Day 2 Part 2 Equation of State Models
- Slides: 42

Day 2 – Part 2: Equation of State Models. § § § Cubic Equation of State models. Two-phase flash calculations (VLE). Stability Analysis Saturation Pressure Calculations. Gradient Calculations Introduction to PVT Simulators Exercise 2 -1 Course in Advanced Fluid Phase Behavior. © Pera A/S 1

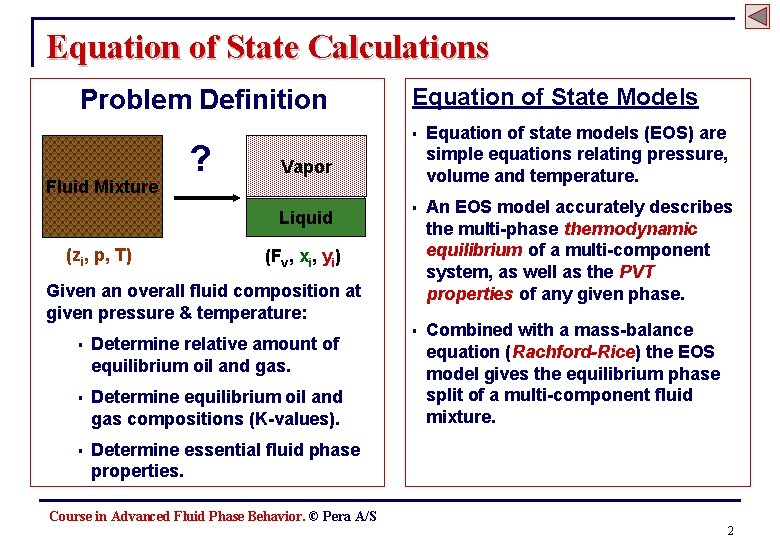
Equation of State Calculations Problem Definition Fluid Mixture ? § Equation of state models (EOS) are simple equations relating pressure, volume and temperature. § An EOS model accurately describes the multi-phase thermodynamic equilibrium of a multi-component system, as well as the PVT properties of any given phase. § Combined with a mass-balance equation (Rachford-Rice) the EOS model gives the equilibrium phase split of a multi-component fluid mixture. Vapor Liquid (zi, p, T) Equation of State Models (Fv, xi, yi) Given an overall fluid composition at given pressure & temperature: § Determine relative amount of equilibrium oil and gas. § Determine equilibrium oil and gas compositions (K-values). § Determine essential fluid phase properties. Course in Advanced Fluid Phase Behavior. © Pera A/S 2

EOS Applications § Reservoir simulation § Generation of black-oil tables § Compositional simulations § § Gas injection. § Near critical fluids. Conversion from black-oil to compositional. Pipeline calculations § § § Pressure loss, liquid dropout, etc. Surface process calculations. Course in Advanced Fluid Phase Behavior. © Pera A/S 3

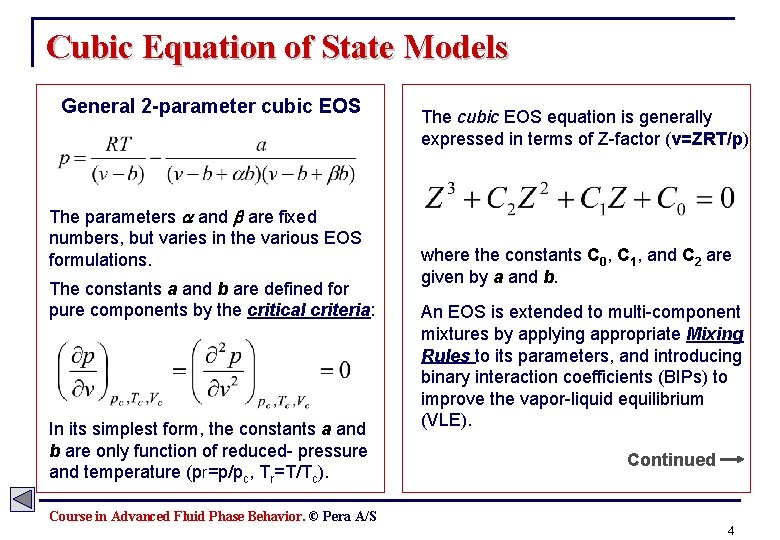
Cubic Equation of State Models General 2 -parameter cubic EOS The parameters and are fixed numbers, but varies in the various EOS formulations. The constants a and b are defined for pure components by the critical criteria: In its simplest form, the constants a and b are only function of reduced- pressure and temperature (pr=p/pc, Tr=T/Tc). The cubic EOS equation is generally expressed in terms of Z-factor (v=ZRT/p) where the constants C 0, C 1, and C 2 are given by a and b. An EOS is extended to multi-component mixtures by applying appropriate Mixing Rules to its parameters, and introducing binary interaction coefficients (BIPs) to improve the vapor-liquid equilibrium (VLE). Continued Course in Advanced Fluid Phase Behavior. © Pera A/S 4

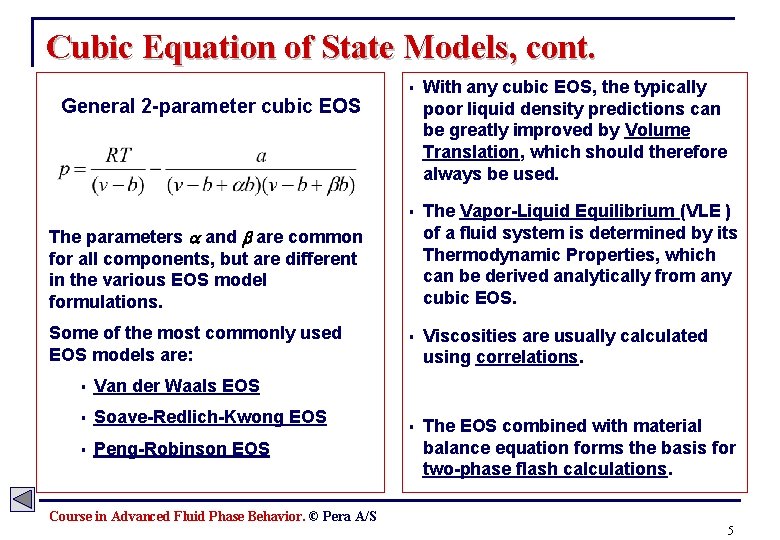
Cubic Equation of State Models, cont. General 2 -parameter cubic EOS § With any cubic EOS, the typically poor liquid density predictions can be greatly improved by Volume Translation, which should therefore always be used. § The Vapor-Liquid Equilibrium (VLE ) of a fluid system is determined by its Thermodynamic Properties, which can be derived analytically from any cubic EOS. § Viscosities are usually calculated using correlations. § The EOS combined with material balance equation forms the basis for two-phase flash calculations. The parameters and are common for all components, but are different in the various EOS model formulations. Some of the most commonly used EOS models are: § Van der Waals EOS § Soave-Redlich-Kwong EOS § Peng-Robinson EOS Course in Advanced Fluid Phase Behavior. © Pera A/S 5

