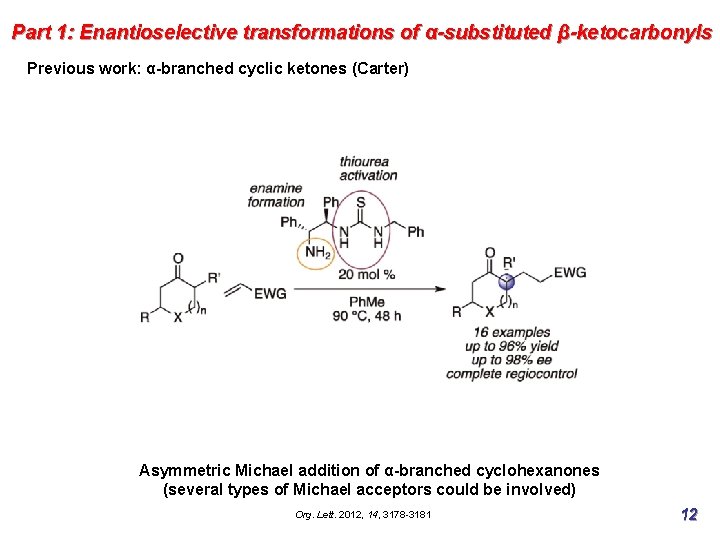
Part 1: Enantioselective transformations of α-substituted β-ketocarbonyls Previous work: α-branched cyclic ketones (Carter) Asymmetric Michael addition of α-branched cyclohexanones (several types of Michael acceptors could be involved) Org. Lett. 2012, 14, 3178 -3181 12

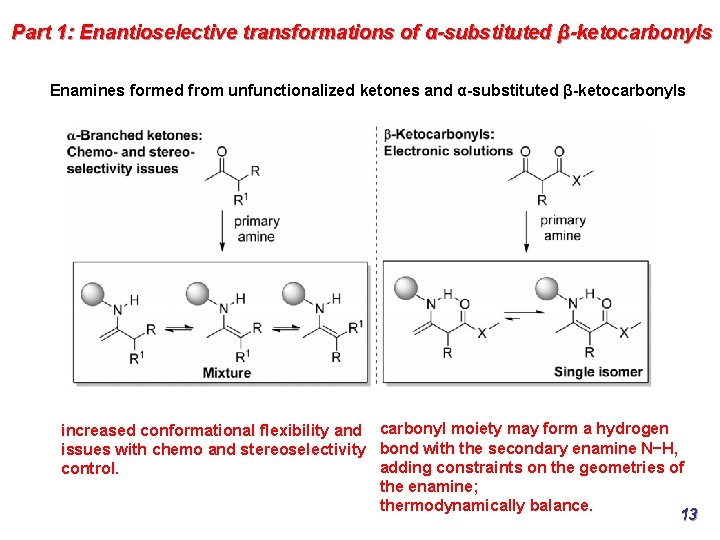
Part 1: Enantioselective transformations of α-substituted β-ketocarbonyls Enamines formed from unfunctionalized ketones and α-substituted β-ketocarbonyls increased conformational flexibility and carbonyl moiety may form a hydrogen issues with chemo and stereoselectivity bond with the secondary enamine N−H, adding constraints on the geometries of control. the enamine; thermodynamically balance. 13

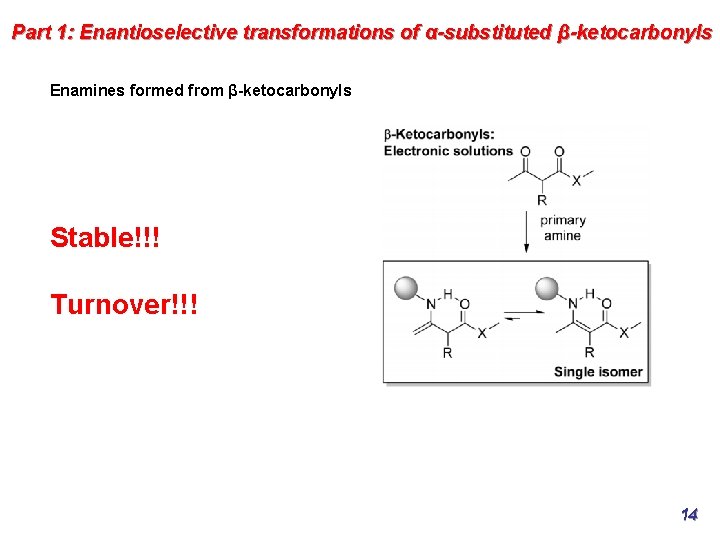
Part 1: Enantioselective transformations of α-substituted β-ketocarbonyls Enamines formed from β-ketocarbonyls Stable!!! Turnover!!! 14

