Group Meeting Reporter Man Li Supervisor Prof Mo


- Slides: 39

Group Meeting Reporter: Man Li Supervisor: Prof. Mo Date: 2016 -09 -30

Contents F. Glorius et al. , Angew. Chem. Int. Ed. , 2012, 51, 10236 -10254

Introduction F. Glorius et al. , Angew. Chem. Int. Ed. , 2012, 51, 10236 -10254

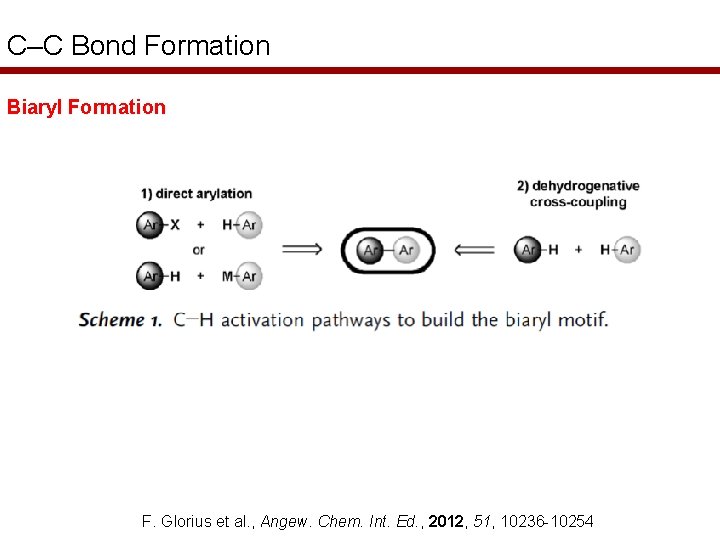
C–C Bond Formation Biaryl Formation F. Glorius et al. , Angew. Chem. Int. Ed. , 2012, 51, 10236 -10254

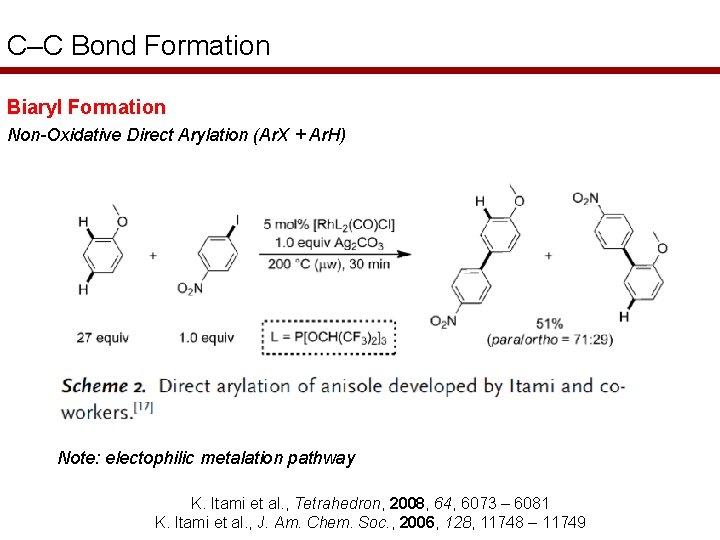
C–C Bond Formation Biaryl Formation Non-Oxidative Direct Arylation (Ar. X + Ar. H) Note: electophilic metalation pathway K. Itami et al. , Tetrahedron, 2008, 64, 6073 – 6081 K. Itami et al. , J. Am. Chem. Soc. , 2006, 128, 11748 – 11749

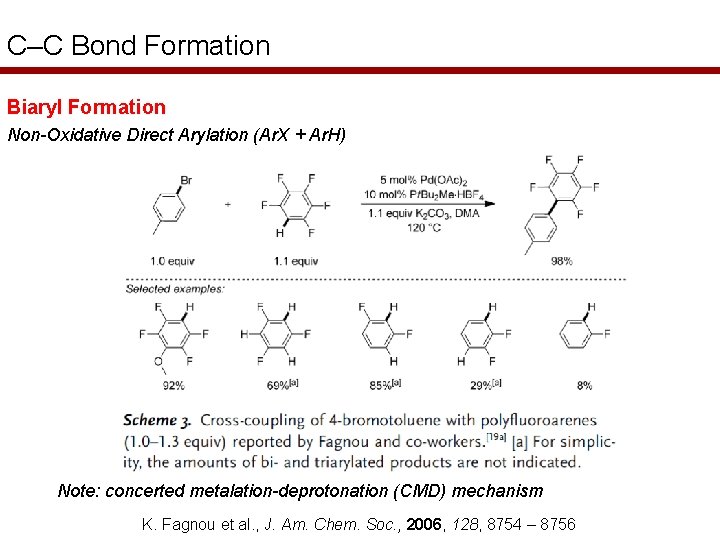
C–C Bond Formation Biaryl Formation Non-Oxidative Direct Arylation (Ar. X + Ar. H) Note: concerted metalation-deprotonation (CMD) mechanism K. Fagnou et al. , J. Am. Chem. Soc. , 2006, 128, 8754 – 8756

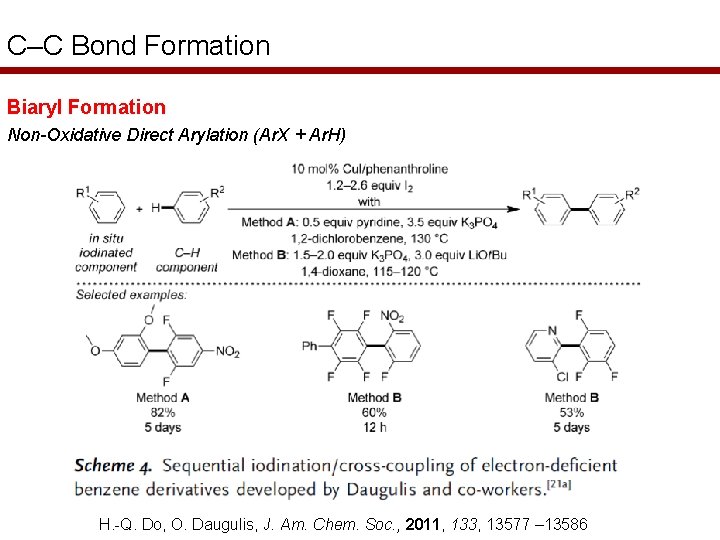
C–C Bond Formation Biaryl Formation Non-Oxidative Direct Arylation (Ar. X + Ar. H) H. -Q. Do, O. Daugulis, J. Am. Chem. Soc. , 2011, 133, 13577 – 13586