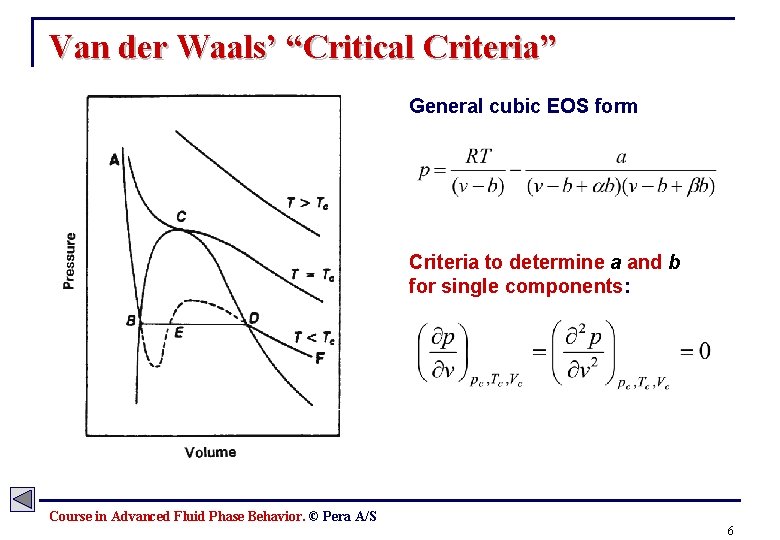
Van der Waals’ “Critical Criteria” General cubic EOS form Criteria to determine a and b for single components: Course in Advanced Fluid Phase Behavior. © Pera A/S 6

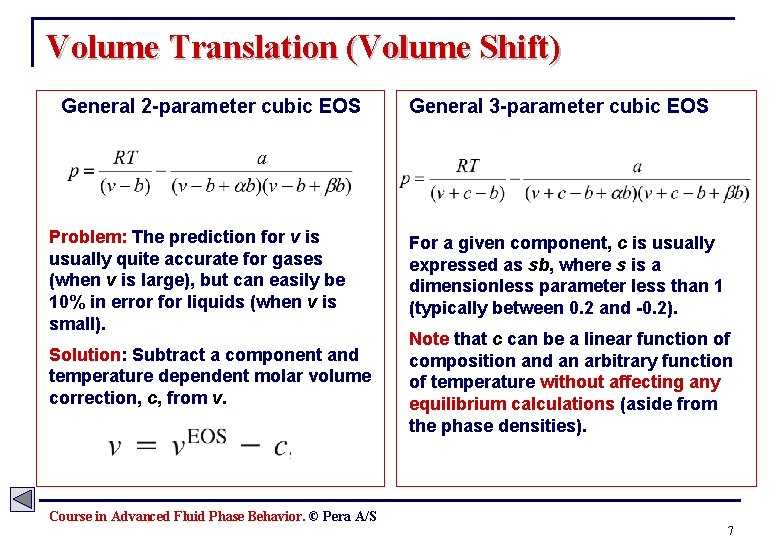
Volume Translation (Volume Shift) General 2 -parameter cubic EOS Problem: The prediction for v is usually quite accurate for gases (when v is large), but can easily be 10% in error for liquids (when v is small). Solution: Subtract a component and temperature dependent molar volume correction, c, from v. General 3 -parameter cubic EOS For a given component, c is usually expressed as sb, where s is a dimensionless parameter less than 1 (typically between 0. 2 and -0. 2). Note that c can be a linear function of composition and an arbitrary function of temperature without affecting any equilibrium calculations (aside from the phase densities). Course in Advanced Fluid Phase Behavior. © Pera A/S 7

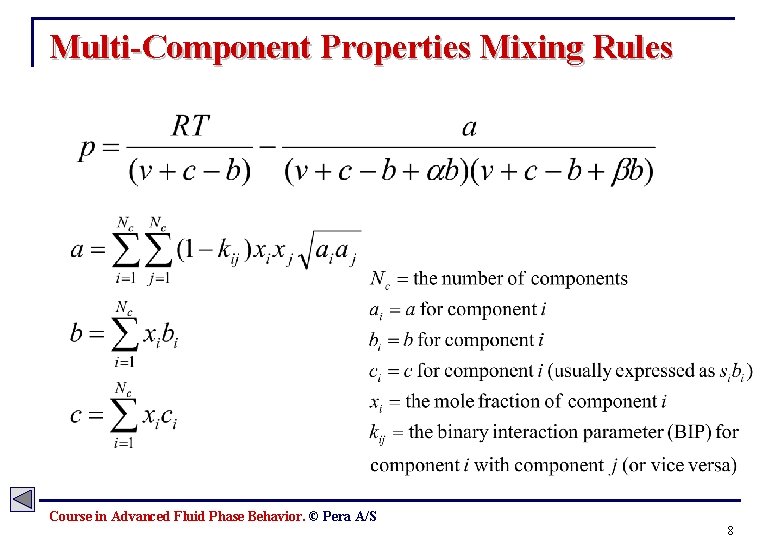
Multi-Component Properties Mixing Rules Course in Advanced Fluid Phase Behavior. © Pera A/S 8

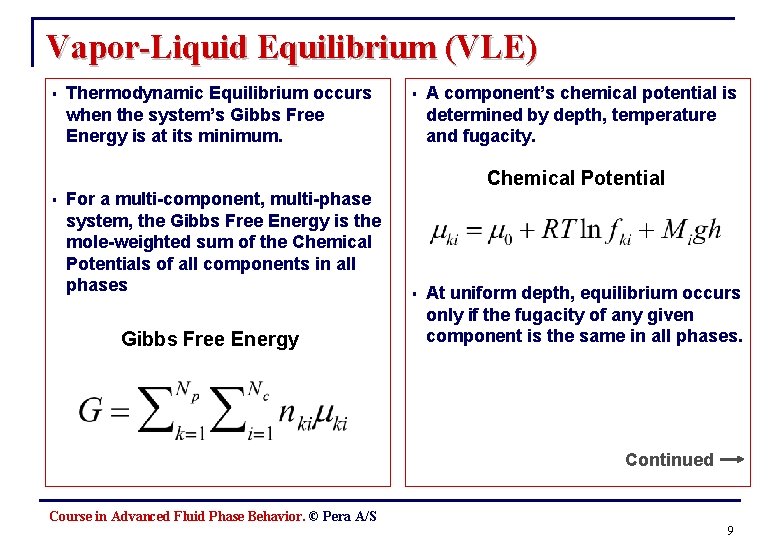
Vapor-Liquid Equilibrium (VLE) § § Thermodynamic Equilibrium occurs when the system’s Gibbs Free Energy is at its minimum. For a multi-component, multi-phase system, the Gibbs Free Energy is the mole-weighted sum of the Chemical Potentials of all components in all phases Gibbs Free Energy § A component’s chemical potential is determined by depth, temperature and fugacity. Chemical Potential § At uniform depth, equilibrium occurs only if the fugacity of any given component is the same in all phases. Continued Course in Advanced Fluid Phase Behavior. © Pera A/S 9