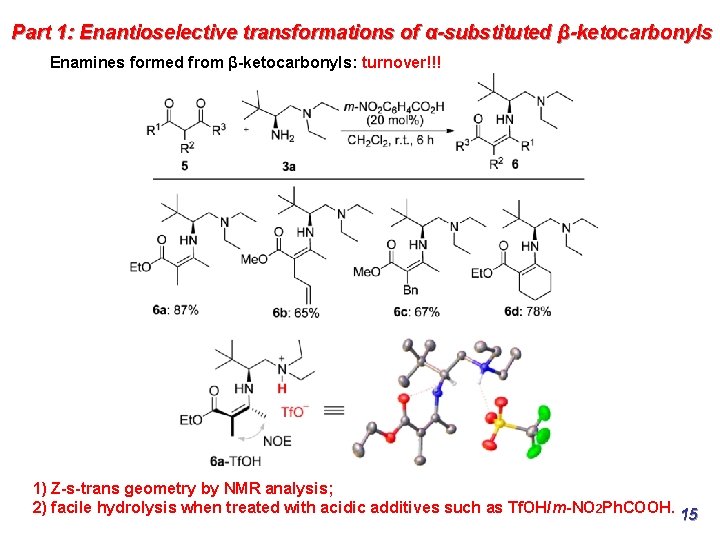
Part 1: Enantioselective transformations of α-substituted β-ketocarbonyls Enamines formed from β-ketocarbonyls: turnover!!! 1) Z-s-trans geometry by NMR analysis; 2) facile hydrolysis when treated with acidic additives such as Tf. OH/m-NO 2 Ph. COOH. 15

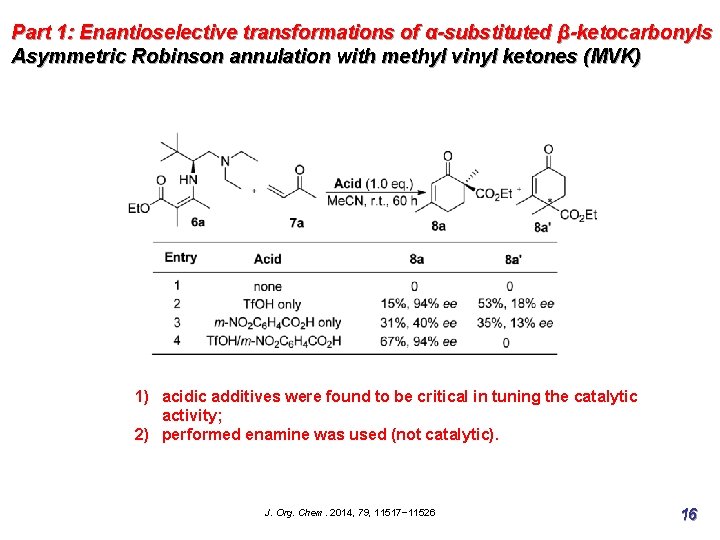
Part 1: Enantioselective transformations of α-substituted β-ketocarbonyls Asymmetric Robinson annulation with methyl vinyl ketones (MVK) 1) acidic additives were found to be critical in tuning the catalytic activity; 2) performed enamine was used (not catalytic). J. Org. Chem. 2014, 79, 11517− 11526 16

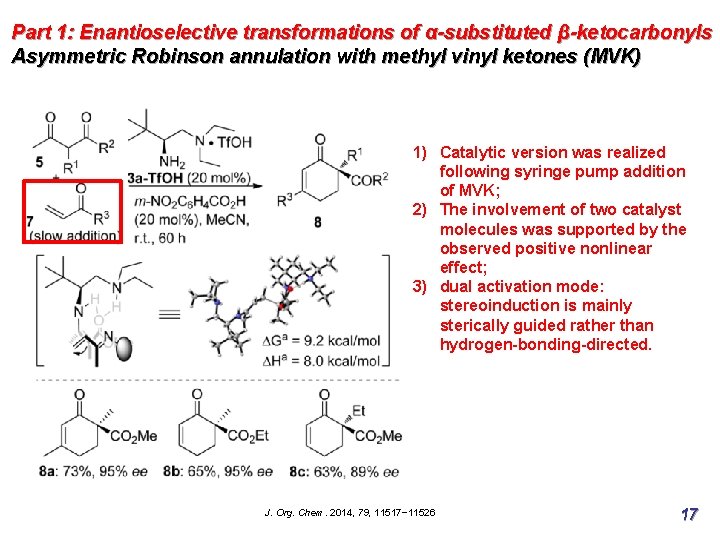
Part 1: Enantioselective transformations of α-substituted β-ketocarbonyls Asymmetric Robinson annulation with methyl vinyl ketones (MVK) 1) Catalytic version was realized following syringe pump addition of MVK; 2) The involvement of two catalyst molecules was supported by the observed positive nonlinear effect; 3) dual activation mode: stereoinduction is mainly sterically guided rather than hydrogen-bonding-directed. J. Org. Chem. 2014, 79, 11517− 11526 17