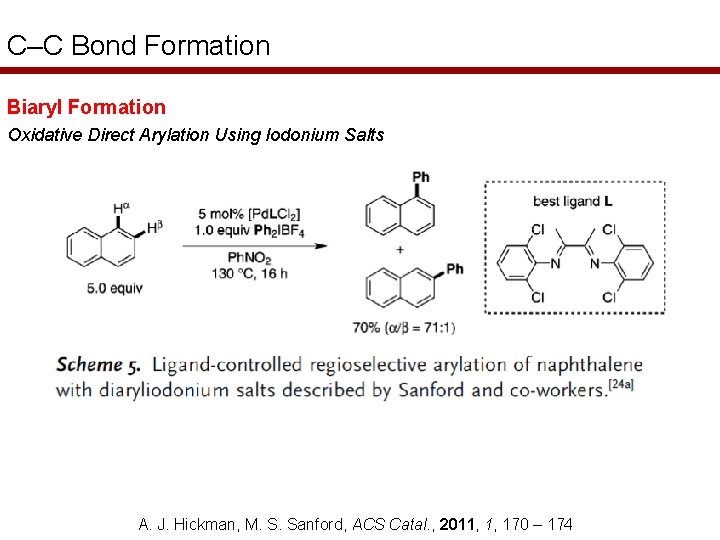
C–C Bond Formation Biaryl Formation Oxidative Direct Arylation Using Iodonium Salts A. J. Hickman, M. S. Sanford, ACS Catal. , 2011, 1, 170 – 174

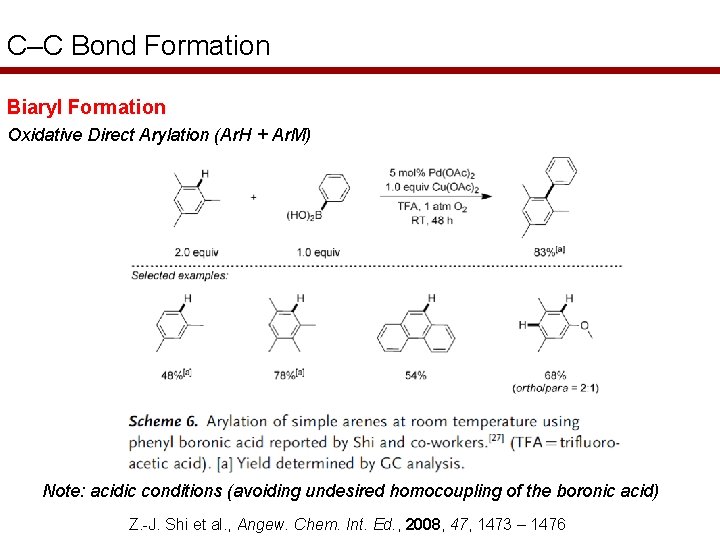
C–C Bond Formation Biaryl Formation Oxidative Direct Arylation (Ar. H + Ar. M) Note: acidic conditions (avoiding undesired homocoupling of the boronic acid) Z. -J. Shi et al. , Angew. Chem. Int. Ed. , 2008, 47, 1473 – 1476

C–C Bond Formation Biaryl Formation Cross-Dehydrogenative Couplings (CDC) W. Lu et al. , Organometallics, 2006, 25, 5973 – 5975

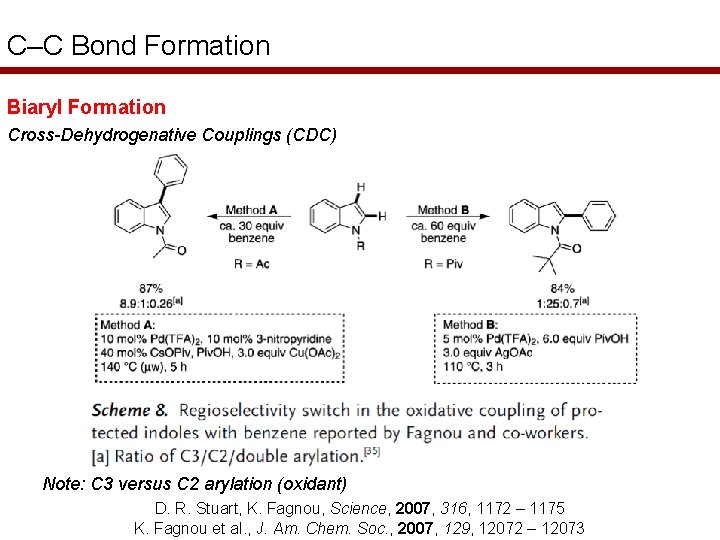
C–C Bond Formation Biaryl Formation Cross-Dehydrogenative Couplings (CDC) Note: C 3 versus C 2 arylation (oxidant) D. R. Stuart, K. Fagnou, Science, 2007, 316, 1172 – 1175 K. Fagnou et al. , J. Am. Chem. Soc. , 2007, 129, 12072 – 12073

C–C Bond Formation Biaryl Formation Cross-Dehydrogenative Couplings (CDC) Note: Regioselectivity was mainly controlled by steric factors. Y. Wei, W. Su, J. Am. Chem. Soc. , 2010, 132, 16377 – 16379

