LIMITING REACTANT q When 2 or more reactants

- Slides: 22

LIMITING REACTANT q When 2 or more reactants are combined in nonstoichiometric ratios, the amount of product produced is limited by the reactant that is not in excess. q This reactant is referred to as limiting reactant. q When doing stoichiometric problems of this type, the limiting reactant must be determined first before proceeding with the calculations. 1

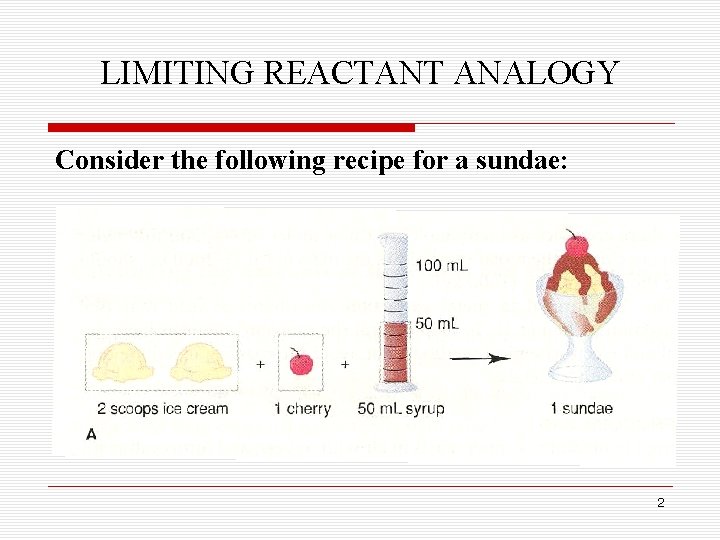
LIMITING REACTANT ANALOGY Consider the following recipe for a sundae: 2

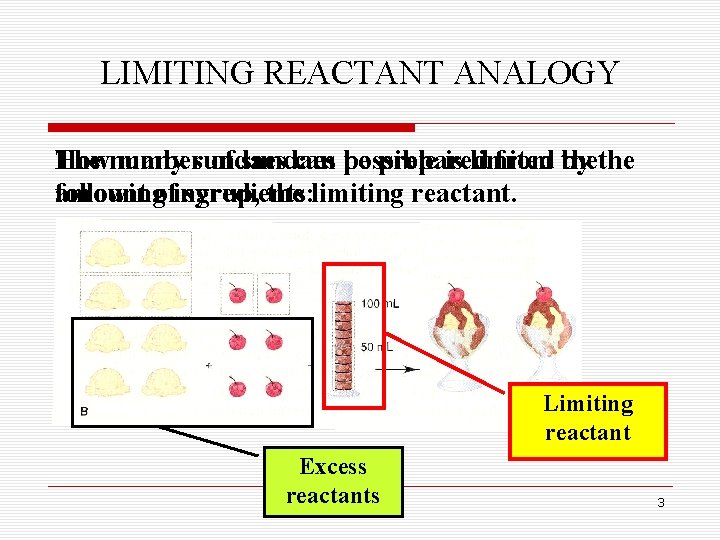
LIMITING REACTANT ANALOGY The number How many sundaes of sundaes can possible be prepared is limited from by thethe followingofingredients: amount syrup, the limiting reactant. Limiting reactant Excess reactants 3

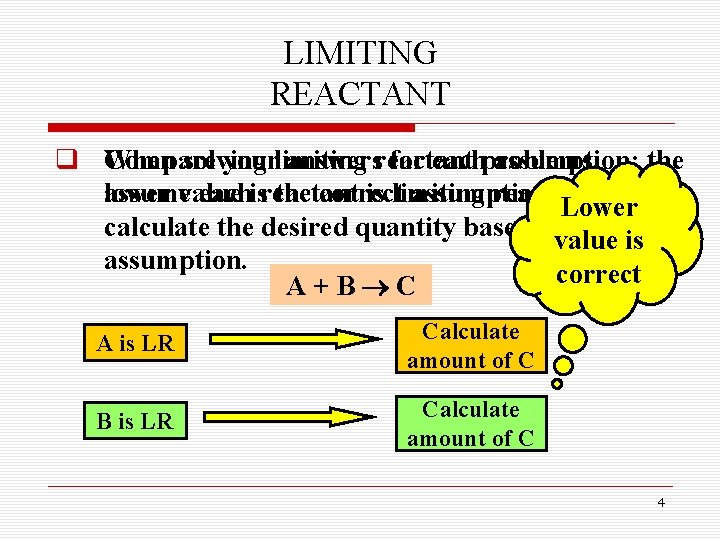
LIMITING REACTANT q When Compare solving yourlimiting answersreactant for eachproblems, assumption; the assumevalue lower eachisreactant the correct is limiting assumption. reactant, and Lower calculate the desired quantity based on that value is assumption. correct A+B C A is LR Calculate amount of C B is LR Calculate amount of C 4