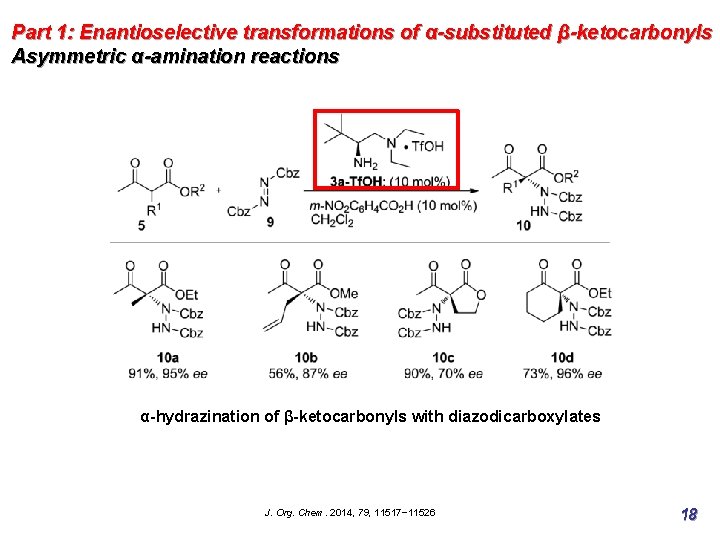
Part 1: Enantioselective transformations of α-substituted β-ketocarbonyls Asymmetric α-amination reactions α-hydrazination of β-ketocarbonyls with diazodicarboxylates J. Org. Chem. 2014, 79, 11517− 11526 18

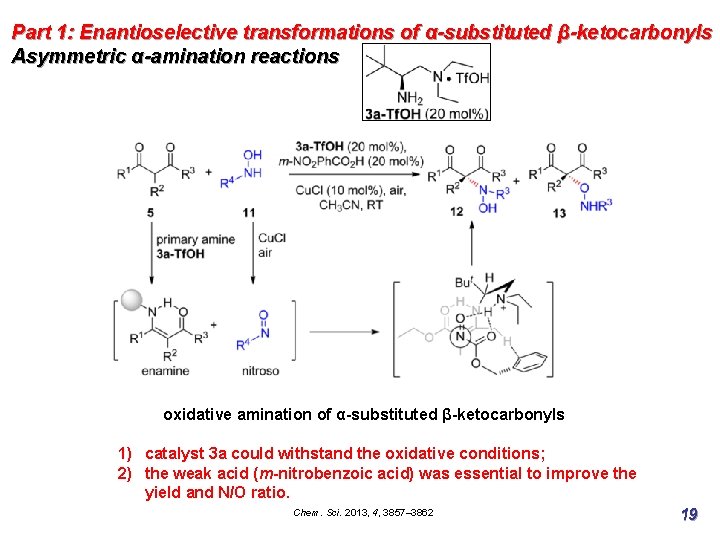
Part 1: Enantioselective transformations of α-substituted β-ketocarbonyls Asymmetric α-amination reactions oxidative amination of α-substituted β-ketocarbonyls 1) catalyst 3 a could withstand the oxidative conditions; 2) the weak acid (m-nitrobenzoic acid) was essential to improve the yield and N/O ratio. Chem. Sci. 2013, 4, 3857– 3862 19

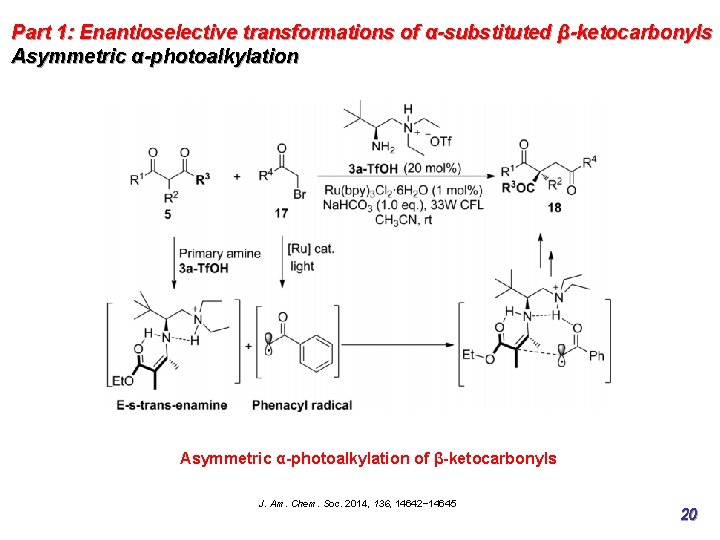
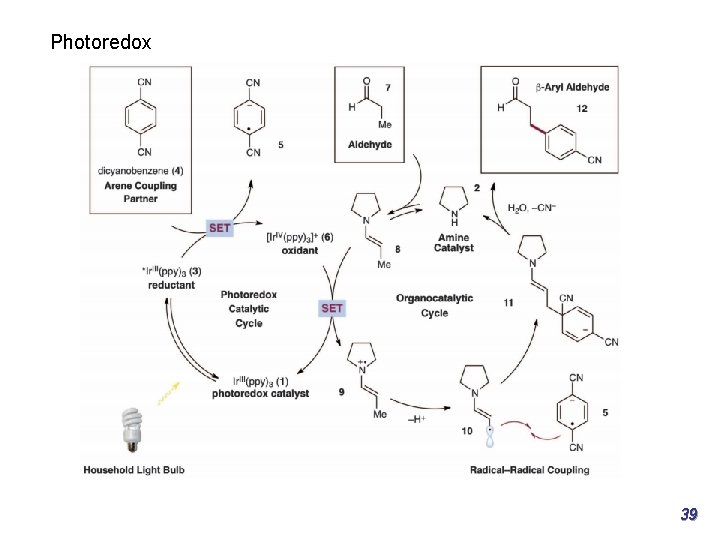
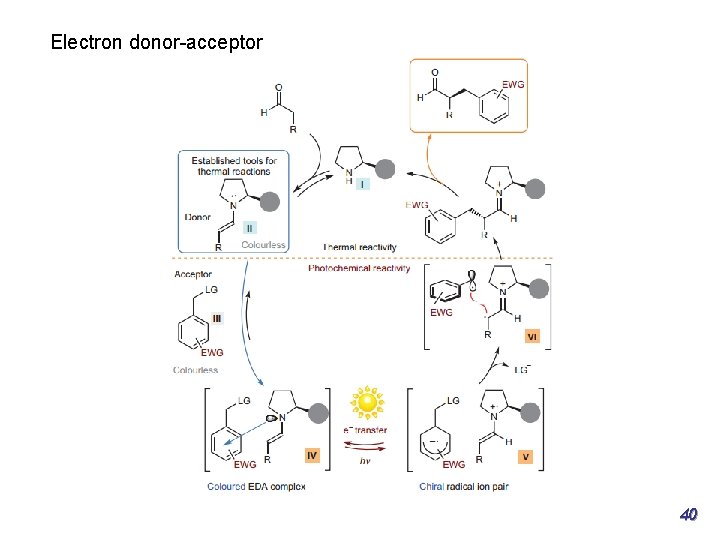
Part 1: Enantioselective transformations of α-substituted β-ketocarbonyls Asymmetric α-photoalkylation of β-ketocarbonyls J. Am. Chem. Soc. 2014, 136, 14642− 14645 20