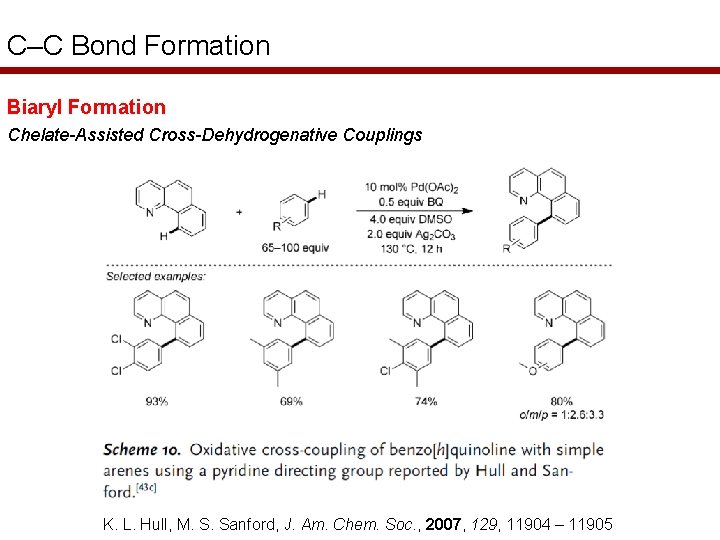
C–C Bond Formation Biaryl Formation Chelate-Assisted Cross-Dehydrogenative Couplings K. L. Hull, M. S. Sanford, J. Am. Chem. Soc. , 2007, 129, 11904 – 11905

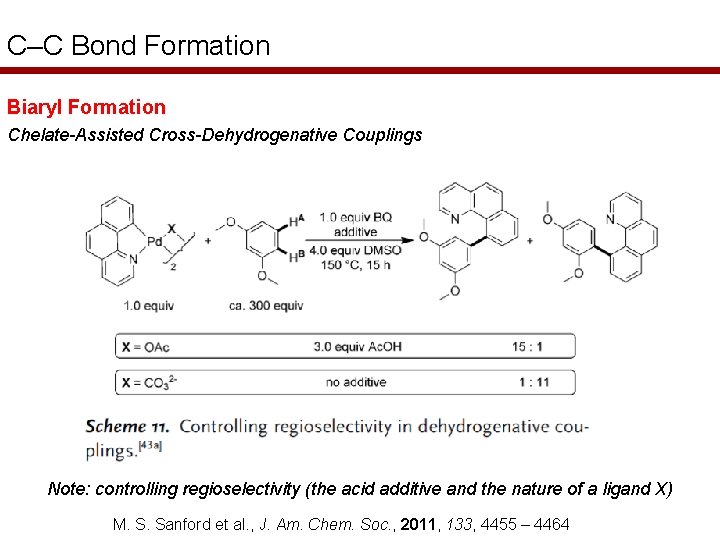
C–C Bond Formation Biaryl Formation Chelate-Assisted Cross-Dehydrogenative Couplings Note: controlling regioselectivity (the acid additive and the nature of a ligand X) M. S. Sanford et al. , J. Am. Chem. Soc. , 2011, 133, 4455 – 4464

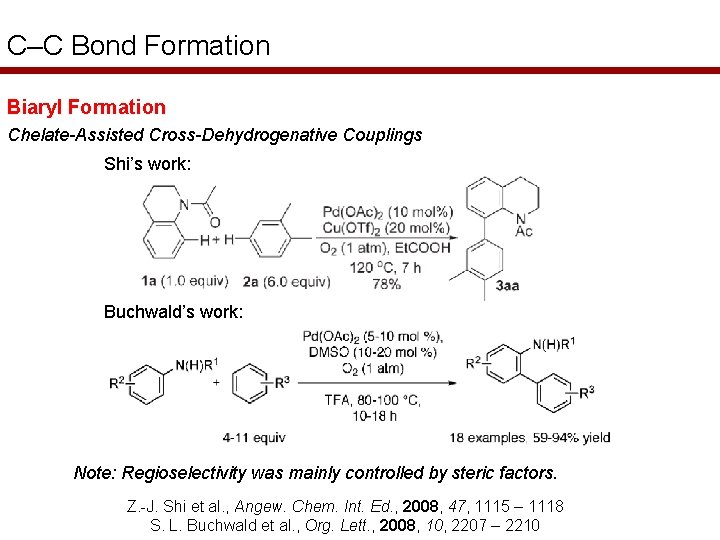
C–C Bond Formation Biaryl Formation Chelate-Assisted Cross-Dehydrogenative Couplings Shi’s work: Buchwald’s work: Note: Regioselectivity was mainly controlled by steric factors. Z. -J. Shi et al. , Angew. Chem. Int. Ed. , 2008, 47, 1115 – 1118 S. L. Buchwald et al. , Org. Lett. , 2008, 10, 2207 – 2210

C–C Bond Formation Biaryl Formation Chelate-Assisted Cross-Dehydrogenative Couplings V. M. Dong et al. , J. Am. Chem. Soc. , 2010, 132, 5837 – 5844 V. M. Dong et al. , Chem. Sci. , 2010, 1, 331 – 336

C–C Bond Formation Biaryl Formation Chelate-Assisted Cross-Dehydrogenative Couplings Note: NFSI (F+ reagents ) was superior with regard to reactivity and para selectivity; electrophilic palladation mechanism was proposed. J. -Q. Yu et al. , J. Am. Chem. Soc. , 2011, 133, 13864 – 13867

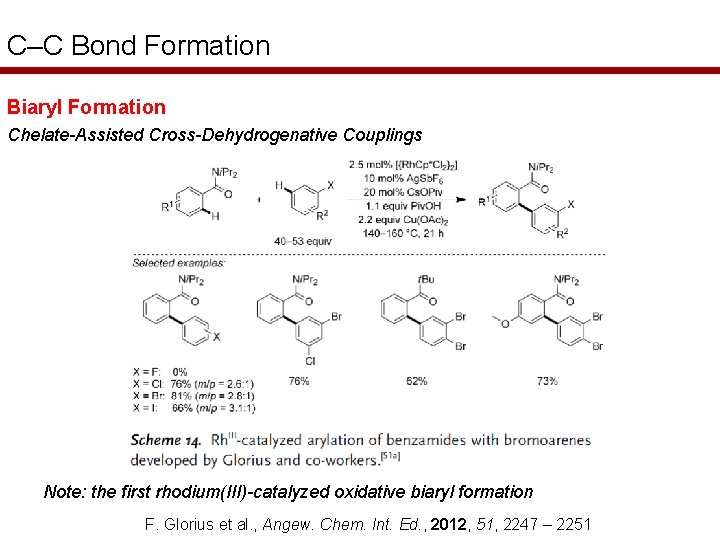
C–C Bond Formation Biaryl Formation Chelate-Assisted Cross-Dehydrogenative Couplings Note: the first rhodium(III)-catalyzed oxidative biaryl formation F. Glorius et al. , Angew. Chem. Int. Ed. , 2012, 51, 2247 – 2251