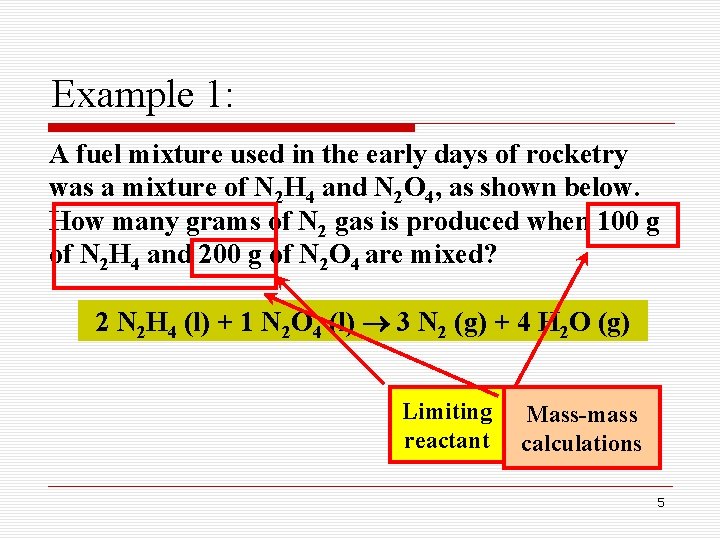
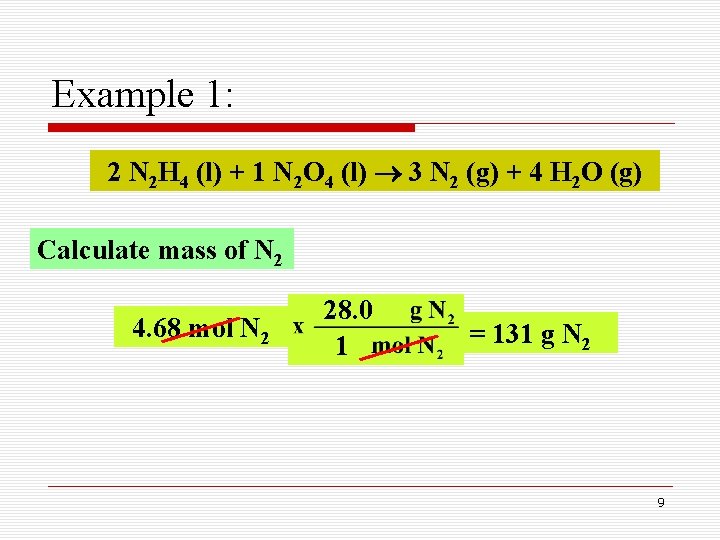
Example 1: A fuel mixture used in the early days of rocketry was a mixture of N 2 H 4 and N 2 O 4, as shown below. How many grams of N 2 gas is produced when 100 g of N 2 H 4 and 200 g of N 2 O 4 are mixed? 2 N 2 H 4 (l) + 1 N 2 O 4 (l) 3 N 2 (g) + 4 H 2 O (g) Limiting reactant Mass-mass calculations 5