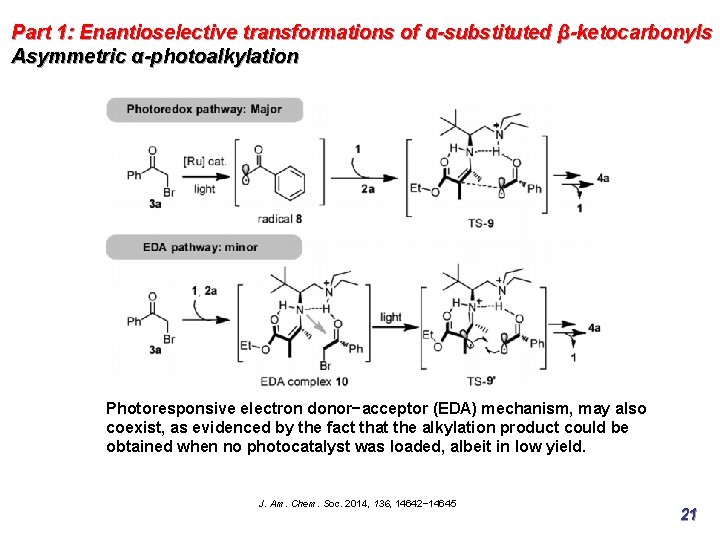
Part 1: Enantioselective transformations of α-substituted β-ketocarbonyls Asymmetric α-photoalkylation Photoresponsive electron donor−acceptor (EDA) mechanism, may also coexist, as evidenced by the fact that the alkylation product could be obtained when no photocatalyst was loaded, albeit in low yield. J. Am. Chem. Soc. 2014, 136, 14642− 14645 21

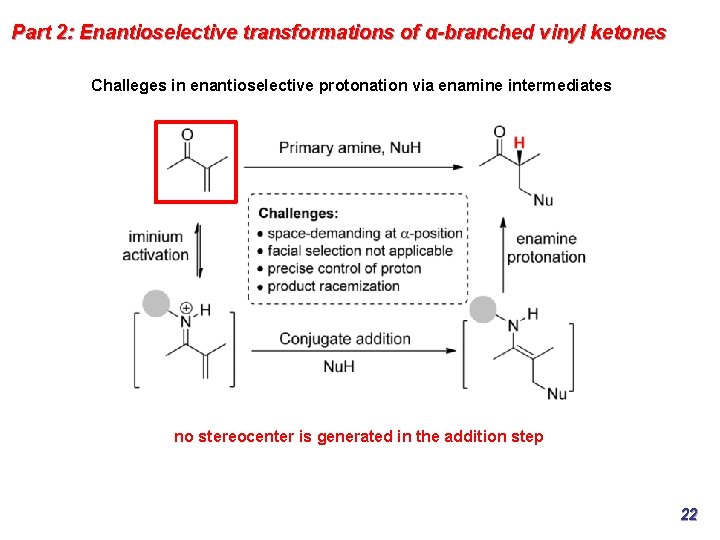
Part 2: Enantioselective transformations of α-branched vinyl ketones Challeges in enantioselective protonation via enamine intermediates no stereocenter is generated in the addition step 22