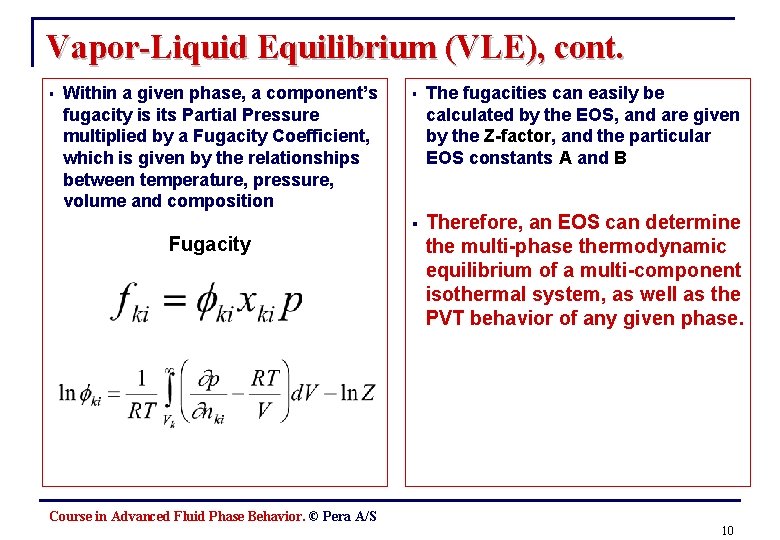
Vapor-Liquid Equilibrium (VLE), cont. § Within a given phase, a component’s fugacity is its Partial Pressure multiplied by a Fugacity Coefficient, which is given by the relationships between temperature, pressure, volume and composition Fugacity § The fugacities can easily be calculated by the EOS, and are given by the Z-factor, and the particular EOS constants A and B § Therefore, an EOS can determine the multi-phase thermodynamic equilibrium of a multi-component isothermal system, as well as the PVT behavior of any given phase. Course in Advanced Fluid Phase Behavior. © Pera A/S 10

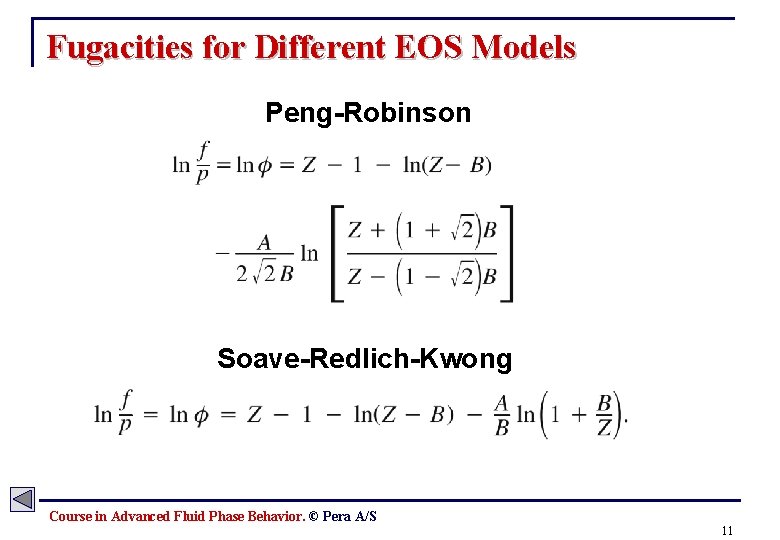
Fugacities for Different EOS Models Peng-Robinson Soave-Redlich-Kwong Course in Advanced Fluid Phase Behavior. © Pera A/S 11

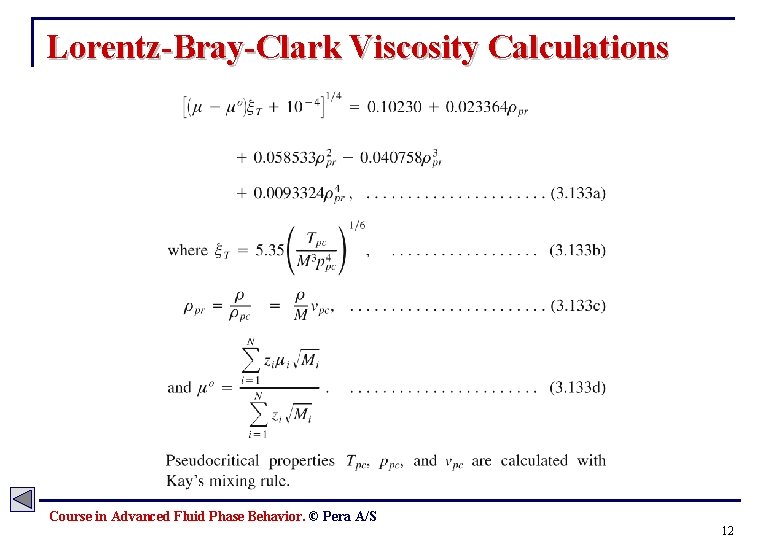
Lorentz-Bray-Clark Viscosity Calculations Course in Advanced Fluid Phase Behavior. © Pera A/S 12

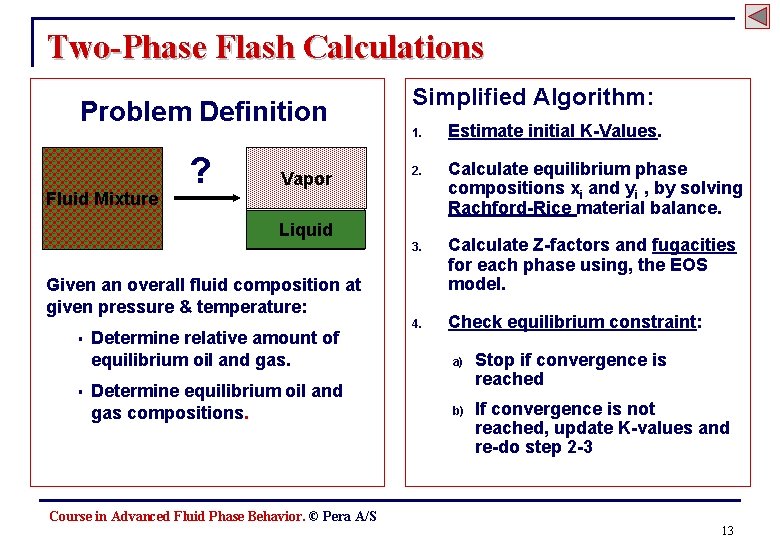
Two-Phase Flash Calculations Problem Definition Fluid Mixture ? Vapor Simplified Algorithm: 1. Estimate initial K-Values. 2. Calculate equilibrium phase compositions xi and yi , by solving Rachford-Rice material balance. 3. Calculate Z-factors and fugacities for each phase using, the EOS model. 4. Check equilibrium constraint: Liquid Given an overall fluid composition at given pressure & temperature: § § Determine relative amount of equilibrium oil and gas. Determine equilibrium oil and gas compositions. a) Stop if convergence is reached b) If convergence is not reached, update K-values and re-do step 2 -3 Course in Advanced Fluid Phase Behavior. © Pera A/S 13