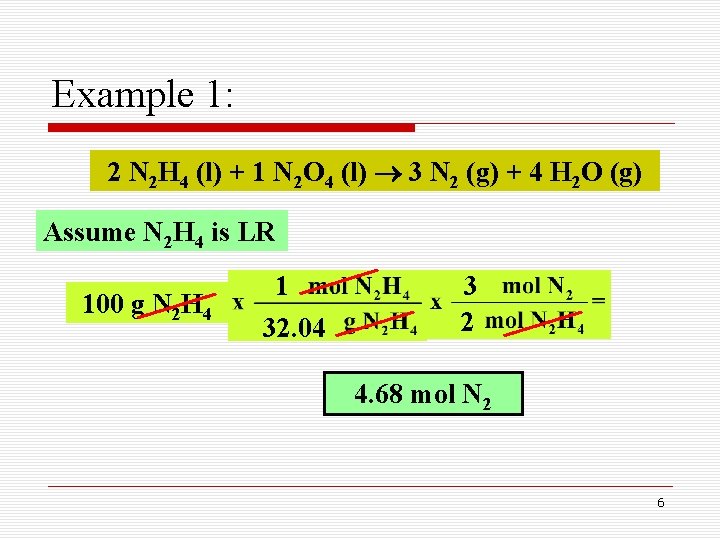
Example 1: 2 N 2 H 4 (l) + 1 N 2 O 4 (l) 3 N 2 (g) + 4 H 2 O (g) Assume N 2 H 4 is LR 100 g N 2 H 4 1 32. 04 3 2 4. 68 mol N 2 6

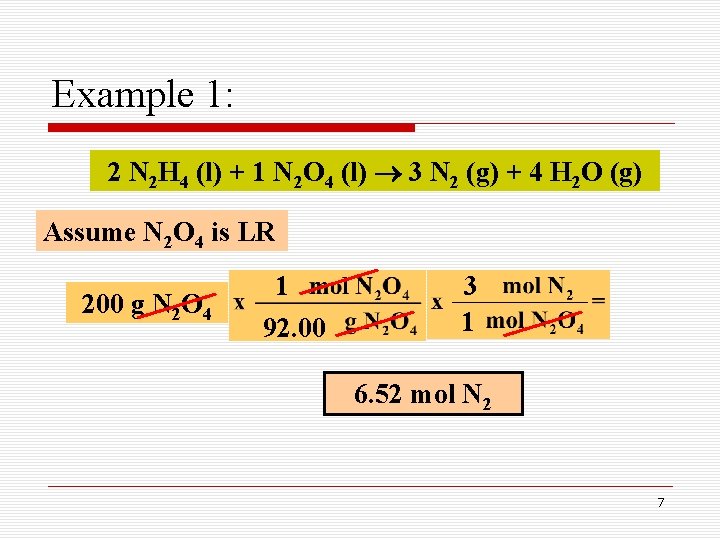
Example 1: 2 N 2 H 4 (l) + 1 N 2 O 4 (l) 3 N 2 (g) + 4 H 2 O (g) Assume N 2 O 4 is LR 200 g N 2 O 4 1 92. 00 3 1 6. 52 mol N 2 7

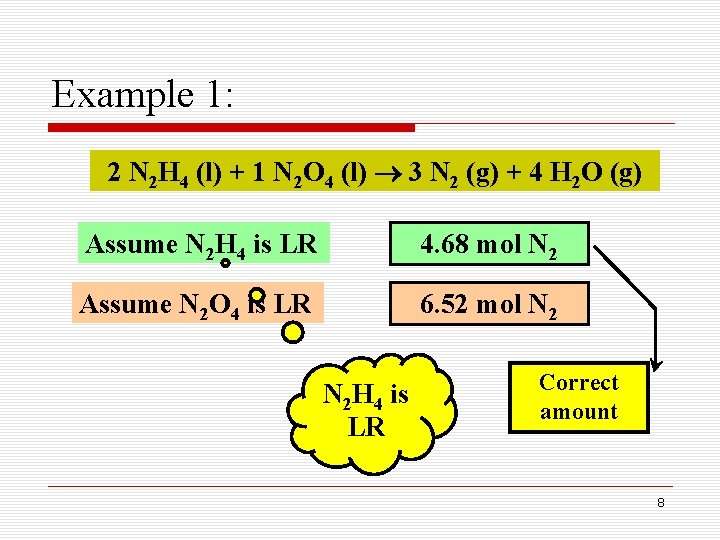
Example 1: 2 N 2 H 4 (l) + 1 N 2 O 4 (l) 3 N 2 (g) + 4 H 2 O (g) Assume N 2 H 4 is LR 4. 68 mol N 2 Assume N 2 O 4 is LR 6. 52 mol N 2 H 4 is LR Correct amount 8

Example 1: 2 N 2 H 4 (l) + 1 N 2 O 4 (l) 3 N 2 (g) + 4 H 2 O (g) Calculate mass of N 2 4. 68 mol N 2 28. 0 1 = 131 g N 2 9