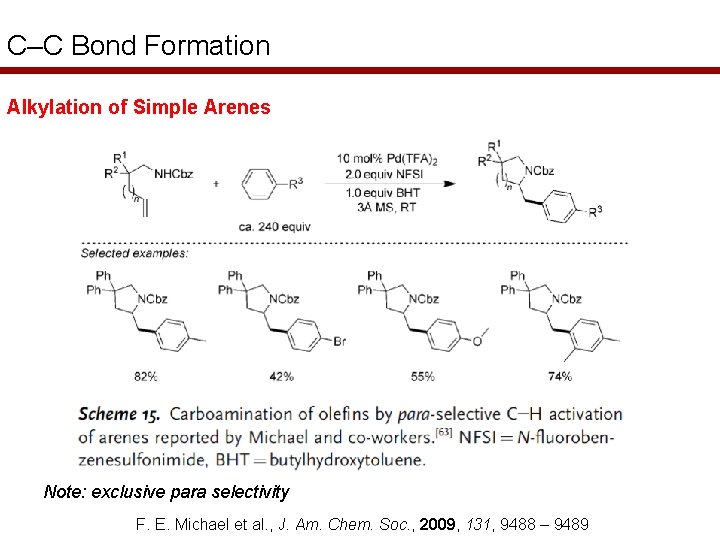
C–C Bond Formation Alkylation of Simple Arenes Note: exclusive para selectivity F. E. Michael et al. , J. Am. Chem. Soc. , 2009, 131, 9488 – 9489

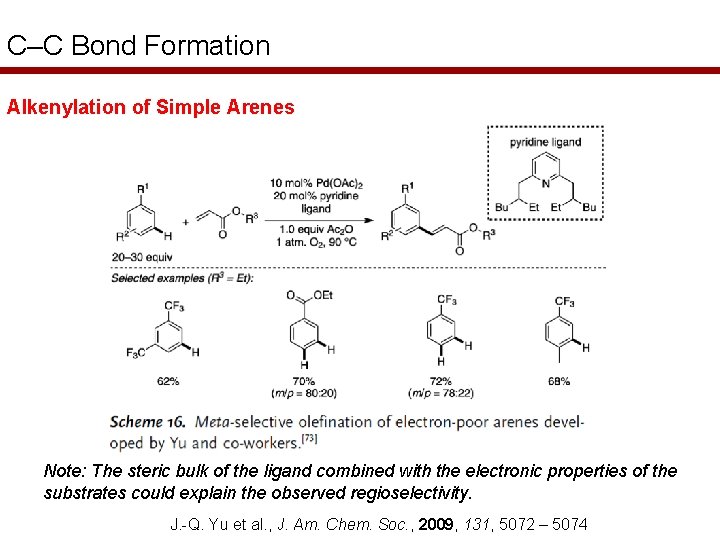
C–C Bond Formation Alkenylation of Simple Arenes Note: The steric bulk of the ligand combined with the electronic properties of the substrates could explain the observed regioselectivity. J. -Q. Yu et al. , J. Am. Chem. Soc. , 2009, 131, 5072 – 5074

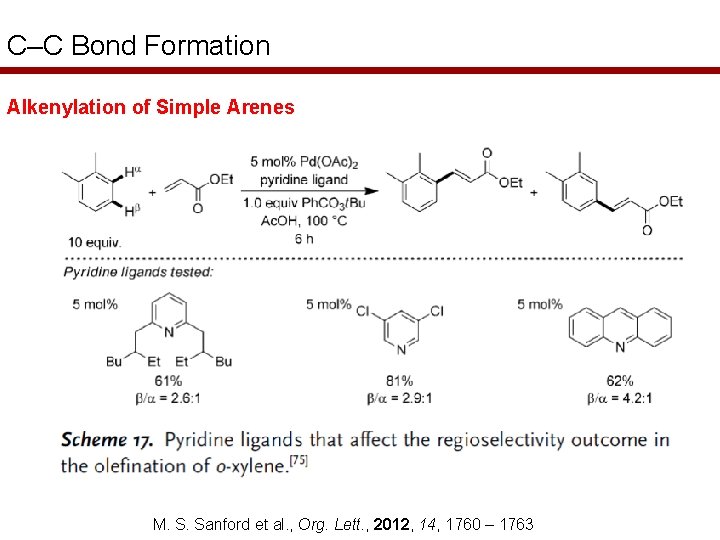
C–C Bond Formation Alkenylation of Simple Arenes M. S. Sanford et al. , Org. Lett. , 2012, 14, 1760 – 1763

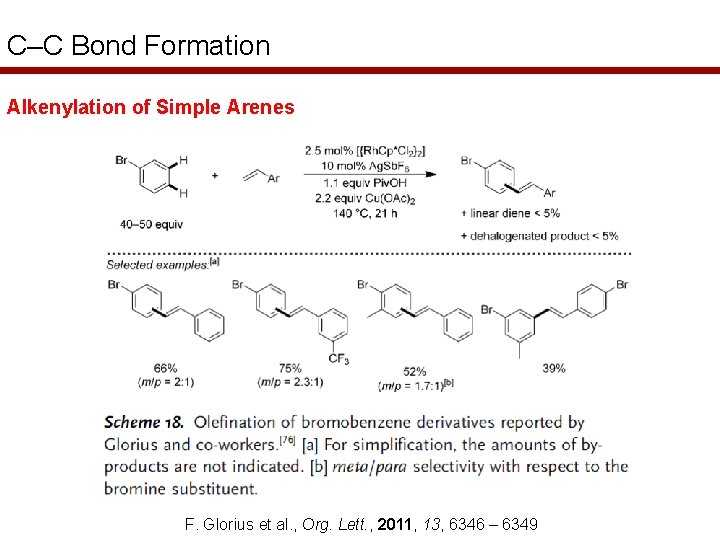
C–C Bond Formation Alkenylation of Simple Arenes F. Glorius et al. , Org. Lett. , 2011, 13, 6346 – 6349