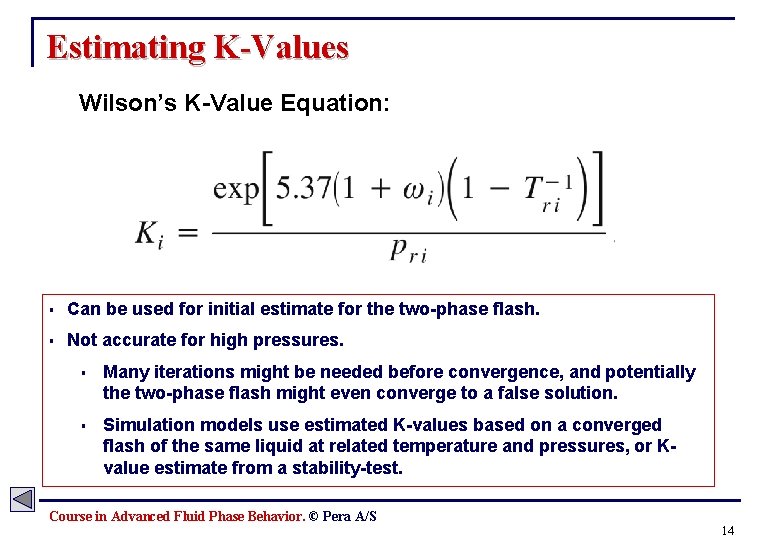
Estimating K-Values Wilson’s K-Value Equation: § Can be used for initial estimate for the two-phase flash. § Not accurate for high pressures. § Many iterations might be needed before convergence, and potentially the two-phase flash might even converge to a false solution. § Simulation models use estimated K-values based on a converged flash of the same liquid at related temperature and pressures, or Kvalue estimate from a stability-test. Course in Advanced Fluid Phase Behavior. © Pera A/S 14

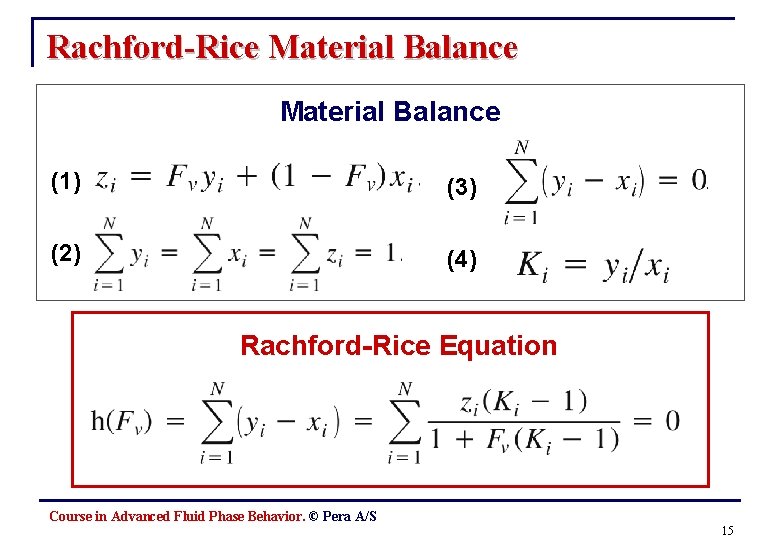
Rachford-Rice Material Balance (1) (3) (2) (4) Rachford-Rice Equation Course in Advanced Fluid Phase Behavior. © Pera A/S 15

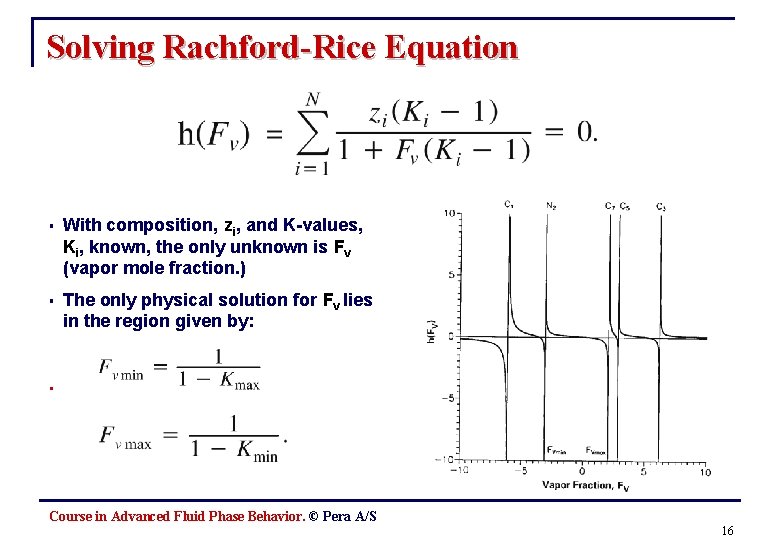
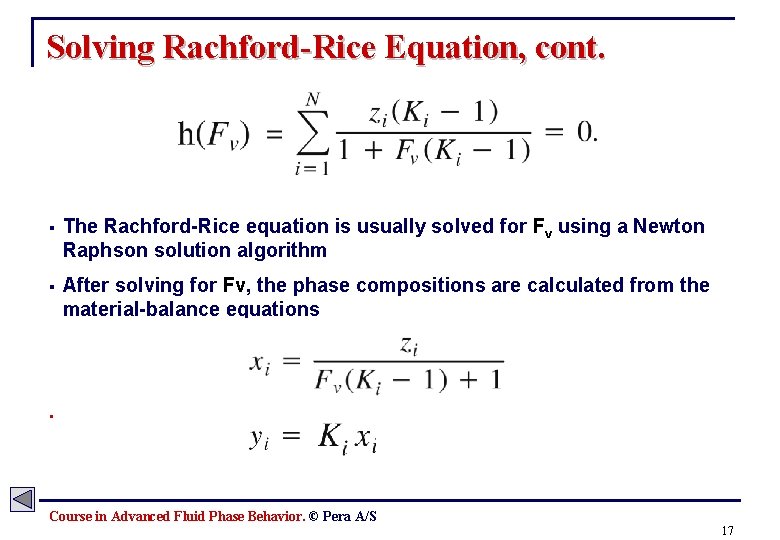
Solving Rachford-Rice Equation § With composition, zi, and K-values, Ki, known, the only unknown is Fv (vapor mole fraction. ) § The only physical solution for Fv lies in the region given by: . Course in Advanced Fluid Phase Behavior. © Pera A/S 16

Solving Rachford-Rice Equation, cont. § The Rachford-Rice equation is usually solved for Fv using a Newton Raphson solution algorithm § After solving for Fv, the phase compositions are calculated from the material-balance equations . Course in Advanced Fluid Phase Behavior. © Pera A/S 17

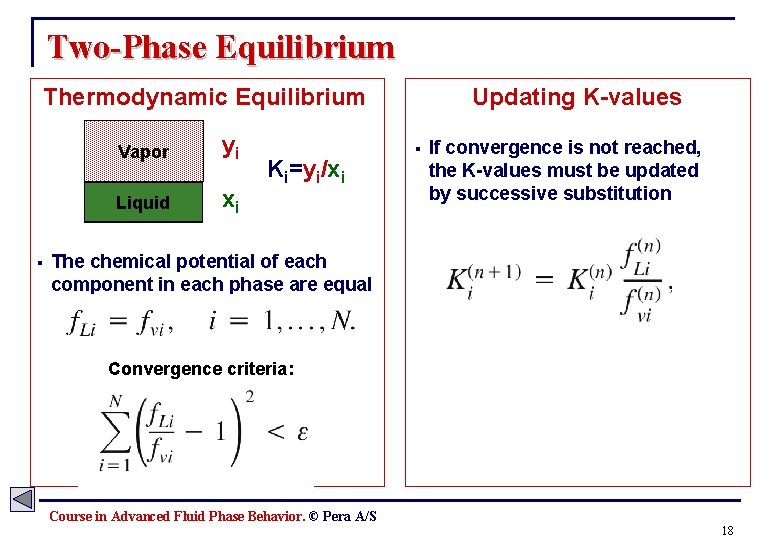
Two-Phase Equilibrium Thermodynamic Equilibrium § Vapor yi Liquid xi Ki=yi/xi Updating K-values § If convergence is not reached, the K-values must be updated by successive substitution The chemical potential of each component in each phase are equal Convergence criteria: Course in Advanced Fluid Phase Behavior. © Pera A/S 18