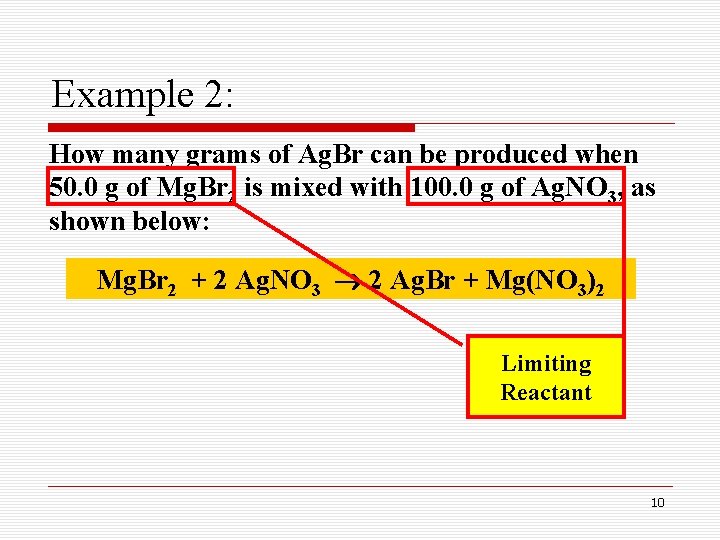
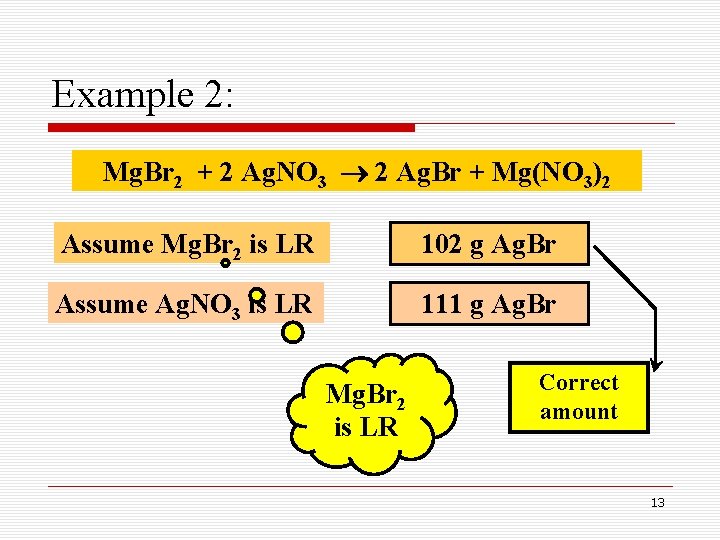
Example 2: How many grams of Ag. Br can be produced when 50. 0 g of Mg. Br 2 is mixed with 100. 0 g of Ag. NO 3, as shown below: Mg. Br 2 + 2 Ag. NO 3 2 Ag. Br + Mg(NO 3)2 Limiting Reactant 10

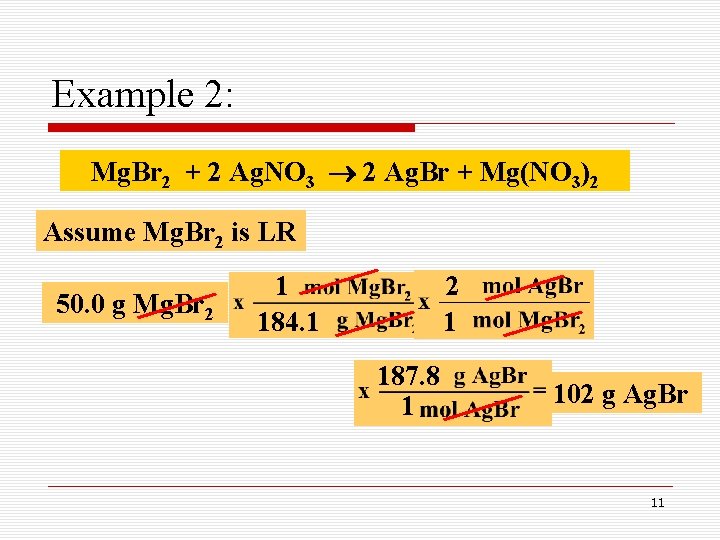
Example 2: Mg. Br 2 + 2 Ag. NO 3 2 Ag. Br + Mg(NO 3)2 Assume Mg. Br 2 is LR 50. 0 g Mg. Br 2 1 184. 1 2 1 187. 8 1 102 g Ag. Br 11