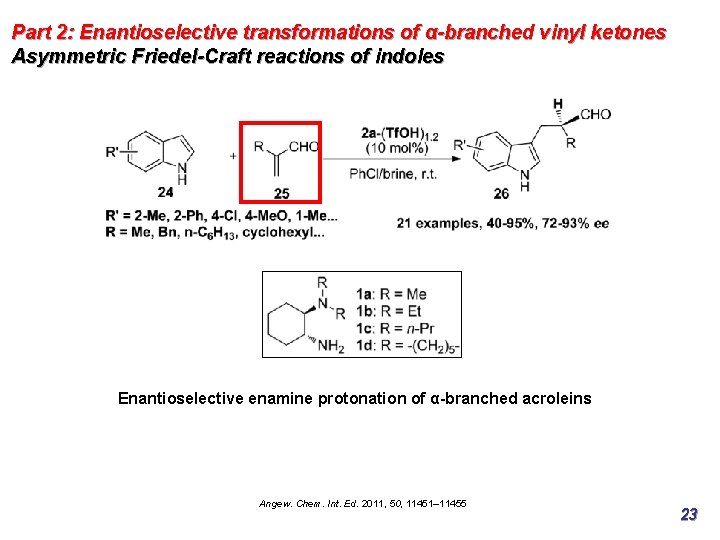
Part 2: Enantioselective transformations of α-branched vinyl ketones Asymmetric Friedel-Craft reactions of indoles Enantioselective enamine protonation of α-branched acroleins Angew. Chem. Int. Ed. 2011, 50, 11451– 11455 23

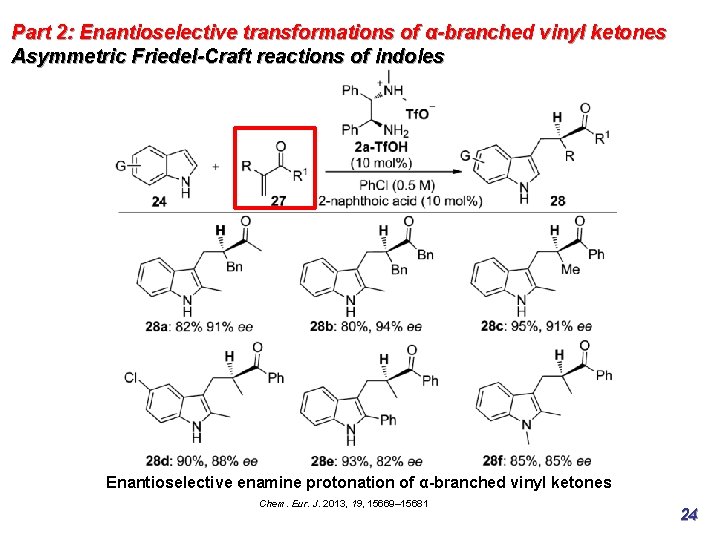
Part 2: Enantioselective transformations of α-branched vinyl ketones Asymmetric Friedel-Craft reactions of indoles Enantioselective enamine protonation of α-branched vinyl ketones Chem. Eur. J. 2013, 19, 15669– 15681 24

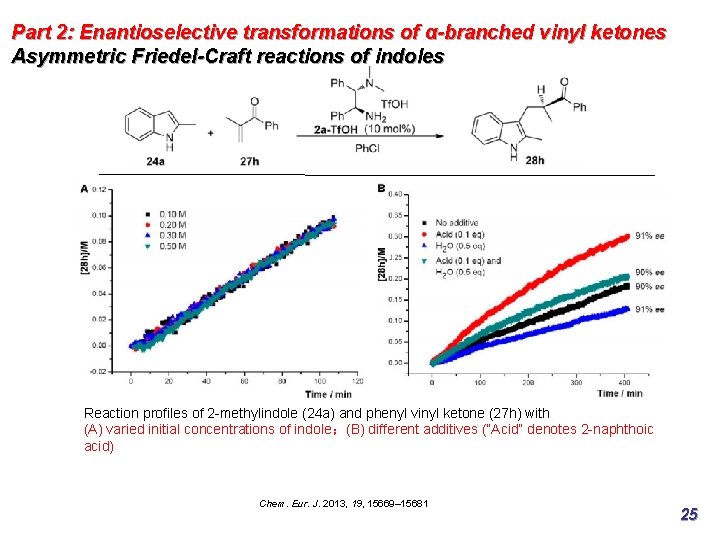
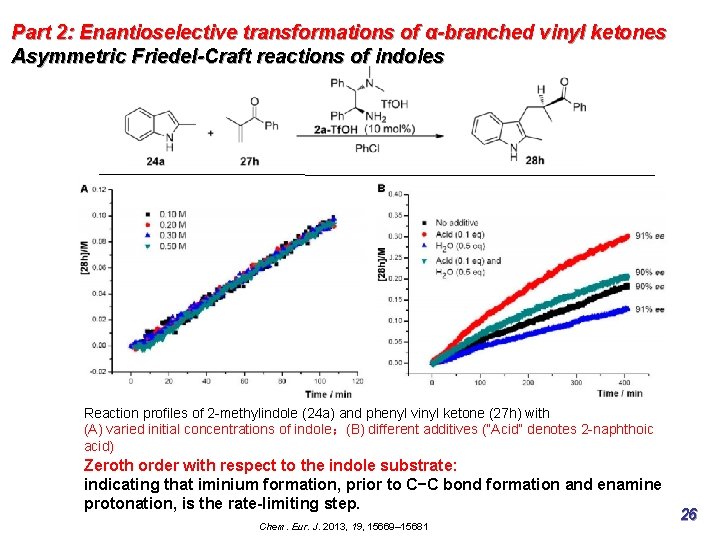
Part 2: Enantioselective transformations of α-branched vinyl ketones Asymmetric Friedel-Craft reactions of indoles Reaction profiles of 2 -methylindole (24 a) and phenyl vinyl ketone (27 h) with (A) varied initial concentrations of indole;(B) different additives (“Acid” denotes 2 -naphthoic acid) Chem. Eur. J. 2013, 19, 15669– 15681 25