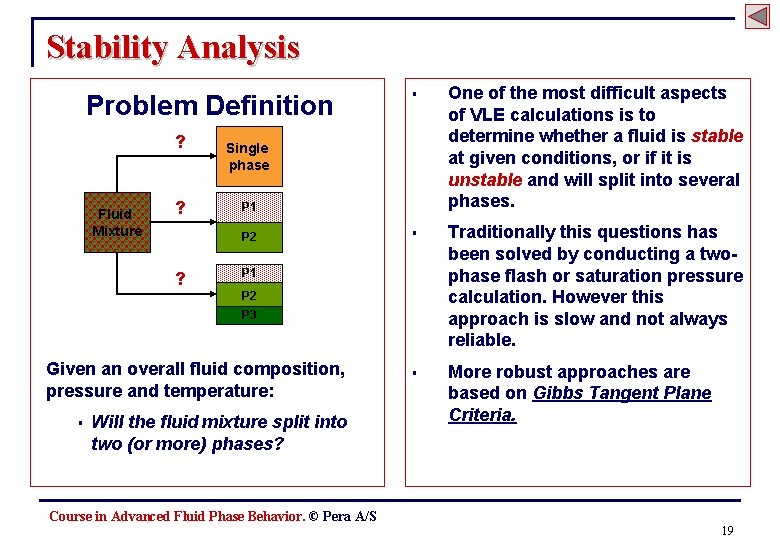
Stability Analysis Problem Definition ? Fluid Mixture ? One of the most difficult aspects of VLE calculations is to determine whether a fluid is stable at given conditions, or if it is unstable and will split into several phases. § Traditionally this questions has been solved by conducting a twophase flash or saturation pressure calculation. However this approach is slow and not always reliable. § More robust approaches are based on Gibbs Tangent Plane Criteria. Single phase P 1 P 2 ? § P 1 P 2 P 3 Given an overall fluid composition, pressure and temperature: § Will the fluid mixture split into two (or more) phases? Course in Advanced Fluid Phase Behavior. © Pera A/S 19

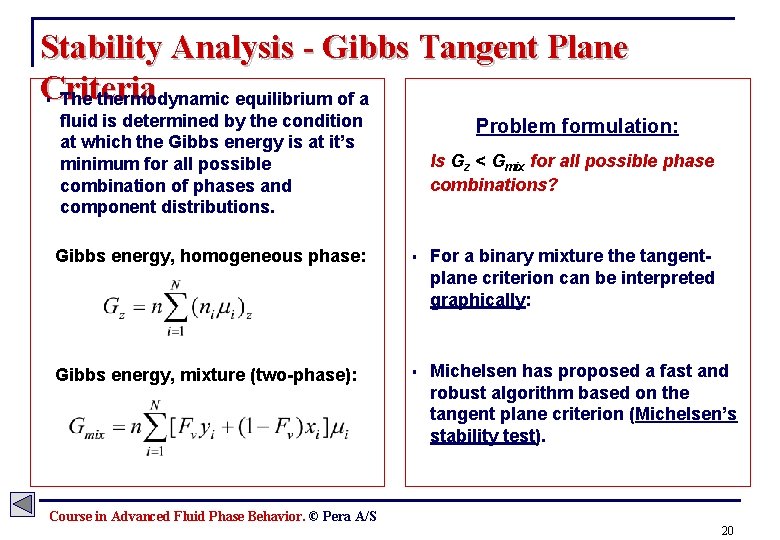
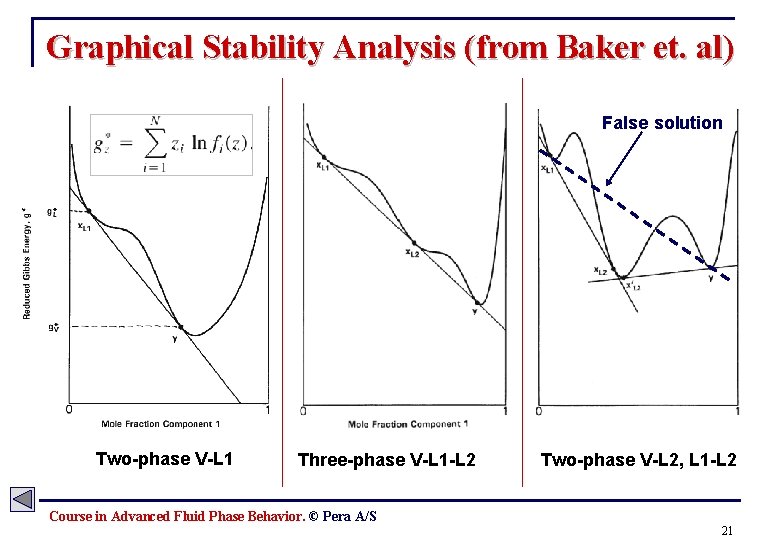
Stability Analysis - Gibbs Tangent Plane Criteria The thermodynamic equilibrium of a § fluid is determined by the condition at which the Gibbs energy is at it’s minimum for all possible combination of phases and component distributions. Problem formulation: Is Gz < Gmix for all possible phase combinations? Gibbs energy, homogeneous phase: § For a binary mixture the tangentplane criterion can be interpreted graphically: Gibbs energy, mixture (two-phase): § Michelsen has proposed a fast and robust algorithm based on the tangent plane criterion (Michelsen’s stability test). Course in Advanced Fluid Phase Behavior. © Pera A/S 20

Graphical Stability Analysis (from Baker et. al) False solution Two-phase V-L 1 Three-phase V-L 1 -L 2 Two-phase V-L 2, L 1 -L 2 Course in Advanced Fluid Phase Behavior. © Pera A/S 21

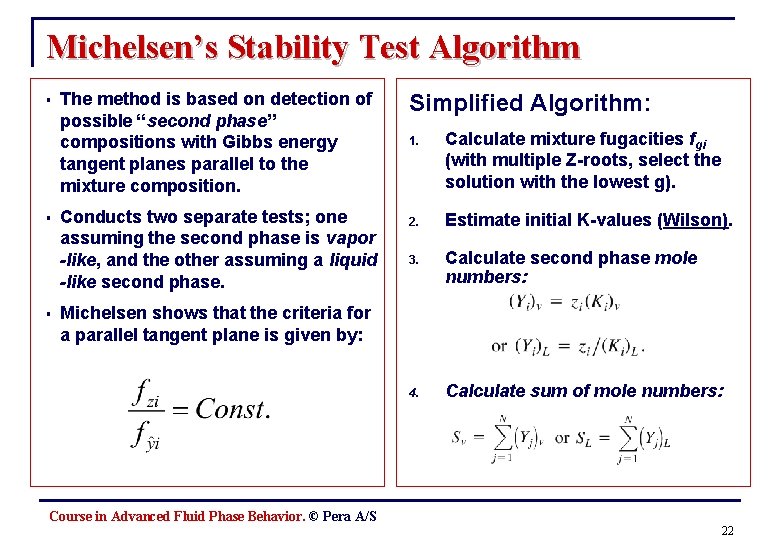
Michelsen’s Stability Test Algorithm § § § The method is based on detection of possible “second phase” compositions with Gibbs energy tangent planes parallel to the mixture composition. Conducts two separate tests; one assuming the second phase is vapor -like, and the other assuming a liquid -like second phase. Simplified Algorithm: 1. Calculate mixture fugacities fgi (with multiple Z-roots, select the solution with the lowest g). 2. Estimate initial K-values (Wilson). 3. Calculate second phase mole numbers: 4. Calculate sum of mole numbers: Michelsen shows that the criteria for a parallel tangent plane is given by: Course in Advanced Fluid Phase Behavior. © Pera A/S 22