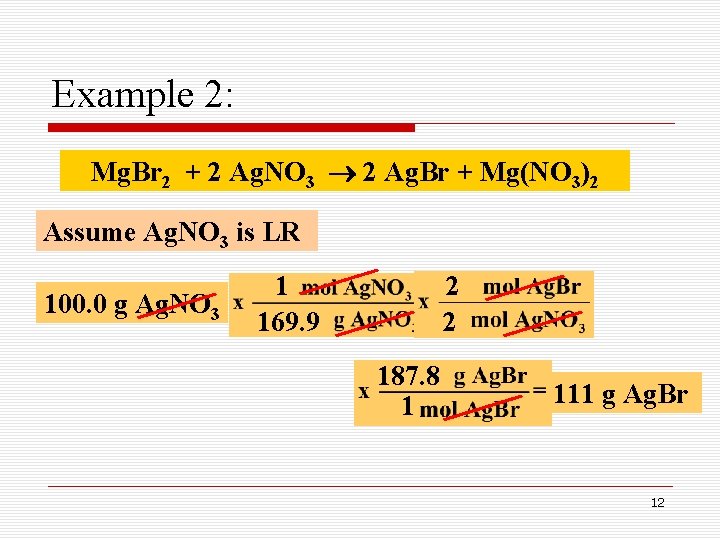
Example 2: Mg. Br 2 + 2 Ag. NO 3 2 Ag. Br + Mg(NO 3)2 Assume Ag. NO 3 is LR 100. 0 g Ag. NO 3 1 169. 9 2 2 187. 8 1 111 g Ag. Br 12

Example 2: Mg. Br 2 + 2 Ag. NO 3 2 Ag. Br + Mg(NO 3)2 Assume Mg. Br 2 is LR 102 g Ag. Br Assume Ag. NO 3 is LR 111 g Ag. Br Mg. Br 2 is LR Correct amount 13

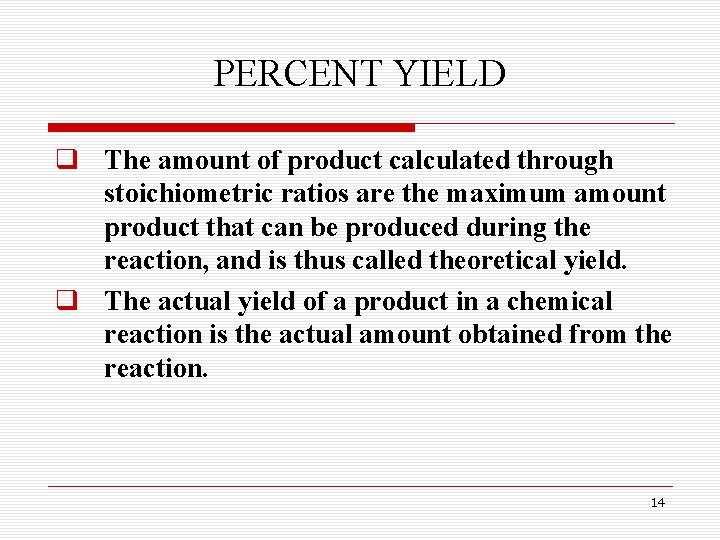
PERCENT YIELD q The amount of product calculated through stoichiometric ratios are the maximum amount product that can be produced during the reaction, and is thus called theoretical yield. q The actual yield of a product in a chemical reaction is the actual amount obtained from the reaction. 14

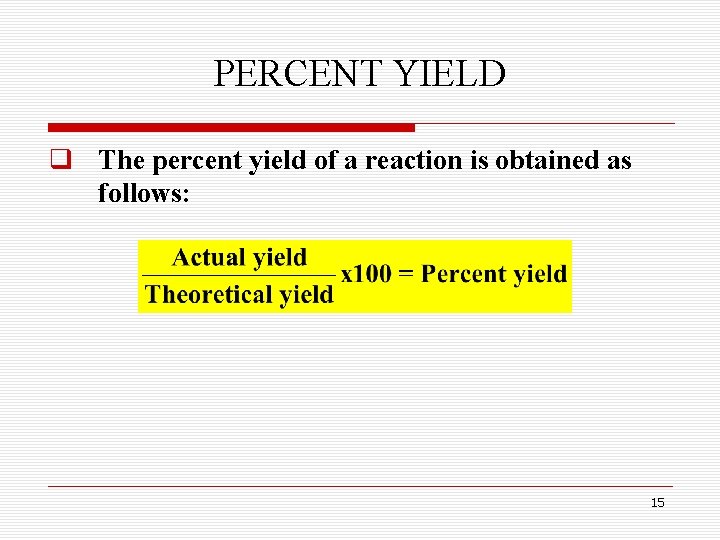
PERCENT YIELD q The percent yield of a reaction is obtained as follows: 15