Part 2: Enantioselective transformations of α-branched vinyl ketones Asymmetric Friedel-Craft reactions of indoles Reaction profiles of 2 -methylindole (24 a) and phenyl vinyl ketone (27 h) with (A) varied initial concentrations of indole;(B) different additives (“Acid” denotes 2 -naphthoic acid) Zeroth order with respect to the indole substrate: indicating that iminium formation, prior to C−C bond formation and enamine protonation, is the rate-limiting step. Chem. Eur. J. 2013, 19, 15669– 15681 26

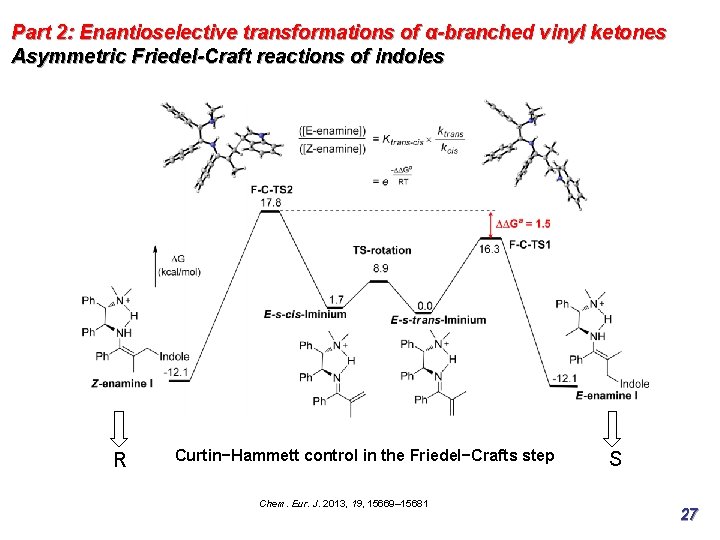
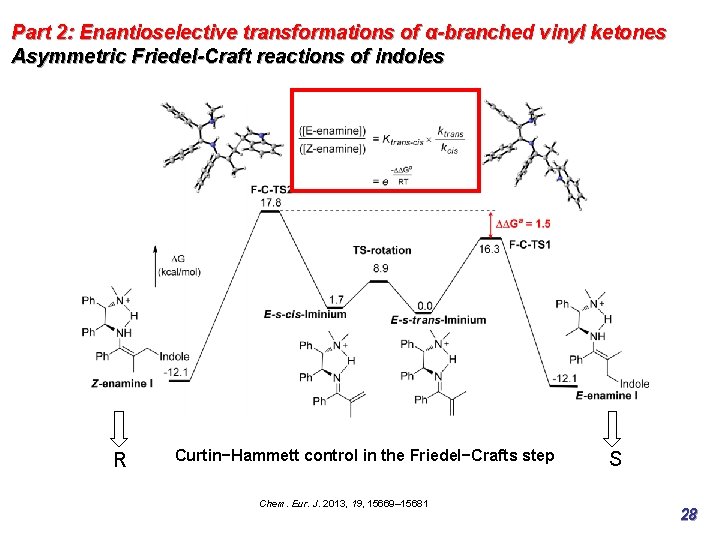
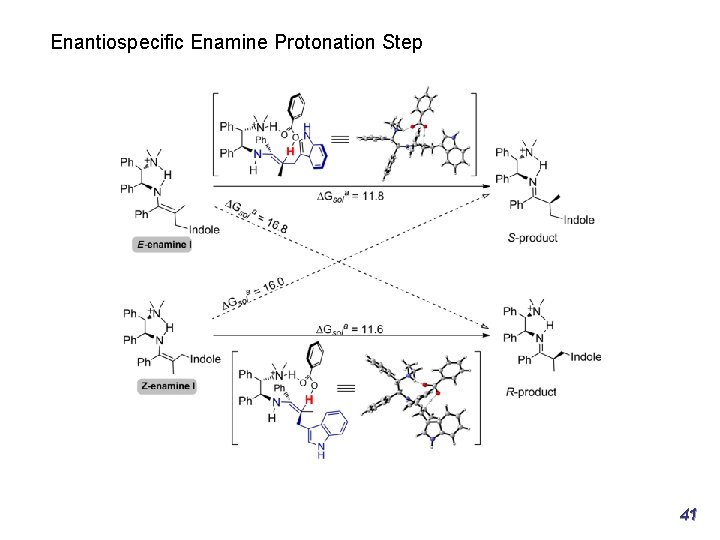
Part 2: Enantioselective transformations of α-branched vinyl ketones Asymmetric Friedel-Craft reactions of indoles R Curtin−Hammett control in the Friedel−Crafts step Chem. Eur. J. 2013, 19, 15669– 15681 S 27