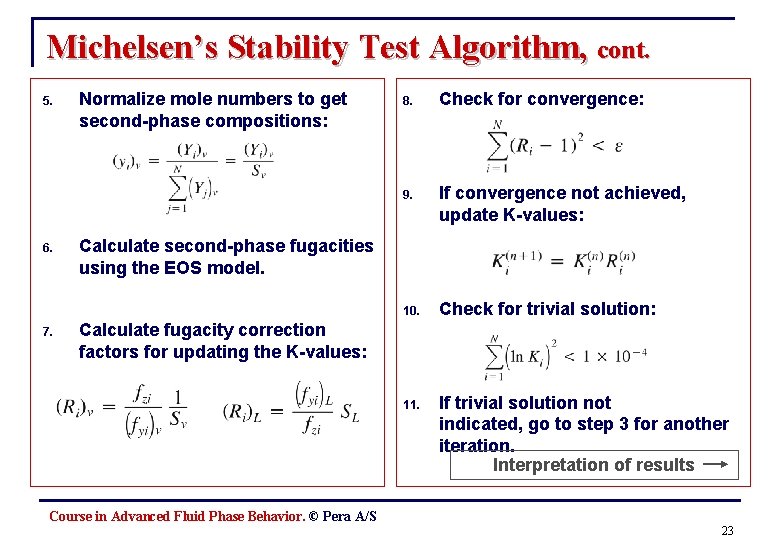
Michelsen’s Stability Test Algorithm, cont. 5. 6. 7. Normalize mole numbers to get second-phase compositions: 8. Check for convergence: 9. If convergence not achieved, update K-values: 10. Check for trivial solution: 11. If trivial solution not indicated, go to step 3 for another iteration. Interpretation of results Calculate second-phase fugacities using the EOS model. Calculate fugacity correction factors for updating the K-values: Course in Advanced Fluid Phase Behavior. © Pera A/S 23

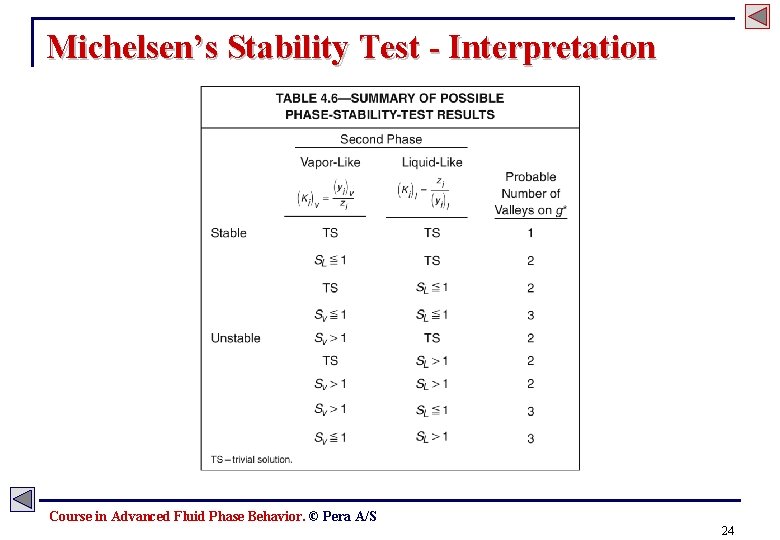
Michelsen’s Stability Test - Interpretation Course in Advanced Fluid Phase Behavior. © Pera A/S 24

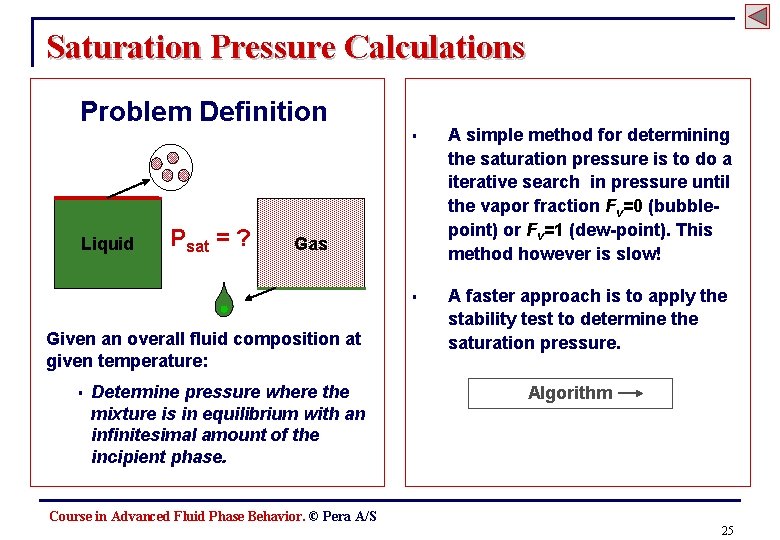
Saturation Pressure Calculations Problem Definition Liquid Psat = ? A simple method for determining the saturation pressure is to do a iterative search in pressure until the vapor fraction Fv=0 (bubblepoint) or Fv=1 (dew-point). This method however is slow! § A faster approach is to apply the stability test to determine the saturation pressure. Gas Given an overall fluid composition at given temperature: § § Determine pressure where the mixture is in equilibrium with an infinitesimal amount of the incipient phase. Algorithm Course in Advanced Fluid Phase Behavior. © Pera A/S 25

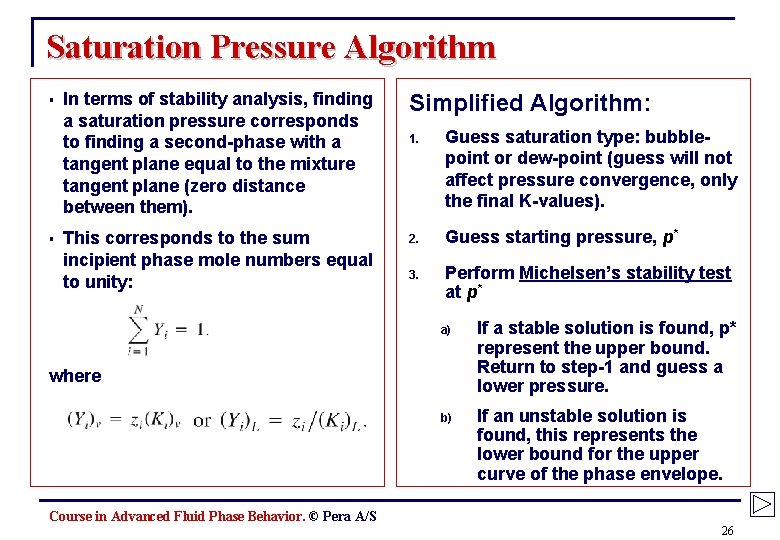
Saturation Pressure Algorithm § § In terms of stability analysis, finding a saturation pressure corresponds to finding a second-phase with a tangent plane equal to the mixture tangent plane (zero distance between them). Simplified Algorithm: 1. Guess saturation type: bubblepoint or dew-point (guess will not affect pressure convergence, only the final K-values). This corresponds to the sum incipient phase mole numbers equal to unity: 2. Guess starting pressure, p* 3. Perform Michelsen’s stability test at p* a) If a stable solution is found, p* represent the upper bound. Return to step-1 and guess a lower pressure. b) If an unstable solution is found, this represents the lower bound for the upper curve of the phase envelope. where Course in Advanced Fluid Phase Behavior. © Pera A/S 26