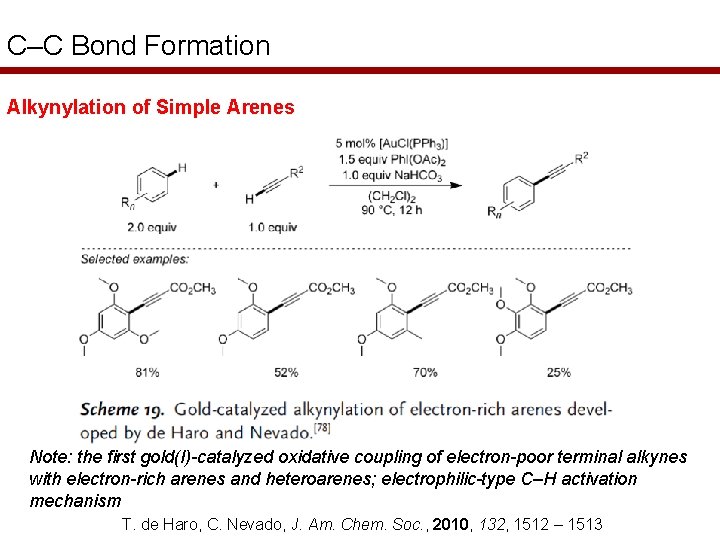
C–C Bond Formation Alkynylation of Simple Arenes Note: the first gold(I)-catalyzed oxidative coupling of electron-poor terminal alkynes with electron-rich arenes and heteroarenes; electrophilic-type C–H activation mechanism T. de Haro, C. Nevado, J. Am. Chem. Soc. , 2010, 132, 1512 – 1513

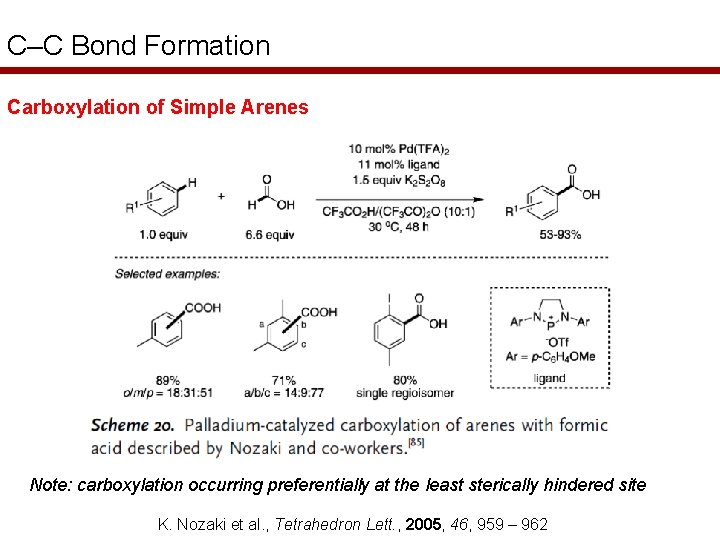
C–C Bond Formation Carboxylation of Simple Arenes Note: carboxylation occurring preferentially at the least sterically hindered site K. Nozaki et al. , Tetrahedron Lett. , 2005, 46, 959 – 962

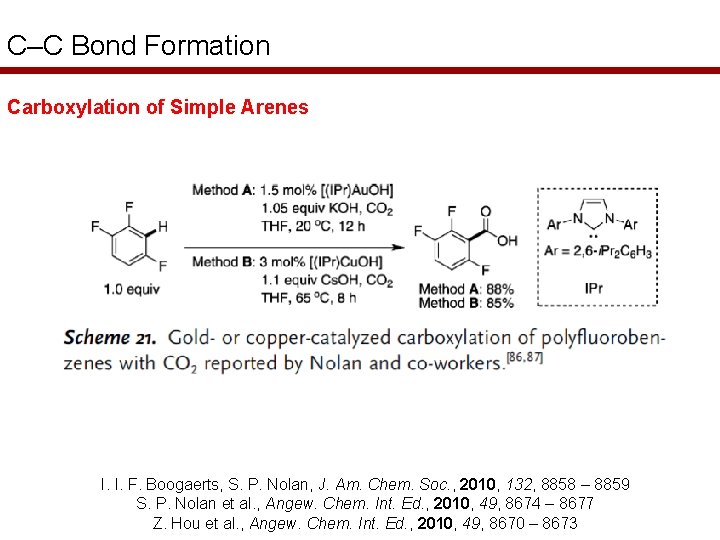
C–C Bond Formation Carboxylation of Simple Arenes I. I. F. Boogaerts, S. P. Nolan, J. Am. Chem. Soc. , 2010, 132, 8858 – 8859 S. P. Nolan et al. , Angew. Chem. Int. Ed. , 2010, 49, 8674 – 8677 Z. Hou et al. , Angew. Chem. Int. Ed. , 2010, 49, 8670 – 8673

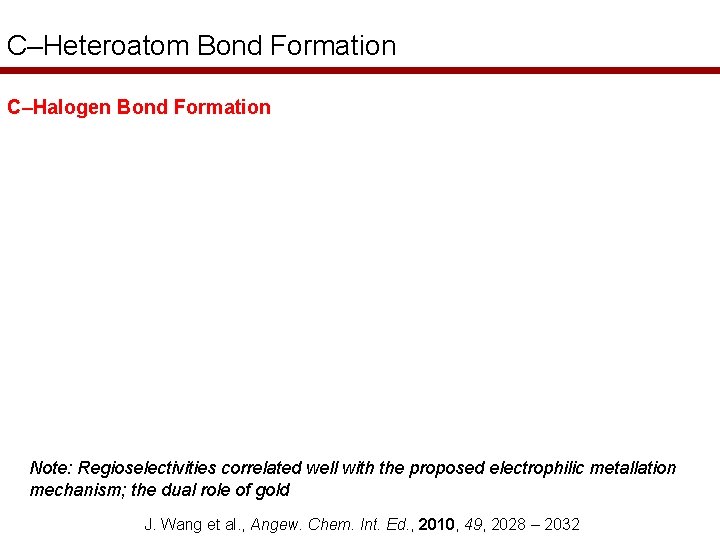
C–Heteroatom Bond Formation C–Halogen Bond Formation Note: Regioselectivities correlated well with the proposed electrophilic metallation mechanism; the dual role of gold J. Wang et al. , Angew. Chem. Int. Ed. , 2010, 49, 2028 – 2032