Part 2: Enantioselective transformations of α-branched vinyl ketones Asymmetric Friedel-Craft reactions of indoles R Curtin−Hammett control in the Friedel−Crafts step Chem. Eur. J. 2013, 19, 15669– 15681 S 28

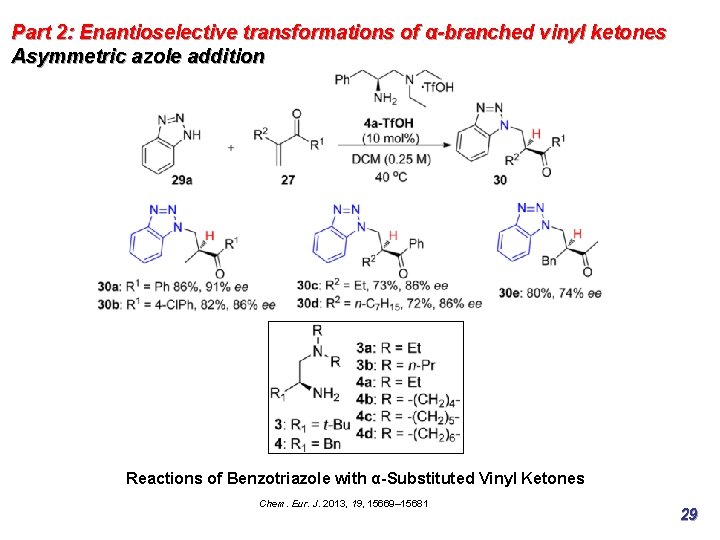
Part 2: Enantioselective transformations of α-branched vinyl ketones Asymmetric azole addition Reactions of Benzotriazole with α-Substituted Vinyl Ketones Chem. Eur. J. 2013, 19, 15669– 15681 29

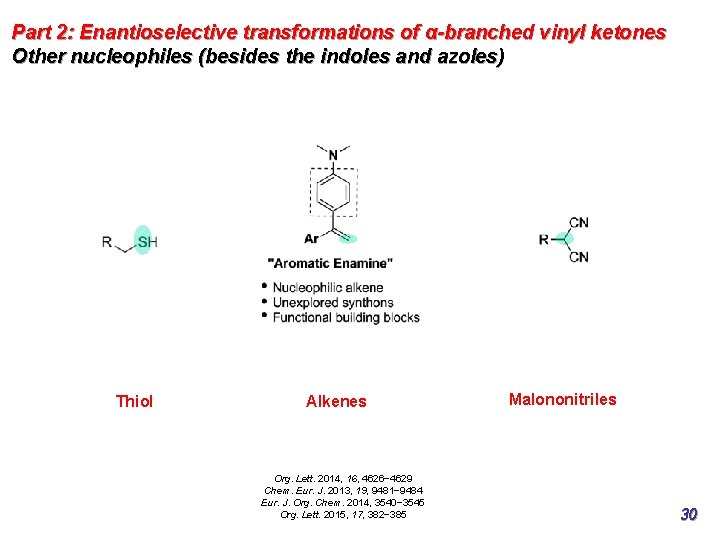
Part 2: Enantioselective transformations of α-branched vinyl ketones Other nucleophiles (besides the indoles and azoles) Thiol Alkenes Org. Lett. 2014, 16, 4626− 4629 Chem. Eur. J. 2013, 19, 9481− 9484 Eur. J. Org. Chem. 2014, 3540− 3545 Org. Lett. 2015, 17, 382− 385 Malononitriles 30

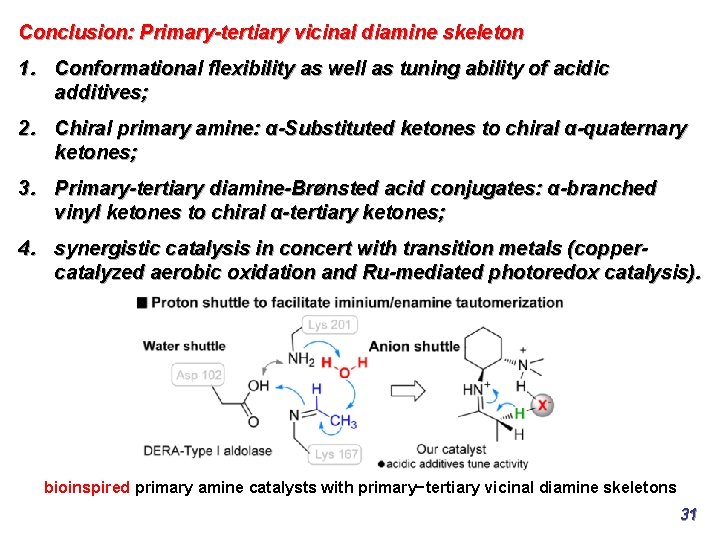
Conclusion: Primary-tertiary vicinal diamine skeleton 1. Conformational flexibility as well as tuning ability of acidic additives; 2. Chiral primary amine: α-Substituted ketones to chiral α-quaternary ketones; 3. Primary-tertiary diamine-Brønsted acid conjugates: α-branched vinyl ketones to chiral α-tertiary ketones; 4. synergistic catalysis in concert with transition metals (coppercatalyzed aerobic oxidation and Ru-mediated photoredox catalysis). bioinspired primary amine catalysts with primary−tertiary vicinal diamine skeletons 31