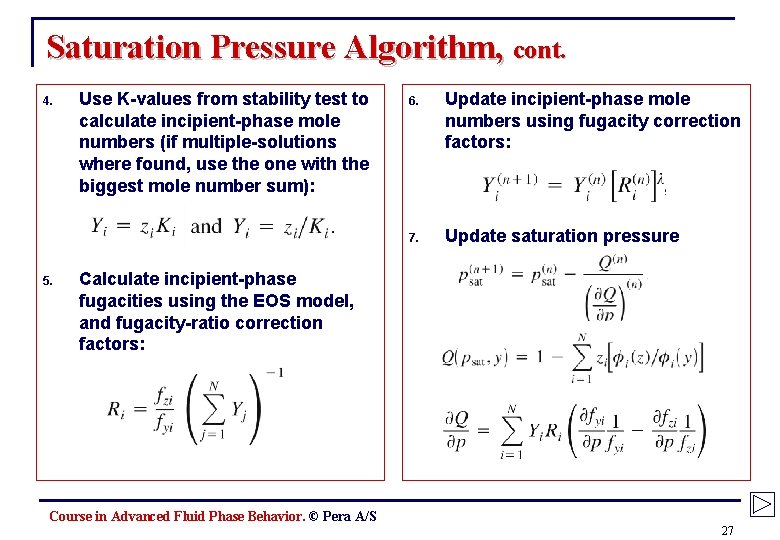
Saturation Pressure Algorithm, cont. 4. 5. Use K-values from stability test to calculate incipient-phase mole numbers (if multiple-solutions where found, use the one with the biggest mole number sum): 6. Update incipient-phase mole numbers using fugacity correction factors: 7. Update saturation pressure Calculate incipient-phase fugacities using the EOS model, and fugacity-ratio correction factors: Course in Advanced Fluid Phase Behavior. © Pera A/S 27

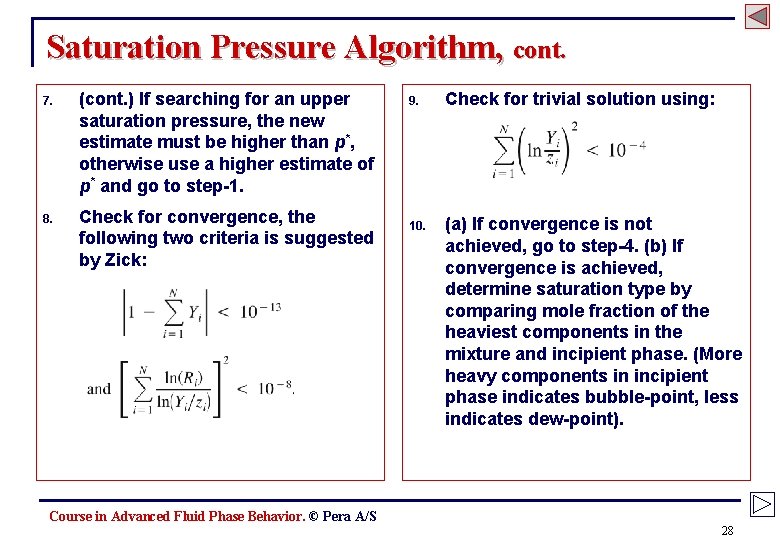
Saturation Pressure Algorithm, cont. 7. (cont. ) If searching for an upper saturation pressure, the new estimate must be higher than p*, otherwise use a higher estimate of p* and go to step-1. 8. Check for convergence, the following two criteria is suggested by Zick: 9. Check for trivial solution using: 10. (a) If convergence is not achieved, go to step-4. (b) If convergence is achieved, determine saturation type by comparing mole fraction of the heaviest components in the mixture and incipient phase. (More heavy components in incipient phase indicates bubble-point, less indicates dew-point). Course in Advanced Fluid Phase Behavior. © Pera A/S 28

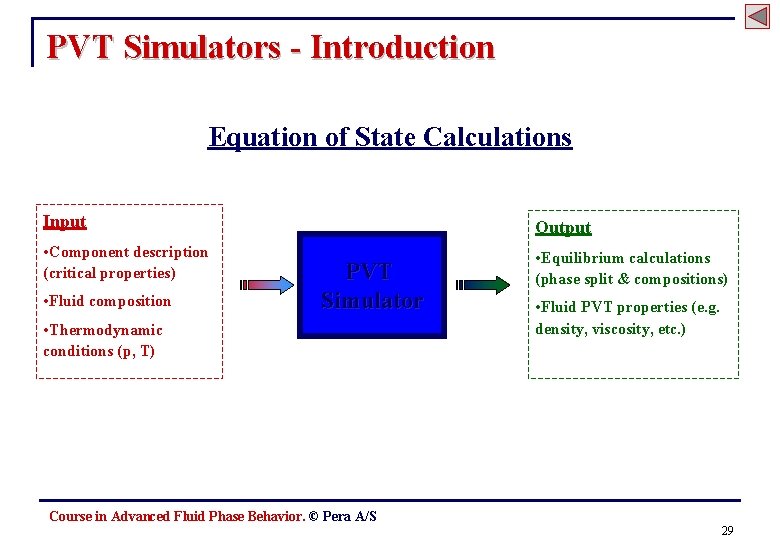
PVT Simulators - Introduction Equation of State Calculations Input Output • Component description (critical properties) • Equilibrium calculations (phase split & compositions) • Fluid composition PVT Simulator • Thermodynamic conditions (p, T) • Fluid PVT properties (e. g. density, viscosity, etc. ) Course in Advanced Fluid Phase Behavior. © Pera A/S 29