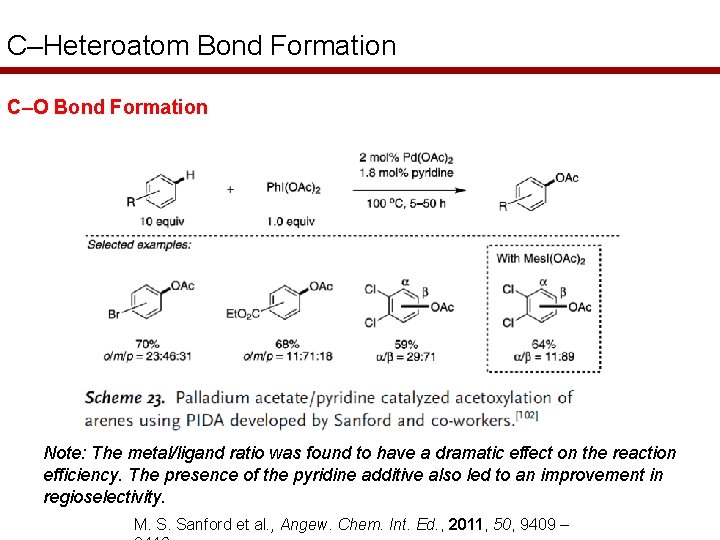
C–Heteroatom Bond Formation C–O Bond Formation Note: The metal/ligand ratio was found to have a dramatic effect on the reaction efficiency. The presence of the pyridine additive also led to an improvement in regioselectivity. M. S. Sanford et al. , Angew. Chem. Int. Ed. , 2011, 50, 9409 –

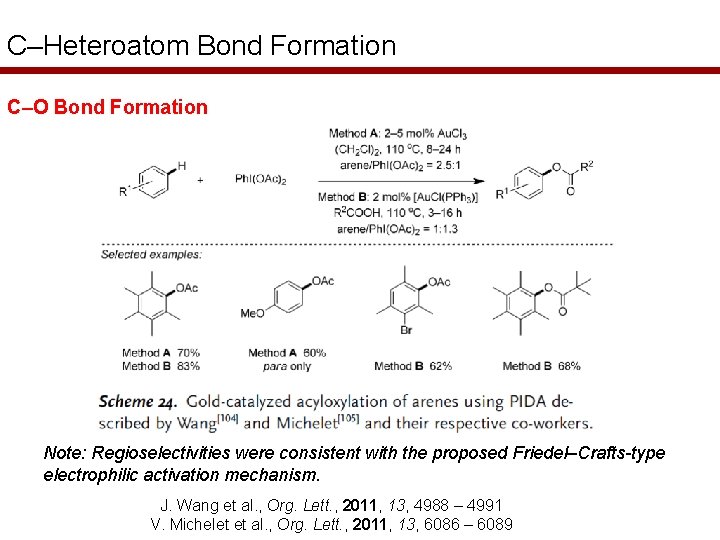
C–Heteroatom Bond Formation C–O Bond Formation Note: Regioselectivities were consistent with the proposed Friedel–Crafts-type electrophilic activation mechanism. J. Wang et al. , Org. Lett. , 2011, 13, 4988 – 4991 V. Michelet et al. , Org. Lett. , 2011, 13, 6086 – 6089

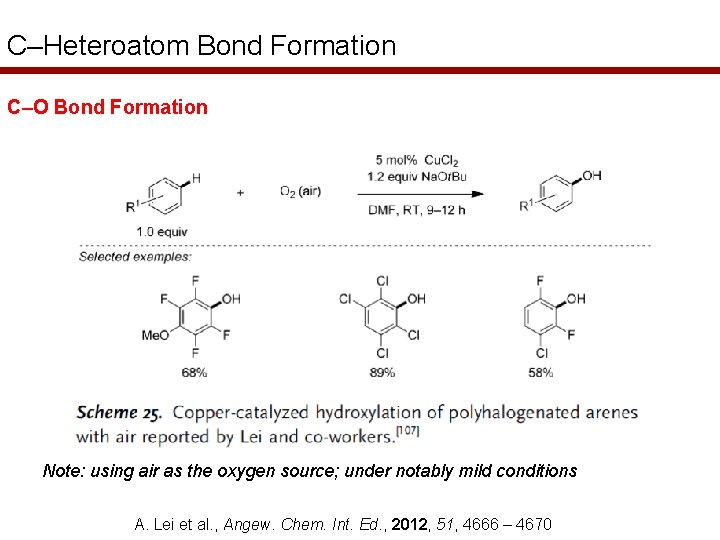
C–Heteroatom Bond Formation C–O Bond Formation Note: using air as the oxygen source; under notably mild conditions A. Lei et al. , Angew. Chem. Int. Ed. , 2012, 51, 4666 – 4670

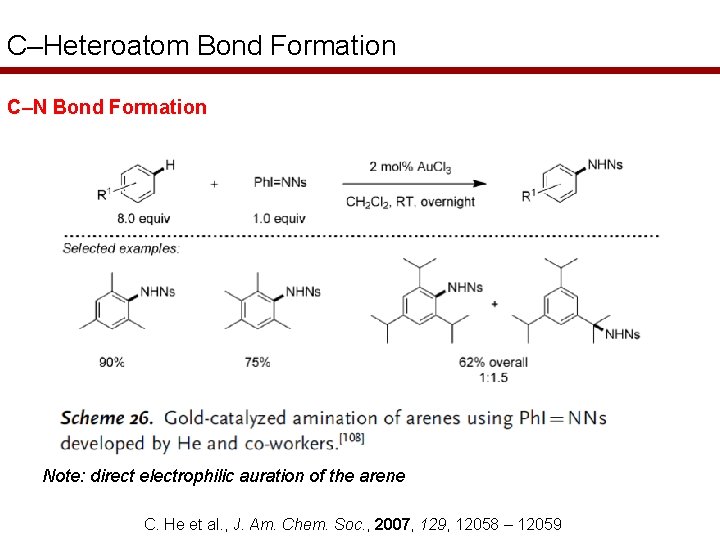
C–Heteroatom Bond Formation C–N Bond Formation Note: direct electrophilic auration of the arene C. He et al. , J. Am. Chem. Soc. , 2007, 129, 12058 – 12059