Conclusion: Primary-tertiary vicinal diamine skeleton 1. Conformational flexibility as well as tuning ability of acidic additives; 2. Chiral primary amine: α-Substituted ketones to chiral α-quaternary ketones; 3. Primary-tertiary diamine-Brønsted acid conjugates: α-branched vinyl ketones to chiral α-tertiary ketones; 4. synergistic catalysis in concert with transition metals (coppercatalyzed aerobic oxidation and Ru-mediated photoredox catalysis). Acidic additives are essential to facilitate enamine/iminium tautomerization via shuttled proton transfer 32

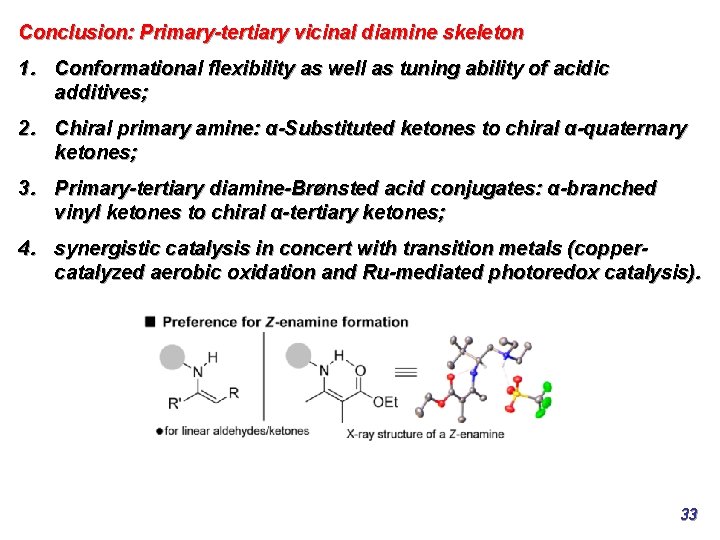
Conclusion: Primary-tertiary vicinal diamine skeleton 1. Conformational flexibility as well as tuning ability of acidic additives; 2. Chiral primary amine: α-Substituted ketones to chiral α-quaternary ketones; 3. Primary-tertiary diamine-Brønsted acid conjugates: α-branched vinyl ketones to chiral α-tertiary ketones; 4. synergistic catalysis in concert with transition metals (coppercatalyzed aerobic oxidation and Ru-mediated photoredox catalysis). 33

THANK YOU!!! 34

35

36

37

38

Photoredox 39

Electron donor-acceptor 40

Enantiospecific Enamine Protonation Step 41
 Herbalife qualifying supervisor vs supervisor
Herbalife qualifying supervisor vs supervisor Yong hoon kim
Yong hoon kim Ban yong
Ban yong Aaron tan tuck choy
Aaron tan tuck choy Cerita salmiah 11
Cerita salmiah 11 Kwon ji yong
Kwon ji yong Sql yong
Sql yong Yong lei
Yong lei Gestalt
Gestalt Yong loo lin school of medicine
Yong loo lin school of medicine Yong lei
Yong lei Sql query
Sql query Yong soon min
Yong soon min Cast of spring, summer, fall, winter... and spring
Cast of spring, summer, fall, winter... and spring Mis yong
Mis yong Yong ung kai v enting
Yong ung kai v enting Yong liu aalto
Yong liu aalto Yong mok hin
Yong mok hin K
K Peng
Peng Peng-robinson equation of state
Peng-robinson equation of state Daniel propson
Daniel propson Bo peng kaggle
Bo peng kaggle Memudaratkan
Memudaratkan ютуб
ютуб Peng
Peng Peng
Peng Peng-robinson equation of state
Peng-robinson equation of state Helen peng
Helen peng Peng
Peng Mindy peng
Mindy peng Stable prediction across unknown environments
Stable prediction across unknown environments Wang peng and li you
Wang peng and li you Zhigang peng
Zhigang peng Peng robinson equation of state
Peng robinson equation of state Peng wang cheese tofu calories
Peng wang cheese tofu calories Peng duhai
Peng duhai Helen peng age
Helen peng age Can mermaid escape love
Can mermaid escape love Chunyi peng
Chunyi peng Peng
Peng Peng
Peng