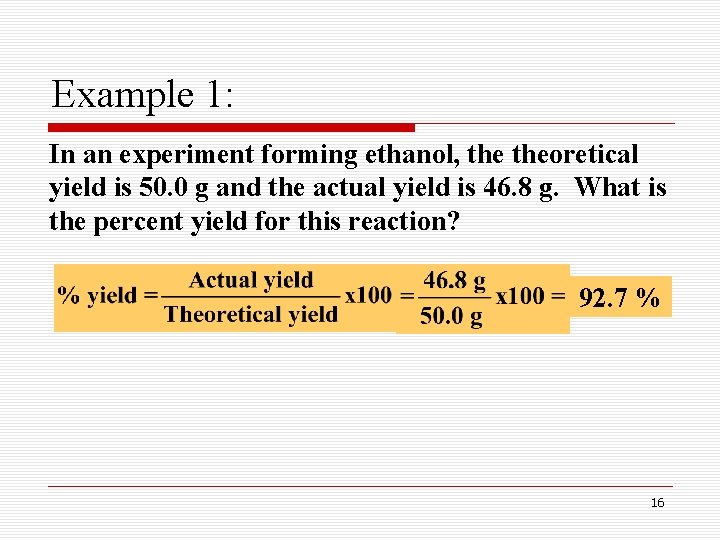
Example 1: In an experiment forming ethanol, theoretical yield is 50. 0 g and the actual yield is 46. 8 g. What is the percent yield for this reaction? 92. 7 % 16

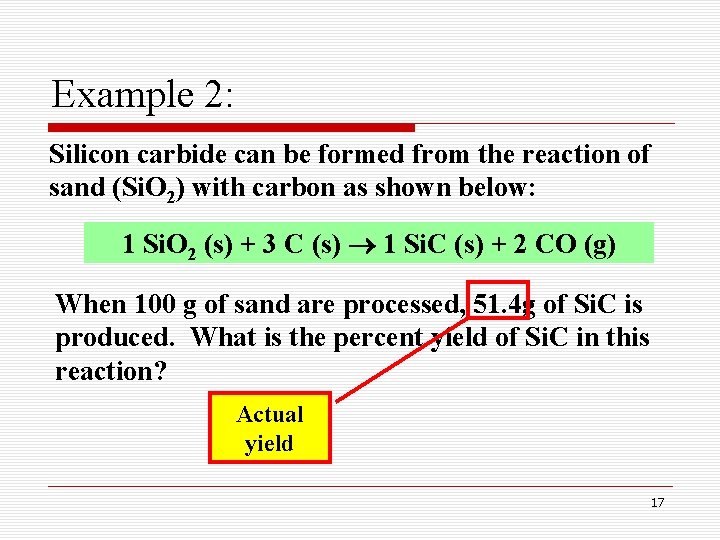
Example 2: Silicon carbide can be formed from the reaction of sand (Si. O 2) with carbon as shown below: 1 Si. O 2 (s) + 3 C (s) 1 Si. C (s) + 2 CO (g) When 100 g of sand are processed, 51. 4 g of Si. C is produced. What is the percent yield of Si. C in this reaction? Actual yield 17