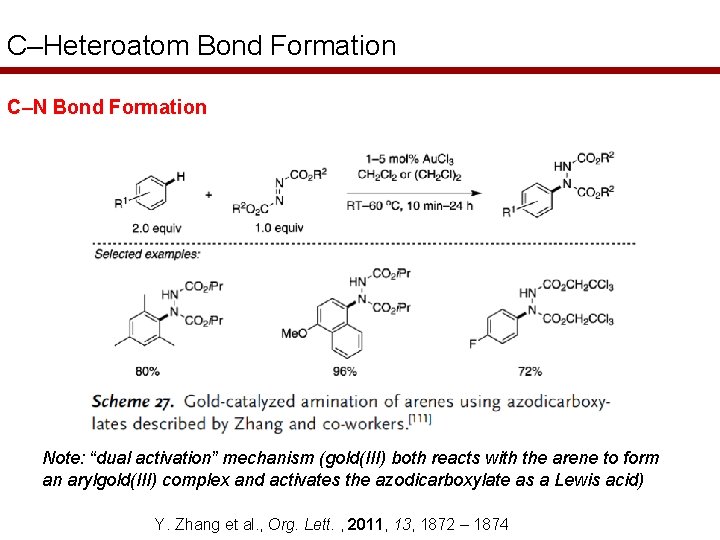
C–Heteroatom Bond Formation C–N Bond Formation Note: “dual activation” mechanism (gold(III) both reacts with the arene to form an arylgold(III) complex and activates the azodicarboxylate as a Lewis acid) Y. Zhang et al. , Org. Lett. , 2011, 13, 1872 – 1874

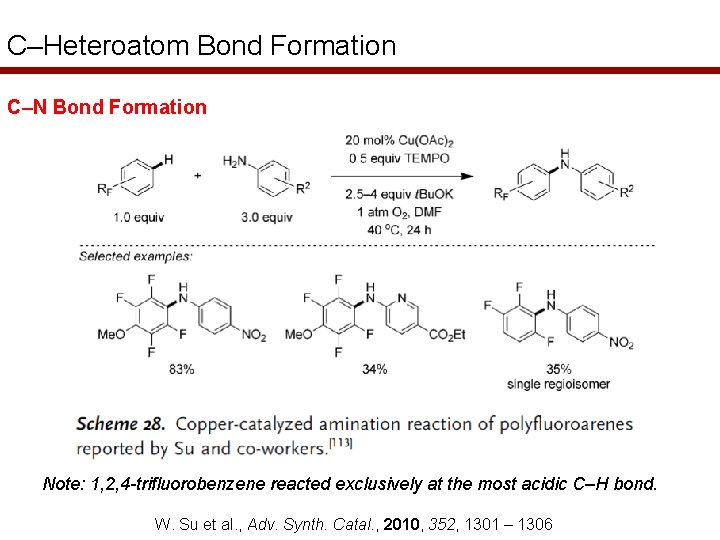
C–Heteroatom Bond Formation C–N Bond Formation Note: 1, 2, 4 -trifluorobenzene reacted exclusively at the most acidic C–H bond. W. Su et al. , Adv. Synth. Catal. , 2010, 352, 1301 – 1306

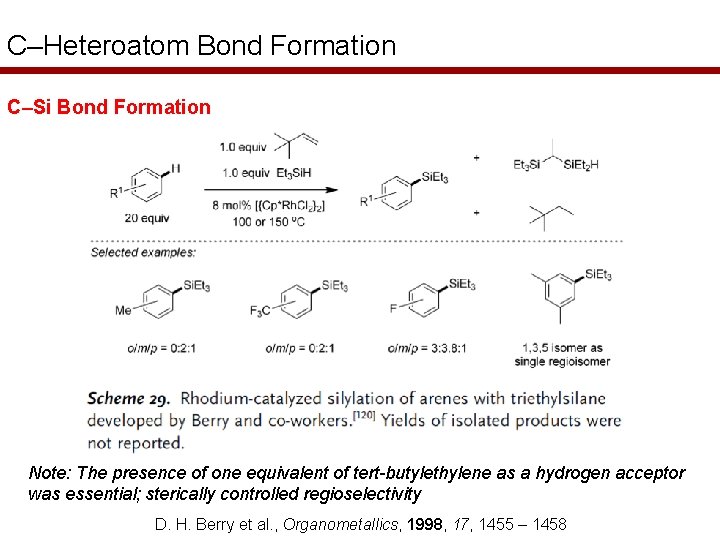
C–Heteroatom Bond Formation C–Si Bond Formation Note: The presence of one equivalent of tert-butylethylene as a hydrogen acceptor was essential; sterically controlled regioselectivity D. H. Berry et al. , Organometallics, 1998, 17, 1455 – 1458

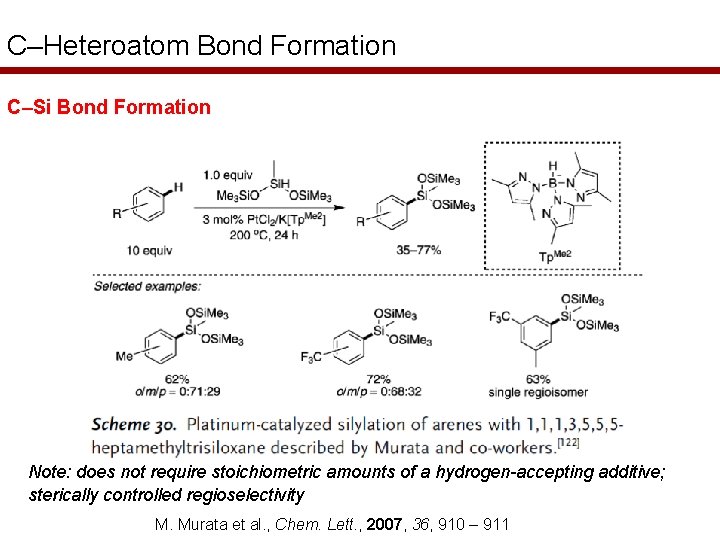
C–Heteroatom Bond Formation C–Si Bond Formation Note: does not require stoichiometric amounts of a hydrogen-accepting additive; sterically controlled regioselectivity M. Murata et al. , Chem. Lett. , 2007, 36, 910 – 911