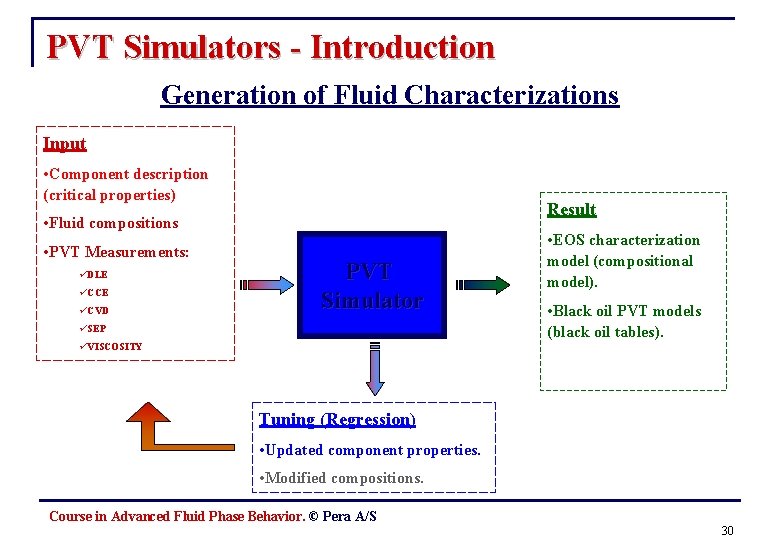
PVT Simulators - Introduction Generation of Fluid Characterizations Input • Component description (critical properties) Result • Fluid compositions • PVT Measurements: üDLE üCCE üCVD PVT Simulator üSEP • EOS characterization model (compositional model). • Black oil PVT models (black oil tables). üVISCOSITY Tuning (Regression) • Updated component properties. • Modified compositions. Course in Advanced Fluid Phase Behavior. © Pera A/S 30

Phaze. Comp – State of the Art, by Zick Technologies § Building and tuning multiple EOS fluid characterizations simultaneously. § Manipulating fluid compositions. § Simulating user-defined experiments. § Matching user-defined data. § Predicting MMPs and MMEs. § Detecting three-phase equilibria. § Gravitational segregation experiments. § User-defined process calculations. § Black-oil table generation. Course in Advanced Fluid Phase Behavior. © Pera A/S 31

The Phaze. Comp Approach § Virtual phase behavior laboratory. § User-programmed sequence of events. § Results from any calculation may be carried over to any other calculation. § Almost any parameter or independent variable can be modified to improve the predictions of experimental data. Course in Advanced Fluid Phase Behavior. © Pera A/S 32

Phaze. Comp – Typical Sequence of Events § Build a fluid characterization. § Define regression variables. § Assign temperatures, pressures, and any number of fluid compositions. § Perform calculations and experiments. § Repeat the above steps as necessary. § Cycle through instructions, regressing on variables to optimize predictions. Course in Advanced Fluid Phase Behavior. © Pera A/S 33

Phaze. Comp – Building Characterizations § Input tables of components and their non-default properties. § Initialize certain missing properties from a base characterization, a component library, and/or a Gamma distribution. § Apply regression variables to properties. § Apply defaults and correlations to define any remaining undefined properties. Course in Advanced Fluid Phase Behavior. © Pera A/S 34

Phaze. Comp – Regression Variables § Defined by name, along with initial and optional bounding values. § May be used to Multiply, Divide, Increase, Decrease, or Replace any parameter or independent variable, in any specified sequence. Course in Advanced Fluid Phase Behavior. © Pera A/S 35

Phaze. Comp – Temperatures and Pressures § Specify manually. § Use, store, and/or restore previously calculated values. § Modify by constants or variables. Course in Advanced Fluid Phase Behavior. © Pera A/S 36

Phaze. Comp – Mixing Fluids § By mole, mass, or tankful. § Individual components. § Previously defined fluids (including those resulting from prior calculations). § Gamma fitting and splitting. § Convert from other characterizations. § Modify by constants or variables. Course in Advanced Fluid Phase Behavior. © Pera A/S 37

Phaze. Comp – Basic Calculations § Two-phase (and negative) flashes. § Saturation pressures (upper and lower). § Vapor and pseudo-vapor pressures. § Convergence pressures. § Three-phase detection. § Two- and three-phase boundaries in temperature, pressure and composition. Course in Advanced Fluid Phase Behavior. © Pera A/S 38

Phaze. Comp – Basic Experiments § Nearly any user-defined single-cell PVT experiment, including, but not limited to: CCEs, DLEs, CVDs, Separator Tests, Multicontact Vaporization and Swelling. § Hundreds of predefined and user-definable quantities can be calculated and compared with experimental data. Course in Advanced Fluid Phase Behavior. © Pera A/S 39

Phaze. Comp – Specialty Experiments § Rigorous MMP and MME experiments. § Gravitational segregation experiments. § User-defined process calculations. § Black-oil table generation. Course in Advanced Fluid Phase Behavior. © Pera A/S 40

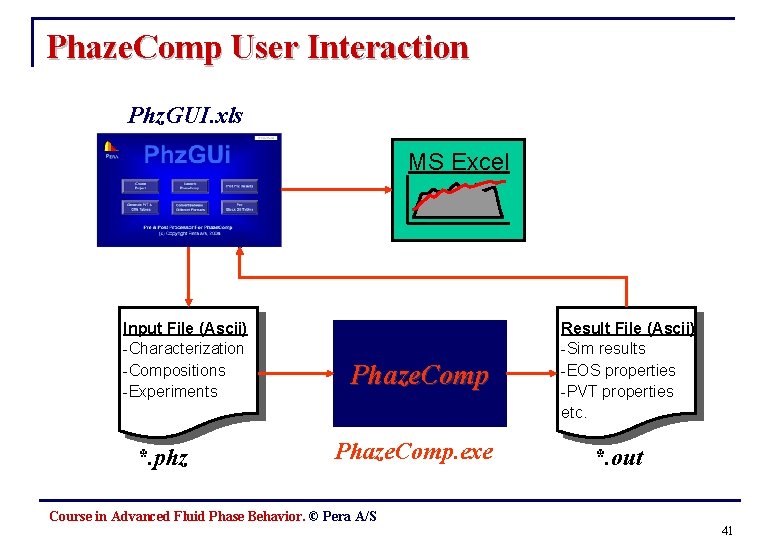
Phaze. Comp User Interaction Phz. GUI. xls MS Excel Input File (Ascii) -Characterization -Compositions -Experiments *. phz Phaze. Comp. exe Result File (Ascii) -Sim results -EOS properties -PVT properties etc. *. out Course in Advanced Fluid Phase Behavior. © Pera A/S 41

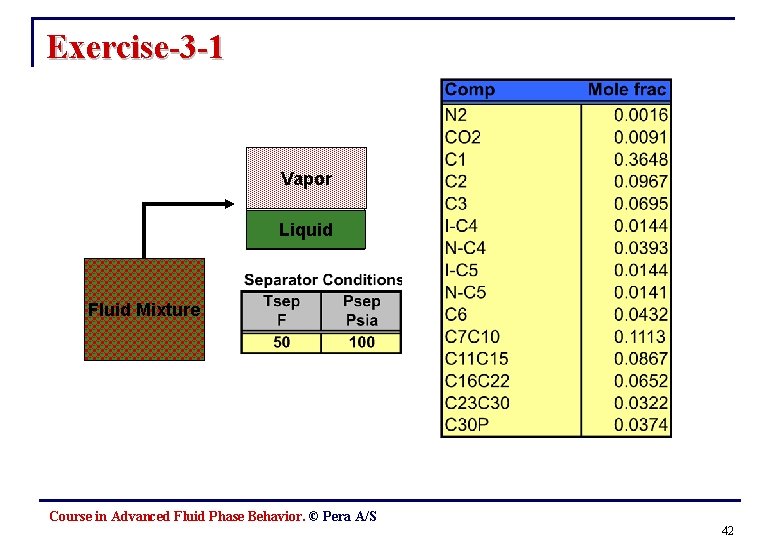
Exercise-3 -1 Vapor Liquid Fluid Mixture Course in Advanced Fluid Phase Behavior. © Pera A/S 42