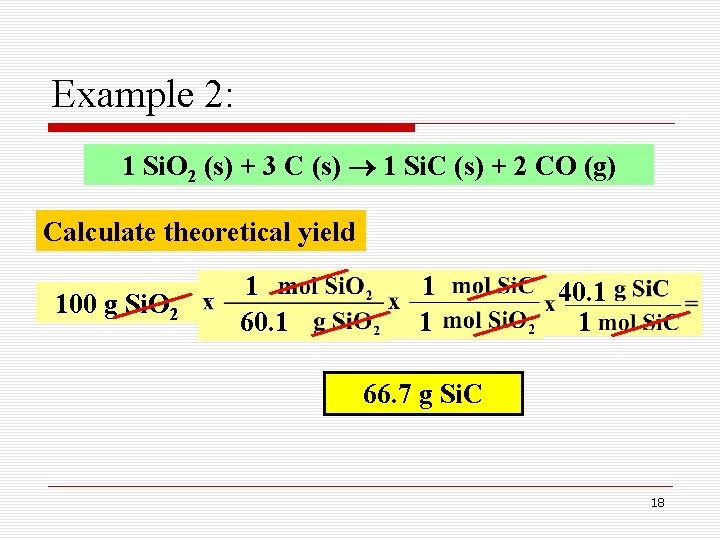
Example 2: 1 Si. O 2 (s) + 3 C (s) 1 Si. C (s) + 2 CO (g) Calculate theoretical yield 100 g Si. O 2 1 60. 1 1 1 40. 1 1 66. 7 g Si. C 18

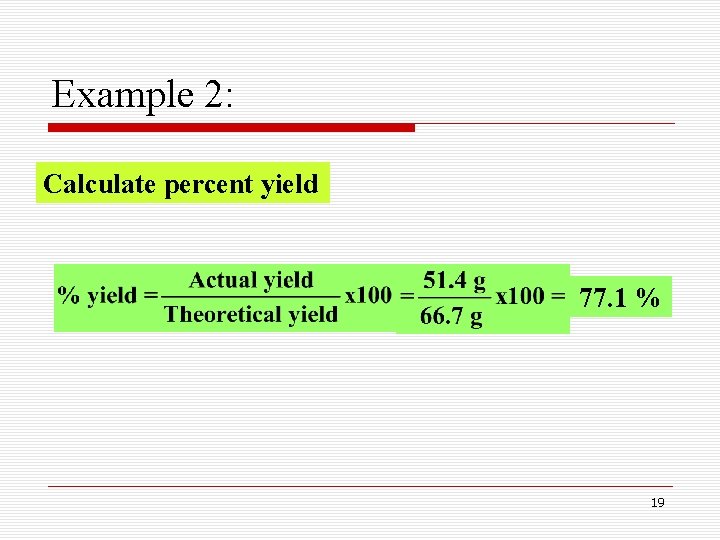
Example 2: Calculate percent yield 77. 1 % 19

Theoretical and Actual Yield • In order to determine theoretical yield, we use reaction stoichiometry to determine the amount of product each of our reactants could make. • The theoretical yield will always be the least possible amount of product. – The theoretical yield will always come from the limiting reactant. • Because of both controllable and uncontrollable factors, the actual yield of product will always be less than theoretical yield.

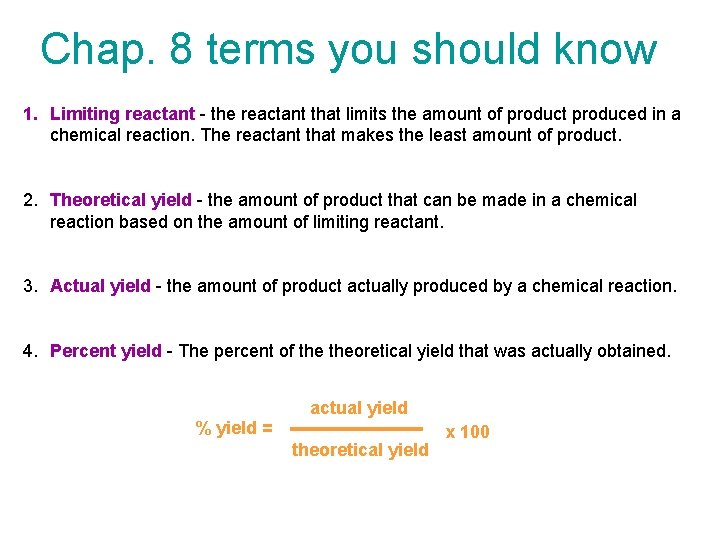
Chap. 8 terms you should know 1. Limiting reactant - the reactant that limits the amount of product produced in a chemical reaction. The reactant that makes the least amount of product. 2. Theoretical yield - the amount of product that can be made in a chemical reaction based on the amount of limiting reactant. 3. Actual yield - the amount of product actually produced by a chemical reaction. 4. Percent yield - The percent of theoretical yield that was actually obtained. actual yield % yield = theoretical yield x 100

THE END 22