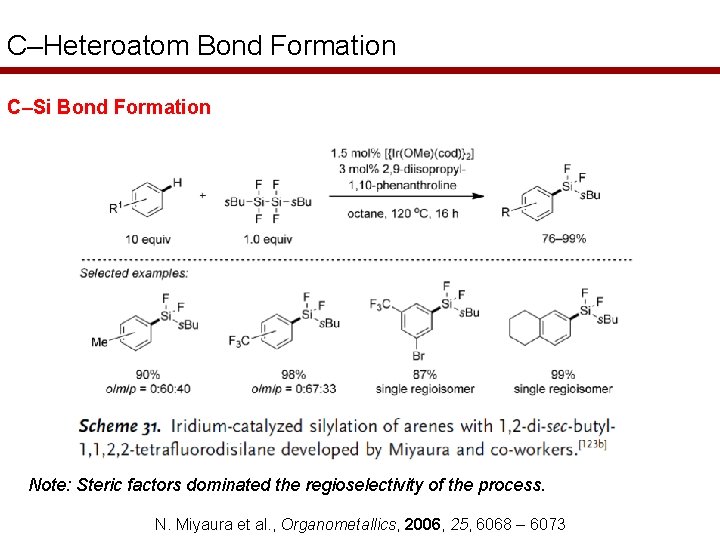
C–Heteroatom Bond Formation C–Si Bond Formation Note: Steric factors dominated the regioselectivity of the process. N. Miyaura et al. , Organometallics, 2006, 25, 6068 – 6073

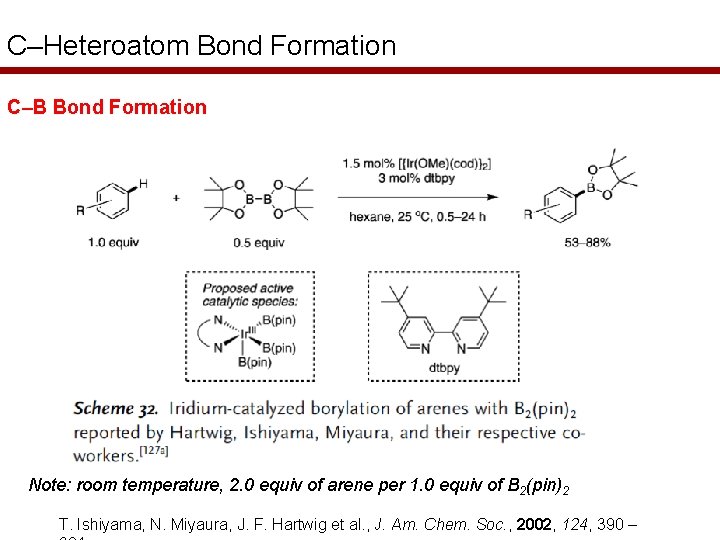
C–Heteroatom Bond Formation C–B Bond Formation Note: room temperature, 2. 0 equiv of arene per 1. 0 equiv of B 2(pin)2 T. Ishiyama, N. Miyaura, J. F. Hartwig et al. , J. Am. Chem. Soc. , 2002, 124, 390 –

C–Heteroatom Bond Formation C–B Bond Formation Note: The site of borylation is essentially completely determined by steric factors. N. Miyaura et al. , Angew. Chem. Int. Ed. , 2002, 41, 3056 – 3058 M. R. Smith et al. , J. Am. Chem. Soc. , 2005, 127, 10539 – 10544

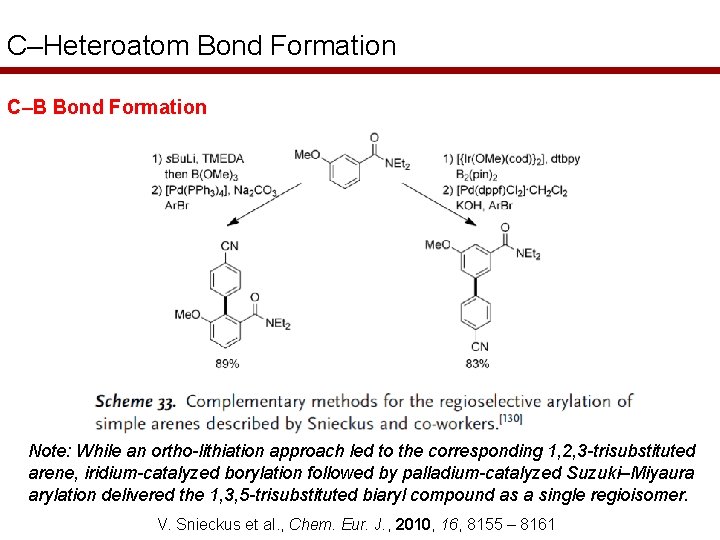
C–Heteroatom Bond Formation C–B Bond Formation Note: While an ortho-lithiation approach led to the corresponding 1, 2, 3 -trisubstituted arene, iridium-catalyzed borylation followed by palladium-catalyzed Suzuki–Miyaura arylation delivered the 1, 3, 5 -trisubstituted biaryl compound as a single regioisomer. V. Snieckus et al. , Chem. Eur. J. , 2010, 16, 8155 – 8161

Conclusion Beyond Directing Groups: Transition-Metal Catalyzed C–H Activation of Simple Arenes Challenge: reactivity and selectivity The efficiency and site of C–H functionalization is governed by factors such as the electronic and steric profiles of the arene. Aryl C–H activation can occur with regioselectivity that is complementary to orthoselective DG-assisted processes. The selectivity can be improved upon judicious selection of the catalyst and ligand combination (Sanford’s work).